Importance of lactate dehydrogenase (LDH) and monocarboxylate transporters (MCTs) in cancer cells
Abstract
Background
In most regions, cancer ranks the second most frequent cause of death following cardiovascular disorders.
Aim
In this article, we review the various aspects of glycolysis with a focus on types of MCTs and the importance of lactate in cancer cells.
Results and Discussion
Metabolic changes are one of the first and most important alterations in cancer cells. Cancer cells use different pathways to survive, energy generation, growth, and proliferation compared to normal cells. The increase in glycolysis, which produces substances such as lactate and pyruvate, has an important role in metastases and invasion of cancer cells. Two important cellular proteins that play a role in the production and transport of lactate include lactate dehydrogenase and monocarboxylate transporters (MCTs). These molecules by their various isoforms and different tissue distribution help to escape the immune system and expansion of cancer cells under different conditions.
1 INTRODUCTION
There is a significant increase in the incidence and prevalence of noncommunicable diseases, despite many successful advances in controlling and preventing contagious diseases in recent decades.1-3 Meanwhile, cancer is the second leading cause of mortality after cardiovascular diseases in some countries4, 5 and is one of the principal causes of death throughout the world, which is heterogeneous from the aspect of genetically. Despite different genetic variations, the common feature of all cancer types is their excessive growth.6, 7 One of the most remarkable changes of cancer cells is altering their metabolism, which has recently been considered as an important and effective factor in the progression of cancer.8, 9 Previous studies have shown that we can inhibit the tumor cells growth and survival throughout halting or reducing their cellular metabolism.10, 11 Changing lactate metabolism as an anaerobic glycolysis by-product that mainly produced in the absence of oxygen in the body is one of the adaptive proceedings of tumor cells for survival and further progression.8 Tumor early development and growth occur in the low vascular regions. There are two different cell types in the tumor lesions included aerobic region and hypoxic regions. Aerobic region included the cells that reside near blood vessels and their energy demands provided through oxidative phosphorylation (OXPHOS), while hypoxic regions are cells that reside far from blood vessels and their energy demands provided through the anaerobic metabolism of glucose. The metabolism of cancer cells has considered equivalent to aerobic glycolysis for a long time. After a while, this theory has been considered imperfect by biologists. The cancer cells metabolic signatures not only do not result in passive response to mitochondrial damage but also because of the reprogramming of the metabolism of cancer cells arose from oncogenes activity needed for anabolic growth.12 Progresses in cancer metabolism studies over the last year have improved our knowledge of how aerobic glycolysis and other metabolic changes detected in cancer cells provide the anabolic needs related two cell proliferation and development. Understanding the mechanisms employed by cancer cell to metabolism and survival can lead to the development of new strategies in fighting cancers and increase our understanding on the behavior of cancer cells. In this article, we review the various aspects of glycolysis with a focus on types of monocarboxylate transporters (MCTs) and the importance of lactate in cancer cells.
2 METHODOLOGY
In this study, data on various aspects of glycolysis with a focus on types of MCTs and lactate in cancer cells were found using PubMed, Scopus, and the Google Scholar database. The Internet searches were done to find published manuscripts with the keywords Cancer metabolism, Monocarboxylate transporters, lactate. All English language articles were found and read independently by two individuals. Literature search strategy for data in tables including inclusion/exclusion criteria and results was provided in Supporting Information: Figure S1.
3 RESULTS AND DISCUSSION
3.1 Advantages of aerobic glycolysis pathway for tumor growth
Even in the presence of ample oxygen, cancer cells demonstrate a distinctive form of cellular metabolism characterized by high levels of glucose uptake and increased conversion of glucose to lactose via the glycolytic pathway (fermentation). This phenomenon called the Warburg effect and known anaerobic glycolysis, has been recognized for many years. Warburg metabolism is not cancer-specific, but instead is a general property of growing cells that is exploited by cancer cells. Aerobic glycolysis provides rapidly dividing tumor cells with metabolic intermediates that are needed for the synthesis of cellular components, whereas mitochondrial OXPHOS does not.
The aerobic glycolysis pathway provides several advantages for tumor cells growth as below. Using aerobic glycolysis, the cancerous cell can survive in variable levels of oxygen pressure (due to unstable hemodynamics of blood vessels) and produce ATP (this condition is associated with risk of death for OXPHOS-dependent cells).13
Tumor cells produce bicarbonate and lactate. These acids make the microenvironment suitable for invasive tumor cells and suppress the immune system.14-16 Lactate produced by tumor cells can be uptake by stromal cells (by Monocarboxylate transporter 1 [MCT1] and MCT2) and used to pyruvate produce.15
The most important advantage is using the glycolysis-pathway mediators for anabolic reactions (e.g., use of glucose 6-phosphate for the synthesis of glycogen and ribose 5- phosphate and nicotinamide adenine dinucleotide phosphate [NADPH], dihydroxyacetone phosphate for the synthesis of triglycerides and phospholipid, pyruvate for the synthesis of alanine and malate).17 The exploitation of the pentose phosphate pathway (PPP) as one of the glycolysis branching commonly increases in tumorgenesis. Moreover, some of the major enzymes of this pathway, such as transketolase 1 and transaldolase, have shown increased expression in many cancers.18-21 Also, one of the reasons for reducing the use of the Krebs cycle is some of the products of this cycle, such as nicotinamide adenine dinucleotide hydrogen (NADH) and adenosine triphosphate (ATP), which are the main inhibitors of glucose metabolism.22
3.2 Metabolic changes in cancer cells
3.2.1 Absorption of glucose and amino acids (increased demand for nitrogen)
Glucose and glutamine (unnecessary amino acid with two reduced nitrogen atoms) are the most important and essential precursors for the biosynthesis of cellular materials). Due to increased carbon consumption in biosynthetic pathways and growth signals, the cell's need for nitrogen increases. Transcription factors such as C-MYC and E2F increase glutamine uptake.22 Lack of glutamine leads to cell cycle arrest in the S-phase in some cells.23, 24 Embryonic stem cells, luminal cells of breast cancer,25, 26 and human glioblastoma tumors continue their growth and proliferation, even in the absence of glutamine in their microenvironment, which represents the glutamine production by these cells de novo.27 It also has been seen an increase in the expression of glutamine synthase (GS) enzyme in some cancers.28
3.2.2 Opportunity to absorb nutrients
The Ras or c-Src mutation enables cells to uptake released amino acids by the lysosomal degradation of extracellular proteins.29 Macropinocytosis is also stimulated by Ras- and c-Src-driven actin cytoskeleton remodeling.22 Moreover, soluble extracellular proteins and free amino acids can be absorbed into cancer cells by entosis or phagocytosis.30, 31
Cancer cells, in addition to the absorption of fatty acids from plasma, can induce the release of stored lipids in adjacent normal cells.22 Furthermore, increased expression of monoacylglycerol lipase (MAGL) and lipoprotein lipase in some of the cancer cells has been related with the invasion of them.32, 33 At the surface of the metastatic ovarian cancer cells, the expression changes of fatty acid binding protein 4 (FABP4) enable these cells to absorb fatty acids from the omental fat adipocytes.34 However, fatty acid de novo biosynthesis in normal cells is very low (except lipogenic tissues such as the liver, adipose tissue, and mammary epithelium during lactation).35, 36
3.2.3 Metabolic interactions with the environment
The increase in reactive oxygen species (ROS) production (due to OXPHOS pathway and excessive ATP production, cellular degradation, cellular overgrowth, and oncogene-induced senescence [OIS]),22 inhibit protein phosphatases such as phosphatase and tensin homolog (PTEN), protein tyrosine phosphatase 1B (PTP1B) and activators of family kinases of Src and mitogen-activated protein kinase (MAPK).37-39 Other effects of enhancement of ROS are facilitating the activation of hypoxia-inducible factor 1-alpha (HIF1-α) and nuclear factor erythroid 2-related factor 2 (NRF2).40, 41 In hypoxia conditions, induced overexpression of mitochondrial SHMT2 (serine hydroxyl methyl transferase 2) protects cells against the toxic effects of oxidative stress.42
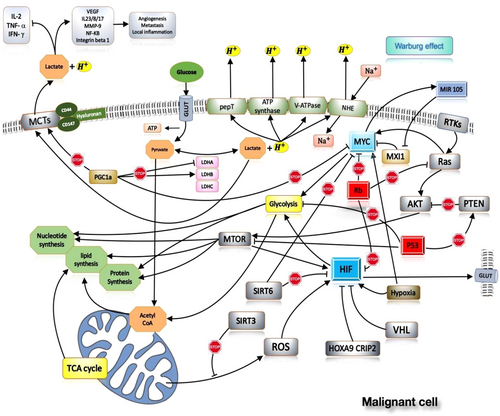
The increase in lactate production in cancer cells has several effects on the other host tissue and cells. Many studies highlighted the effects of lactate in suppressing on the immune cells such as activation of dendritic cell and T cell, migration of monocytes, and stimulation of M2 macrophage. M2 macrophages play an important role in the inhibition of the immune system and wound healing.14, 43-46 Several other effects have been described for lactate on the host body cells such as induction of angiogenesis,47 induction of HIF1a stability,48 activating of nuclear factor kappa-light-chain-enhancer of activated B cells and PI-3k signaling pathways,49 triggering the secretion of angiogenesis factors such as vascular endothelial growth factors (VEGFs)47, 50-52 stimulate and stimulating the production of acid hyaluronic by fibroblasts (may play a role in cellular invasiveness).53 Some important factors involved in tumor cell metabolism and their effects listed in Table 1 and Figure 1.
| Factor | Effect | References | ||
|---|---|---|---|---|
| SREBP |
|
[143-146] | ||
| FBI-1 (Pokemon/ZBTB7A) |
|
[143] | ||
| MYC (Figure 1) |
|
[147-154] | ||
| mTORC1 |
|
[155-157] | ||
| HIF1 (Figure 1) |
|
[7, 158] | ||
| PI3K |
|
[159] | ||
| Akt (Figure 1) |
|
[160] | ||
| ErbB2 (Her2) |
|
[161] | ||
| PD-L1 |
|
[162-172] | ||
| CAV1/caveolin 1 |
|
[173, 174] | ||
| CD147 (Figure 1) |
|
[175, 176] | ||
| Ecdysoneless |
|
[177] | ||
| FAK |
|
[178] | ||
| GRP78 |
|
[179] | ||
| KSHV |
|
[180] | ||
| LMP1 |
|
[181] | ||
| P2X7R |
|
[182] | ||
| Skp2 |
|
[183] | ||
| BIRC5/Survivin |
|
[184] | ||
| Wnt/β-catenin |
|
[152] | ||
| p53 (Figure 1) |
|
[185-188] | ||
| Fumarate hydratase (fumarase) |
|
[189] | ||
| abhd5 (other name: CGI-58) |
|
[190] | ||
| GRIM-19 |
|
[191] | ||
| HSP40 |
|
[192] | ||
| KLF4 |
|
[193] | ||
| TRAP1 |
|
[194] | ||
| FBI-1 (also known as Pokemon, ZBTB7A) |
|
[143, 195] | ||
- Abbreviations: abhd5, α/β-hydrolase domain-containing 5; Ang-1, angiopoietin 1; Ang-2, angiopoietin 2; EMT, epithelial-mesenchymal transition; FAK, focal adhesion kinase; FAS, fatty acid synthetase; FBI-1, factor that binds to the inducer of short transcripts of human immunodeficiency virus-1; FGFR1, fibroblast growth factor receptor 1; G3PDH. glyceraldehyde 3-phosphate dehydrogenase; GRIM-19, gene associated with retinoid-interferon-induced mortality-19; GRP78, glucose-regulated protein 78; HIF, hypoxia-inducible factor; HSP40, heat shock protein 40; IGF2, insulin-like growth factor 2; ITGB4, integrin β 4; KLF4, Krüppel-like factor 4; KSHV, Kaposi's-sarcoma-associated herpesvirus; LDHA, lactate dehydrogenase A; LMP1, latent membrane protein 1; MAPK, mitogen-activated protein kinase; MCT1, monocarboxylate transporter 1; P2X7R, P2X7 receptor; PDHA, pyruvate dehydrogenase A; PDHB, pyruvate dehydrogenase B; PDHK1, pyruvate dehydrogenase kinase 1; PI3K, Phosphoinositide 3-kinase; PKB, protein kinase B; PFK, phosphofructokinase; PKM2, pyruvate kinase M2; ROS, reactive oxygen species; SIRT, selective internal radiation therapy; Sp1, specificity protein 1; SRE, sterol-responsive element; SREBP, SRE-binding protein 1; TRAP1, TNF receptor-associated protein; VEGF, vascular endothelial growth factor.
3.3 Glycolysis pathway and cancer cells
The result of the anaerobic metabolism of glucose in cancer cells that reside far from vessels is lactate production, which releases from these cells and entered into the intercellular spaces. This metabolite is entered to near vasculature cancer cells from intercellular space and provides their energy supply.9
The glycolysis pathway in cancer cells allows them to consume most of the cell glucose storage and convert it to lactate. Lactate stimulates and enhances cancer cell growth and proliferation. Therefore, it is imperative to recognize and survey the genes involved in this pathway, especially the genes that their product plays an important role in lactate metabolism.54 For example, some cancer cells such as cells in glioblastoma use aerobic glycolysis pathway for energy production even in the presence of oxygen (Warburg effect) (Figure 1).55
As noted earlier, a large quantity of lactate and proton is produced due to the increasing glycolysis. This extra proton led to an acidic space inside the cell, which can induce normal cell apoptosis under normal conditions.56 However, this extra proton is sent out or used by cancer cells through different mechanisms57 such as using the family of MCTs,58 H/Na exchanger (NHE),59 V-ATPase pump,60, 61 carbonate anhydrase enzyme,16 and the conversion of glutamate to gamma aminobutyrate.62 Since this acidity increases the cancer cells invasion potentials as well as drug resistance; therefore, these molecules are overexpressed in cancerous cells than normal cells.55 Moreover, not only lactate acts as a signal to stimulate angiogenesis but also is an immunosuppressive agent.63
There are two main checkpoints for regulating the production and transport of lactate in the cell included lactate dehydrogenase (LDH) and the family of MCTs. LDH has an important role in the conversion of pyruvate to lactate, and MCTs are involved in the transport of lactate into and out of the cells.8 These monocarboxcylates, like lactate, can be transferred to other cancer cells, or use for OXPHOS.
3.3.1 LDH
LDH is produced in normal cells such as the brain, heart, liver, kidney, skeletal muscle, red blood cells, and lungs.64, 65 However, LDH is overexpressed in some conditions such as hypothyroidism, anemia, meningitis, myocardial infarction, acute pancreatitis, acquired immunodeficiency syndrome (AIDS), liver/pulmonary disease, as well as in most of cancerous cells. Therefore, it can be used as a cancer cell marker or as a therapeutic option for cancer.66
This enzyme is found in different organisms. LDH converts pyruvate to lactate when oxygen is absent or trace and it performs the reverse reaction in the Cori cycle in the liver.64 Therefore, LDH is an enzyme with two forward and reverse functions. Structurally, this enzyme is a tetrameric enzyme composed of two major subunits (A, B), which are encoded by two the Lactate dehydrogenase A (LDHA) (11p15) and LDHB (12p12) genes. These two subunits produce five isoenzymes.65, 67 Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) by reducing the mRNA transcriptions of the LDHA and MYC genes and increasing the mRNA transcriptions of the LDHB and MCT1 genes regulate the conversion of pyruvate to lactate gene68 (Figure 1).
3.3.2 MCTs
Monocarboxylate is referred to the compounds such as lactate, pyruvate, ketone bodies, and thyroid hormones that play an important role in the energy metabolism of different tissues (i.e., skeletal muscle, heart, brain, and blood cells).69, 70 Among monocarboxylates, lactate is considered as one of the most important glycolysis products. MCTs facilitate the transport of lactate and other monocarboxylates, thus have an important role in cellular metabolism.71
This family of monocarboxylic transporters (MCTs) is considered as one component of the SLC16 family. These soluble carriers structurally consist of 12 transmembranes domain with a cytoplasmic C-terminal and N-terminus and one intracellular loop between domain 6 and 7.72, 73 MCTs in their transmembrane domains have the most conservative sequence, especially in the transmembrane one and five domains. However, in N and C terminal regions more diversity was seen.74 This family constitutes of 14 members that are identified by similar conserved amino acid sequence and often they are different in the type of substrate and their tissue distribution (Table 2). The first four members of this family (MCT1-4) are proton-linked transporter that facilitate transporting of short-chain monocarboxylates such as lactate, pyruvate, butyrate, and ketone bodies, and another this family members are involved in the transport of sodium-coupled monocarboxylic (SMCT) such as thyroid hormones.71 More precisely, MCT1, 2, 4 is involved in the transport of lactate, pyruvate, butyrate, acetate, and beta-hydroxybutyrate. MCT3 plays a role in the transport of lactate.73, 75 MCT5-14 transfer certain substances,76 for example, MCT9 in the transfer of carnitine and hemostasis of urate,76, 77 MCT8-10 in the transfer of thyroid hormones,73 and MCT 10 in the transfer of aromatic amino acids,78, 79 MCT 12 in the transfer of creatine,80 and guanidine acetate homeostasis,76 and MCT6 in the transfer of xenobiotic compounds such as bumetanide, nateglinide, and probenecid have an important role.76
| Protein | Gene name and locus | Subcategories | Properties | Tissue distribution | References | ||
|---|---|---|---|---|---|---|---|
| MCT-1 | SLC16A1 1p13.2 | MOT1 MEV MCT1D HHF7 |
Specific substrates
|
Most tissues except pancreatic β cells | [72, 75, 119, 196-203] | ||
| MCT-2 | SLC16A7 12q14 | MOT2 |
|
Liver, kidney, spleen, heart, brain, testis, pancreas | [72, 75, 119, 197, 199, 204-208] | ||
| MCT-3 | SLC16A8 22q13 | REMP |
|
Eye | [74, 197] | ||
| MCT-4 | SLC16A3 17q25.3 | MCT3 |
|
Heart, brain, skeletal muscle, lung, placenta, kidney, leukocyte, chondrocytes, testis | [72, 73, 75, 119, 197, 203, 209] | ||
| MCT-5 | SLC16A4 1p13.3 | MCT4 |
|
Placenta, heart, egg, liver, muscle, brain, kidney | [74, 75, 119, 197] | ||
| MCT-6 | SLC16A5 17q25.1 | MCT5 |
|
Lung, intestine, prostate, muscle, kidney, spleen, heart, brain, pancreas | [73-75, 119] | ||
| MCT-7 | SLC16A6 17q24.2 | MCT6 |
|
Brain, muscle, pancreas | [74, 75, 119] | ||
| MCT-8 | SLC16A2 Xq13.2 | MCT7, MRX22DXS128, AHDXPCT |
|
Most tissues | [73, 75, 119, 197, 210-212] | ||
| MCT-9 | SLC16A9 10q21.2 | YKW1, OXlT-2C10orf36 | Orphan transporter | Testis, ovule, brain, kidney, adrenal, eye, breast | [75, 119] | ||
| MCT-10 (TAT1) | SLC16A10 6q21-q22 | TAT1PRO0813 |
|
Skeletal muscle, heart, kidney, placenta, liver, intestine | [73, 75, 78, 119] | ||
| MCT-11 | SLC16A11 17p13 | - |
|
Eye, kidney, lung, skin, breast, ovary | [75, 119] | ||
| MCT-12 | SLC16A12 10q23.3 | CJMGCRT2CTRCT47 |
|
Kidney, testis, eye | [75, 119] | ||
| MCT-13 | SLC16A13 17p13.1 | - |
|
Breast, ovary, brain, heart | [75, 119] | ||
| MCT-14 | SLC16A14 2q36.3 | - |
|
Eye, pancreas, lung, breast, brain | [75, 119] | ||
- Abbreviations: AICAR, Aminoylamidazole carboxymide-ribonuclease; AMPK, active protein kinase AMP; CHC, a-cyano-4-hydroxycinnamate; DBDS , 4,4′-dibenzamidostilbene-2,2′-disulphonate; DIDS, 4′-diisothiocyanostilbene-2,2′-disulphonate; pCMBS, p-chloromercuribenzene sulfonate.
The MCTs have numerous physiological functions. They are often expressed in different tissues and involved in regulating of activities such as gluconeogenesis, lymphocyte activation, spermatogenesis, thyroid hormones metabolism, beta-pancreatic cells activity, and drug delivery.63 In the tumor tissue, the process of transporting and exchanging of lactate among different tumor cells is also carried out MCTs.8 The export of lactate from cancer cells is an important factor in maintaining the acidic phenotype and survival of these cells, with considering this assumption, studies showed repression of the MCT-4 gene reduces tumor growth.81, 82 Several factors affect the regulation of MCTs expression, for example, it has been shown that hypoxia increases the expression of MCTs.83
In patients with (rs1049434) polymorphism, a reduction of 35%–40% in lactate transmission in erythrocytes has been observed.84 Two polymorphisms (rs10506398 and rs10506399) in MCT2 have been associated with reduced sperm count and infertility in men.76, 85 Polymorphism (rs2242206) in MCT-9 causes lysine to be transformed into threonine at position 258, which is associated with renal overload gout.86 A nonsense mutation in the MCT-12 gene, which causes Q215X amino acid mutation, has been observed in patients with cataract and increased levels of urinary glucose.87 In Figure 1, MCT molecules and the effect of them on the process and intracellular molecules in one malignant cell are shown.
The proper expression of MCTs often requires an ancillary protein that for MCT1, 3, and 4 is CD147 (other names are EMMPRIN, OX-47, HT7, and Basigin) which is MCT chaperone. Ancillary protein for MCT-2 is Embigin or gp70. These ancillary proteins are a multipurpose glycoprotein of the immunoglobulins family.8, 88 Upregulation of CD147 has been reported in metastatic breast cancer cells and in partnership with MCT-4, plays an important role in the invasion of the cancer cells.89 CD147 is a highly glycosylated membrane protein belonging to the immunoglobulin family, which in human located at 19p13.3.90 The human gene has 10 exons91 and encoded 269 amino acids that consist of four types: CD147-1, CD147-2, CD147-3, and CD147-4. Among these isoforms, CD147-2 has the most expression and distribution.92-95 This molecule plays a role in regulating of lymphocyte response, cancer metastasis, inducing of MCT, inflammatory reaction, and spermatogenesis.96 Interactive proteins with CD147 and their functions have been shown in Table 3.
| Protein | Functions | References |
|---|---|---|
| Integrins | Adherence, proliferation, migration signal transmission | [97] |
| CD98 | Cell aggregation, skeletal structure | [98, 99] |
| MCT1, 3, 4 | Glycosylation and removal of lactate | [100] |
| Cavolin-1 | Inhibition of CD147 dimerization and activity, CD147 glycosylation increasing | [101, 102] |
| S100A | Cell migration | [99] |
| E-selectin | Neutrophilic Infiltration and adherence | [103] |
| CyPB | Cell migration | [104] |
| yPA | Cell migration, adherence, chemotaxis, Induction of metalloproteinase matrix, Increasing the regulation of metalloproteinase matrix 9, NF-κB pathway activation | [105-107] |
| CD147 | Adherence, induction of metalloproteinase matrix, NF-κB pathway activation | [99, 108] |
- Abbreviations: CyPA, cyclosporine B; CyPA, cyclosporine A; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells.
Hyaluronan is another protein that interacts with MCTs.109 Hyaluronan is an extracellular polysaccharide that is present in most tissues and has an instructive, cell signaling function in addition to its structural role.110-113 The concentration of hyaluronan is more in malignant cells than in normal tissues.109, 114, 115 Hyaluronan binds to various cell surface receptors such as CD44, Rhamm, TLR1/4, Hare and LYVE-1.110-113 Among these receptors, CD44 is the most known. The binding of this receptor to hyaluronan regulates signaling pathways such as apoptosis and drug resistance to anticancer agents.109 Emmprin as an essential ancillary protein for MCTs by stimulating the production of hyaluronan plays an important role in the invasion and division of cancer cells.112, 116, 117 Studies have shown that lactate produced in the glycolysis pathway stimulates the production of hyaluronan and the expression of CD44 in fibroblasts and melanoma cells.53, 118 The interaction of Emmprin-CD44 is one of the main causes of malignancy and drug resistance in cancer cells.109 Several studies have been conducted on the MCTs that the history of major studies and their results from 1999 to 2018 is shown in Table 4.
| Year | Gene | Result | References |
|---|---|---|---|
| 1999 | MCT1-4 |
|
[119] |
| 2005 | MCT3 |
|
[120] |
| 2010 | MCT3 |
|
[121] |
| 2012 | MCT1, 2, and 4 |
|
[122] |
| 2013 | MCT8 |
|
[123] |
| 2014 | MCT1-4 |
|
[124] |
| 2007 and 2014 | MCT9 |
|
[125, 126] |
| 2014 | MCT4 |
|
[127] |
| 2016 | MCT1 |
|
[55] |
| 2017 | MCT1 and 4 |
|
[128] |
| 2017 | MCT8 |
|
[129] |
| 2018 | LDH and MCT1 | In ovarian cancer cells, knockdown of SATB1significantly
|
[130] |
| 2018 | LDH-A and MCT1 | miR-124:
|
[131] |
- Abbreviation: HIF, hypoxia-inducible factor.
3.4 Diagnostic and therapeutic applications of metabolic changes in the cancer cells
Cancer is a disease that is known to alter cellular metabolism; therefore, metabolomics can play a major role in the early detection and diagnosis of cancer and in the evaluation of medical interventions and therapies to cancer.132 It has been established that aerobic glycolysis increases in cancer and this is known as the “Warburg effect.”133 The ultimate goal of most metabolomics cancer studies is to discover cancer-specific diagnostic, prognostic, or predictive biomarkers for a patient. Metabolomics research is being used to discover diagnostic cancer biomarkers in the clinic, to better understand its complex heterogeneous nature, to discover pathways involved in cancer that could be used for new targets and to monitor metabolic biomarkers during therapeutic intervention.134 These metabolomics approaches may also provide clues to personalized cancer treatments by providing useful information to the clinician about the cancer patient's response to medical interventions. Therapeutics in oncology is touching toward the use of medications that specifically target abnormal pathways involved in growth, proliferation, and metastases. Biomarkers are being increasingly applied in the early clinical development of such agents to identify, validate, and optimize therapeutic targets and agents; determine and confirm the mechanism of medications action, as a pharmacodynamic endpoint; and in predicting or monitoring responsiveness to treatment, toxicity, and resistance.135 Current examples of using metabolomics in developmental therapeutics are with tyrosine kinase inhibitors, proapoptotic agents, and heat shock protein inhibitors.136-138 Numerous metabolic inhibitors have been reported for cancer treatment both in preclinical studies and in clinical trials, included amino acid metabolisms inhibitor, lipid metabolisms inhibitor, glutamines inhibitor, coenzyme/nucleotide inhibitor, mitochondrial inhibitors, and transporter inhibitors. The development of metabolic inhibitors has been well addressed in a recently published review paper.139
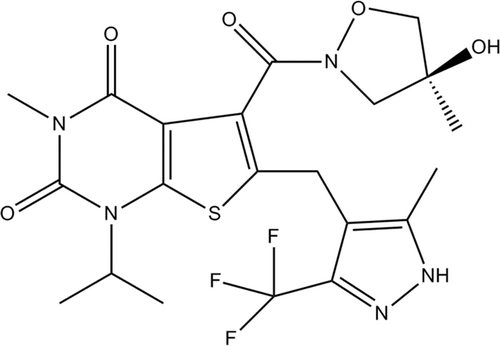
AZD3965 (Figure 2) is a first-in-class, strong (K1 = 1.6 nM), orally bioavailable, MCT-1 selective inhibitor. As cancer cells exhibit an elevated dependence on glycolysis lead to lactic acid formation, they up-regulate MCTs as a protective system to transport lactate and inhibit its intracellular accumulation.140 Significant antiproliferative effects of AZD3965 have been reported in some lymphoma cell lines as well as small-cell lung tumor systems.141 A pharmacodynamics indicator investigation has been shown that AZD3965 therapy lead to reduced lipid metabolisms including reduced choline concentration. Decreased choline levels has been attributed to reduced choline kinase A because of elevated intracellular levels of lactate in cancer cells.141 AZD3965-treated cancers show an increased frequency of dendritic cells and natural killer cells.142 Currently, AZD3965 is being evaluated in a phase I dose-ranging study.139
4 CONCLUSION
Changed energy metabolism is emerged as one of the very important cancer biochemical fingerprint which could be introduced as a cancer hallmark. These metabolic events are characterized by preferential dependence on glycolysis for the production of energy in an oxygen-independent condition. Glycolysis is one of the major underlying energy-related processes, which play critical roles in the initiation, and progression of all malignancies. Pyruvate and lactate are the main products of glycolysis. Given that, some cellular proteins (i.e., LDH and MCTs) involved in the production and transport of lactate. Considering the vital role of LDH and MCTs in the metabolism of cancer cells, these molecules can be investigated as a target for treatment or as markers in cancer diagnosis in future studies. Mounting evidence confirmed that these molecules could contribute to escape cancer cells from the immune system and promote the expansion of tumor cells under distinct conditions.
AUTHOR CONTRIBUTIONS
Hamed Hatami: Investigation; methodology; software; validation; writing – original draft; writing – review and editing. Atefe Sajedi: Data curation; investigation; writing – original draft. Seyed Mostafa Mir: Conceptualization; investigation; methodology; software; supervision; writing – original draft; writing – review and editing. Mohammad Yousef Memar: Conceptualization; methodology; software; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
The author is grateful to Golestan University of Medical Sciences, Gorgan, Iran, for providing all kinds of facilities to prepare this manuscript.
TRANSPARENCY STATEMENT
The lead author Seyed Mostafa Mir, Mohammad Yousef Memar affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Seyed Mostafa Mir and Mohammad Yousef Memar had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.