Potential clinical benefits of warfarin in end-stage cancers: A retrospective analysis
Abstract
Background and Aims
Coagulopathy and thromboembolism are common comorbidities in cancer, and anticoagulants, such as warfarin, are needed in specific situations. This study aimed to determine the clinical relevance of prothrombin time (PT) monitoring and the clinical usefulness of warfarin in patients with malignancy.
Methods
We retrospectively investigated patients with PT lower than 10% treated in our hospital between April 2006 and March 2013. Cases of false coagulopathy, including those due to technical errors during blood sampling, were excluded. The cause of coagulopathy was determined or estimated by physicians.
Results
This study included 338 cases comprising 155 females and 183 males with a median age was 68 (0–97) years. Among them, 89 (26.3%) had cancer, and 163 (48.2%) received warfarin at a median dose of 2.23 (0.5–8.0) mg/day. PT prolongation caused by warfarin overdose and malignancy exacerbation were observed in 75 (22.2%) and 64 (18.9%) patients, respectively. The leading reasons for warfarin administration were arterial fibrillation, chronic heart failure, and deep vein thrombosis. Univariate analysis revealed that the overall survival was higher in the warfarin and nononcology groups than in the nonwarfarin and oncology groups (both p < 0.001). In multivariate analysis, survival was significantly decreased in older adults (p = 0.049), those with malignancy (p < 0.001), and those without warfarin therapy (p < 0.001). Early mortality (within 3 days after PT prolongation) was observed in 65 patients and was mostly related to emergent diseases (36.9%, 24/65) and end-stage malignancy (32.3%, 21/65).
Conclusion
Patients with malignancy may experience subclinical PT prolongation upon disease progression. Warfarin treatment mitigates panic PT values in patients with malignancy. Conversely, those not treated with warfarin have poor survival, suggesting that coagulopathy without warfarin treatment can lead to death. Warfarin enhances hemostatic conditions, thereby preventing malignancy-related lethal hemorrhagic or thromboembolic events.
1 INTRODUCTION
Prothrombin time (PT) is often monitored in patients undergoing warfarin treatment for optimal anticoagulation therapy control. Sudden prolonged PT may indicate a comorbidity-related warfarin dysregulation. However, patients with cancer without warfarin treatment may also unpredictably manifest emergent PT dysregulation. Despite the use of warfarin in clinical oncology, the clinical significance of emergent PT dysregulation in patients with malignancies remains insufficiently studied. Therefore, this retrospective, longitudinal, cohort study aimed to investigate the clinical manifestation of a disorder related to panic PT levels in patients with an extremely low PT value (PT < 10%) at our institute.
PT level is used to monitor coagulation function and reflect the hemostatic conditions mediated by extrinsic pathway coagulation factors, such as factor VII and tissue factor. The activation of factor VII depends on vitamin K; however, warfarinization may hinder the extrinsic pathway through factor VII inhibition.1 Generally, PT is monitored during warfarin therapy,2 especially in patients at risk for thromboembolism. The prolonged status of PT levels should remain within 1.5–2.5 to ensure an adequate anticoagulant effect. PT monitoring is also crucial upon initiating vitamin K antagonist oral anticoagulants. However, the clinical significance of PT monitoring without anticoagulant therapy remains unknown. Therefore, we extracted all data of patients with panic PT levels (i.e., <10%) and investigated their clinical details retrospectively.
2 MATERIALS AND METHODS
2.1 Study population and sample preparation
This study retrospectively included consecutive patients with a PT value of <10% who were treated in our hospital between April 2006 and March 2013. Patient characteristics, PT dysregulation causes, and clinical outcomes were reviewed. However, cases of false coagulopathy, such as technical errors during blood sampling, were excluded. The cause of coagulopathy and the final diagnosis were determined by more than two hematologists/specialists. Furthermore, we checked the STROBE statement, a checklist that ensures a clear presentation of cohort studies.
2.2 Statistical analysis
The cohort's survival status was followed up until treatment termination or all-cause death, whichever occurred first. The overall survival was compared between the warfarin and nonwarfarin groups and between the oncology and nononcology groups. In addition, survival significance was determined by the log-rank test. Using a Cox regression model, we conducted a multivariate analysis according to the following parameters: age, sex, warfarin therapy, and malignancy coexistence. We hypothesized that any of the clinical parameters were identified as contributing factors to survival. Afterward, subgroup analyses were conducted by using some explanatory contributing factors, including age, sex, disease, warfarin treatment, and PT value as an exploratory parameter. This manuscript conforms to the SAMPL guidelines for statistical reporting. Two-sided t-test was used, and p < 0.05 were considered statistically significant. For the statistical analysis, we used the JMP5 software (version 5.0.1J).
3 RESULTS
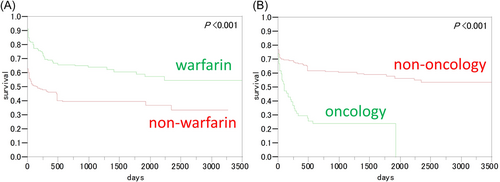
This study included 338 patients, consisting of 155 females and 183 males (median age: 68 [range: 0–97] years). The most common underlying disease was cardiovascular disease, followed by emergency and gastrointestinal diseases (n = 81, 61, and 44, respectively). Other underlying diseases were 2 respiratory, 20 renal/genitourinary, 14 hematological, 21 immunological, 19 neurological, 25 pediatric, 12 gynecological, 11 orthopedic, and 28 other diseases. Among the 338 patients, 163 (48.2%) received warfarin at a median dose of 2.23 (0.5–8.0) mg/day, and 89 (26.3%) had malignancies. Table 1 shows the patients' PT and PT–international normalized ratio (PT-INR). Between the warfarin and non-warfarin groups, both PT and PT-INR were within comparable ranges. In all patients, the most common causes of prolonged PT was warfarin overdose (including drug interaction) (75 patients, 22.2%) and malignancy exacerbation (at any cancer site) (64 patients, 18.9%). In 68 additional patients (not included in the study population), prolonged PT was pseudo-abnormal, as confirmed by re-examination of newly drawn blood samples. Warfarin was administered mainly because of atrial fibrillation, chronic heart failure, and deep venous thrombosis. Univariate analysis revealed that the overall survival was higher in the warfarin group than in the nonwarfarin group (Figure 1A, p < 0.001) and in the nononcology group than in the oncology group (Figure 1B, p < 0.001). A qualitative interaction was identified between warfarin use and malignancy coexistence; thus, patients' survival was also analyzed by combining the two factors (warfarin and malignancy). The median survival time was not attained in the warfarin/oncology group (estimated survival at 10 years: 62.9%). Nonwarfarin/nononcology, warfarin/oncology, and nonwarfarin/oncology groups showed a median survival time of 492, 241, and 62 days, respectively. The estimated survival at 3 years was 31.8% and 18.2% in the warfarin/oncology and nonwarfarin/oncology groups, respectively. The median follow-up time was 6 years and 100 (46–3642) days. In the multivariate analysis, parameters such as high age and malignancy showed a significant adverse effect. In addition, survival was significantly decreased among older adults (p = 0.049; hazard ratio [HR] = 1.006, 1.000–1.014) and those with malignancies (p < 0.001; HR = 1.902, 1.347–2.669). Conversely, warfarin use improved survival (p < 0.001; HR = 0.417, 0.293–0.587). Within 3 days after PT prolongation (early mortality), 65 patients died because of an emergent disease (36.9%, 24/65) and end-stage malignancy (32.3%, 21/65). A subgroup analysis was then conducted according to the cause of death. The last pathogenicity during the measurement of PT associated with poor prognosis included emergent diseases (visited the emergency unit via an ambulance), infectious diseases, cardiovascular diseases, oncological terminal status, and chronic heart failure, with a median survival time of 0 (0–1), 2 (0–13), 8 (0–262), 27 (4–63), and 98 (12–254) days, respectively. Therefore, emergent diseases were significantly associated with poor prognosis (p < 0.001).
| Total (n = 338) | With warfarin (n = 163) | Without warfarin (n = 175) | |
|---|---|---|---|
| PT (%) | 5.89 (below detectable range–9.00) | 7.00 (2.0–9.0) | 5.83 (BDR–9.0) |
| PT-INR | 14.66 (6.92–45.49) | 14.72 (6.92–45.49) | 14.53 (8.46–27.49) |
- Abbreviation: BDR, below detectable range.
4 DISCUSSION
Prolonged PT occurs in most patients treated with warfarin or presenting malignancies.3 In our cohort with panic PT values, warfarin administration enhanced the survival of the oncology group (recovery rate of 13.6%), as supported by our multivariate analysis results. In the nonwarfarin/oncology group, panic PT values were associated with poor survival, indicating that coagulopathy leads to mortality.4 Therefore, warfarin may ameliorate hemostatic conditions and avoid malignancy-related lethal hemorrhagic or thromboembolic events. Hence, we advocate that warfarin treatment may help rescue patients with end-stage cancer suffering from a PT-related emergent crisis; thus, its prophylactic use may also mitigate coagulopathy in these patients.5
Low-molecular-weight heparin (LMWH) is controversial in this setting.6, 7 All existing guidelines and recommendations aim to prevent venous thromboembolism (VTE), which has an estimated prevalence of 10% and may accelerate death in patients with cancer.8, 9 In some populations, warfarin is used in an end-of-life setting as a standard prophylactic regimen for VTE according to the experience and preference of health professionals,8 although some randomized controlled trials (RCTs) failed to demonstrate the survival benefits of anticoagulation treatments.7 When prescribing warfarin, oncologists are expected to estimate the risk of bleeding,10 especially for palliative patients with cancer. Further studies are required to estimate bleeding risk in patients with cancer.
Patients with persistent risk factors, such as cancer, have a significantly higher risk of experiencing recurrent thrombosis.4 Some pivotal clinical studies reported that direct oral anticoagulants (DOAC) are not inferior to LMWH in cancer-related thrombosis (CAT) treatment.11-14 Four kinds of DOACs are available as prophylactic drugs for VTE in patients with cancer. Among the DOACs, such as edoxaban, rivaroxaban, and apixaban, edoxaban exerted noninferior efficacy in an RCT compared with LMWH (dalteparin).11 Edoxaban is the most effective for VTE recurrence and major bleeding occurrence.11 This finding has been pivotal evidence for standard care for patients with cancer with VTE risk for a long period in Japan. Additionally, an RCT revealed that rivaroxaban has relatively lower VTE recurrence but higher clinically relevant nonmajor bleeding than dalteparin.12 Recently, apixaban has shown to be noninferior to subcutaneous dalteparin for the prevention of recurrent VTE.13 Apixaban is highly convenient because it does not require dose adjustment by renal function, except for patients with severe renal dysfunction. A guideline published by SCC and the Journal of Thrombosis and Hemostasis14 stated that VTE is manageable by DOACs, including edoxaban, rivaroxaban, and apixaban. According to the National Comprehensive Cancer Network Guideline 2022, apixaban is one of the recommended options as category 1 for ambulatory medical patients with cancer with high VTE risk.15 Additionally, apixaban is reportedly effective for CAT in a Japanese cohort,16 as well as the appropriateness of DOAC. However, the efficacy of DOAC for patients with cancer with high VTE risk requires further investigation.
A meta-analysis of two RCTs showed that the 6-month rate of recurrent VTE was lower in patients receiving DOACs than in those receiving LMWH, with a statistically significant risk reduction.17 However, the major bleeding rate, as well as the clinically relevant nonmajor bleeding rate, was higher in patients receiving DOACs than in those receiving LMWH. The 6-month mortality rates were lower in studies included in the meta-analysis than in the RCTs that compared LMWHs with vitamin K antagonists for managing cancer-associated thrombosis.18, 19 Presently, the insight into whether these differences in mortality reflect the selection of higher risk patients in the trials assessing LMWH remains unclear. However, the prophylaxis of recurrent VTE and bleeding incidence always offset each other. Optimally selected patients with higher risk would gain benefits from anticoagulants. Therefore, we should carefully and properly designate the target patients.
The most important oncologic emergency concerning coagulopathy is disseminated intravascular coagulation (DIC),20 which results from tumor lysis or progression and involves a crucial and lethal complication. All cases presenting DIC showed progression of underlying malignancies; thus, cancer-related DIC may lead to death. This finding was reflected in the shorter survival of this group of patients than of those with PT value/PT-INR index abnormality caused by other reasons.21, 22 Moreover, PT pseudo-abnormalities were observed in 20% of patients, emphasizing the need to avoid technical errors by re-examining those with asymptomatic coagulopathy. However, half of the panic PT episodes occurred in patients treated with warfarin, indicating that warfarin administration is not closely associated with disorders related to PT at panic levels. Additionally, half of the patients with such disorders had underlying malignancies. Therefore, PT should be closely monitored, irrespective of warfarin administration. Currently, the most useful prophylactic anticoagulant regimen for VTE is LMWH, which replaces warfarin because of its safety and manageability. Thereby, LMWH should be used as an anticoagulant regimen whenever possible. This regimen should be adopted to each community setting following the physician's preference, medical situation, hospitalization place, and patient's preferences.8
This retrospective cohort study focused on PT/PT-INR alarming cases. Approximately one-fifth of the study population (68 events in 328 patients) had false coagulopathy, indicating that rechecking the samples for sampling errors should be initially performed when faced with positive results. The limitations of this study were (1) its retrospective nature, (2) the absence of a control–case study, and (3) the lack of concomitant sampling of coagulation factors. In patients with cancer-related DIC, the routine administration of vitamin K can result in adverse events, such as thrombosis.
5 CONCLUSIONS
Incidentally prolonged PT mainly occurred in patients treated with warfarin or complicated with malignancies. In our panic PT cohort, warfarin administration enhanced survival in patients with malignancies (recovery rate: 13.6%). The multivariate analysis results support that warfarin use in patients with cancer is beneficial. Disorders related to PT at panic levels in patients with cancer without taking warfarin treatment led to poor survival, indicating that coagulopathy can result in death. Our cohort study suggests that warfarin ameliorates hemostatic conditions and prevents lethal hemorrhagic or thromboembolic events in patients with malignancies. In conclusion, PT/PT-INR coagulation is associated with disease progression in patients with malignancies irrespective of warfarin administration.
AUTHOR CONTRIBUTIONS
Osamu Imataki: Conceptualization; data curation; formal analysis; investigation; methodology; supervision; validation; writing – original draft; writing – review & editing. Takeshi Arai: Data curation; methodology; project administration; resources; writing – review & editing. Makiko Uemura: Funding acquisition; investigation; project administration; resources; writing – review & editing.
ACKNOWLEDGMENTS
This work was supported by internal funding (JSPS KAKENHI grant numbers: JP16K19315, JP15K09570, and JP19K17927). The role of these sources was publication fee.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
We obtained approval from the Kagawa University Internal Review Board (IRB). The protocol was approved by Kagawa University IRB following the relevant guidelines and regulations. Informed consent was waived under the approval of IRB in the study.
TRANSPARENCY STATEMENT
The lead author Osamu Imataki affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data sets used and analyzed during the current study are available from the corresponding author on reasonable request.