Short-term effects of a novel bronchial drainage device: A pilot cohort study in subjects with cystic fibrosis
Abstract
Background and Aims
In cystic fibrosis (CF) airways, impaired airway mucociliary clearance and mucus accumulation due to cystic fibrosis transmembrane conductance regulator defects contribute to inflammation, progressive structural lung damage, and decline of lung function. Physiotherapy is essential to promote mucus mobilization and removal in CF and is a key element of rehabilitation measures, but conventional techniques may be suboptimal to mobilize viscous mucus. This study aimed to test the specific effects of a novel bronchial drainage device (BDD) (Simeox®; PhysioAssist) in subjects with CF and evaluate lung function, diaphragm mobility, and sputum properties.
Methods
This prospective monocentric clinical cohort study in the setting of outpatient physiotherapy of CF patients (n = 21) with stable CF lung disease collected pulmonary lung function tests (PFT), diaphragm mobility, and sputum properties before and after two physiotherapy sessions using the novel BDD. PFT was assessed using spirometry and diaphragm mobility using m-mode ultrasound analysis. Spontaneous sputum samples were collected before and after using the BDD and analyzed for microstructure and DNA concentrations.
Results
PFT parameters (FEV1, FVC, MEF25/50/75) were not affected by the use of the BDD. Ultrasound analysis of diaphragm mobility revealed an increase in maximum diaphragm excursion upon the intervention. Mucus analysis demonstrated altered microstructure and higher DNA concentrations collected after using the BDD compared to samples collected before. Pearson correlation analysis showed significant correlations between changes in mucus properties and DNA levels in respective mucus samples.
Conclusion
Our results demonstrate that the novel BDD improves diaphragm mobility and alters sputum properties in subjects with CF. The novel BDD with unique properties may be further studied as a device in CF-specific physiotherapy to facilitate sputum mobilization of CF patients.
1 INTRODUCTION
Cystic fibrosis (CF) is the most common autosomal recessive inherited disease in Caucasians, affecting approximately 100,000 individuals worldwide.1 Due to viscous secretion in various organs, frequent symptoms that compromise quality of life are chronic bronchitis with recurrent pulmonary infections and inflammation, the gradual decline of lung function, chronic sinusitis, pancreatic insufficiency with failure to thrive, and hepatopathy. Despite continuous therapy improvement, patients still suffer from frequent symptoms, compromised quality of life, and shortened life expectancy. Being a multisystemic disease, CF affects almost all organ systems, however, its pulmonary manifestation represents the main cause of CF-related morbidity and mortality. In the airways, genetic cystic fibrosis transmembrane conductance regulator (CFTR) defects lead to impaired mucociliary clearance, mucus plugging, and obstruction.2, 3 Accumulation of thickened mucus in the airways contributes to persistent infection and inflammation and subsequent progressive structural lung damage and decline of lung function.2, 4
In CF lung disease, airway clearance techniques (ACT) have been a cornerstone in bronchial drainage and mucus removal. Current guidelines recommend continuous ACT from the time point of diagnosis.5 Methods for mucus mobilization include manual techniques and vibrating devices applying intra- or extrathoracic oscillations.6 For most of the commonly used bronchial drainage methods, improvement of thoracic mobility, pulmonary function, or further patient-related outcomes have been demonstrated in both infants and adults with CF.7-9 Although beneficial effects of airway clearance physiotherapy are unquestionable, among the established techniques there is no distinct evidence for the advantages of one method over another.8, 9 Therefore, patient-related factors such as individual tolerability, symptoms, and preferences frequently contribute to the selection of airway clearance methods for the individual patient.
The recently approved bronchial drainage device (BDD) Simeox® (Physio-Assist S.A.S.) provides a novel technique for airway clearance therapy. It applies intrathoracic oscillatory negative pressure impulses during exhalation via a mouthpiece. Vibratory signals are considered to spread along the bronchial tree and thereby impact biophysical mucus properties. According to the manufacturers' information, the technology particularly aims at the mobilization of mucus from distal airway areas. The technique is based on a pneumatic vibration signal intended to liquefy airway mucus and facilitate its expectoration. After professional instruction, therapy with the device is designated to be performed independently from the physiotherapist.
The application of the novel BDD for CF airway clearance has had limited investigation. Only one small trial with pediatric subjects with CF examined the use of the novel method during exacerbation and concluded, that the BDD is a safe and appropriate ACT in this group.10 The effect of the BDD in clinically stable subjects with CF has not been systematically evaluated.
Diaphragm ultrasound can be used to assess air trapping and airway obstruction, as has been demonstrated in patients with chronic obstructive pulmonary disease (COPD).11-14 In a very recent study, an ultrasound of the diaphragm has been used in CF patients for disease severity.15
Sputum viscosity correlates with a change in microstructure, measurable by fluorescence recovery after photobleaching (FRAP) and an increase in DNA concentrations.16-19
In this pilot study, we investigated the short-term effects of therapy with a novel BDD in a cohort of adult subjects with stable CF lung disease. The study objectives were to evaluate: (1) Does the BDD affect lung function even after short-term use? (2) Has the BDD had an impact on diaphragm mobility, assessed by diaphragm excursion during the ultrasound? (3) Does the BDD affect sputum properties, reflected by FRAP and DNA concentration? As the device is supposed to remove mucus from distal lung areas, we hypothesized, that therapy using the BDD affects pulmonary function, thoracic mobility, and properties of expectorated sputum.
2 METHODS
2.1 Study design and recruitment
This study was approved by the Ethics Committee of Ulm University (Chairman Florian Steger; no. 98/20, approved May 8, 2020), registered at the German Clinical Trials Register (DRKS), study ID: DRKS00024819, and conducted according to the Declaration of Helsinki.
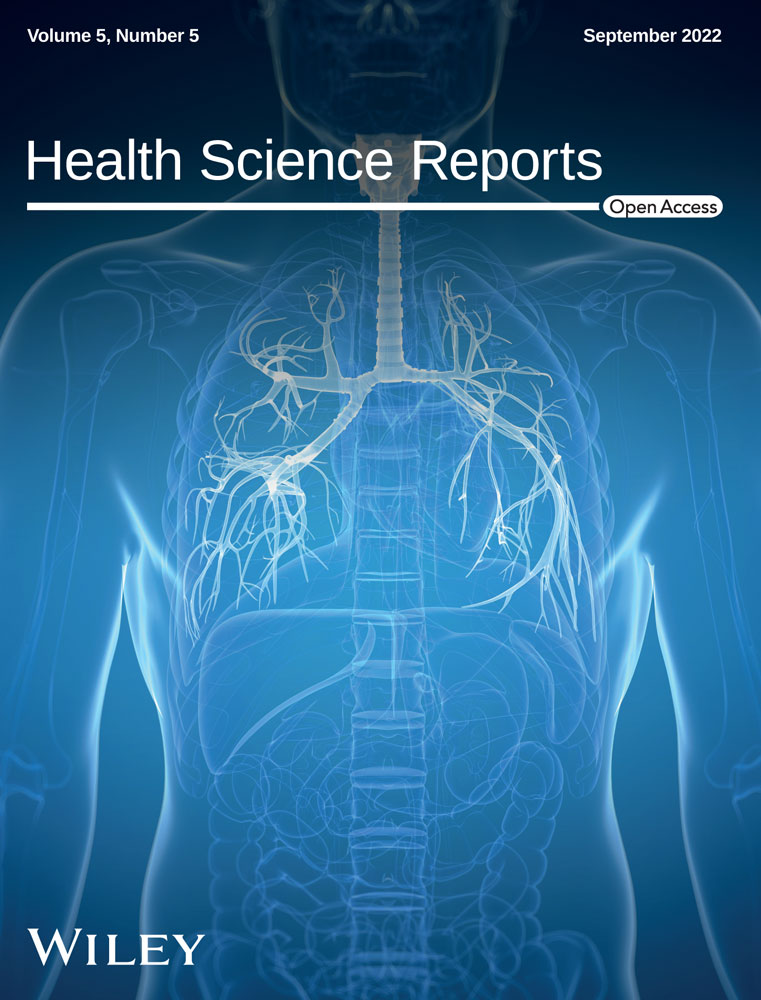
Patients were informed about the study during a regular visit, by e-mail, or additionally by telephone, and recruitment was finalized in our clinic during regular checkups or physiotherapy sessions. Written consent was obtained from all participants after education about the study was provided and before any type of intervention took place. 21 adult individuals with genetically confirmed CF from the Department of Pediatric Pulmonology and Cystic Fibrosis of Ulm University Hospital were invited for ACT sessions with the BDD. Inclusion criteria were: a definitive diagnosis of classic CF based on CF Foundation diagnostic criteria, age >18 years, and clinical stability, defined as the absence of exclusion criteria. Exclusion criteria were: Acute infection, unstable pulmonary or cardiovascular disease, pregnancy, and recent pneumothorax (<6 weeks) or relevant hemoptysis (old blood >5 ml or fresh blood more than traces <4 weeks). None of the participants had used the device previously. All subjects participated in two sessions with a mean interval of 3.4 weeks. Both before and after using the BDD (20 min), we performed diaphragm ultrasound (10–15 min), spirometry (10 min), and collected spontaneous sputum samples (1–2 min), as indicated in a flow sheet (Figure 1). No complications or adverse events occurred related to the intervention.
2.2 Diaphragm ultrasound
Diaphragmatic ultrasound was performed the first time immediately before spirometry, and the second time within 10–15 min after the use of the BDD (Figure 1). The diaphragmic excursion was evaluated during tidal breathing in a supine position and 30% thorax elevation. Ultrasound analysis of the diaphragm was performed according to previous studies.20 The diaphragm was imaged in the anterior axillary line with B-mode using an Affiniti 70 ultrasound system with a 5.1-MHz convex transducer (Philips GmbH Market DACH). Diaphragm mobility was assessed using M-mode analysis in the dorsal third of the diaphragm. In each measurement, M-mode curves of a series of breaths were recorded. Five different breaths were analyzed for maximum amplitude of diaphragmic excursion (excmax) and the slopes of the amplitude increase or decrease over time during inspiration (slopeins) and expiration (slopeexp), respectively. Values of five breaths were averaged for further analysis. To exclude changes in tidal breathing as much as possible, patients could not observe the ultrasound screen where the diaphragm was visible. While a physician performed the ultrasound, a physiotherapist observed the patient without seeing the screen and made sure the patient kept a natural breathing pattern. In addition, there was blinding of the physician during ultrasound measurements.
2.3 Spirometry
Spirometry was performed immediately before and 10–15 min after BDD intervention (Figure 1) according to common procedure standards for diagnostic application21 using an Easy One PC Spirometer No. 2000-3 (ndd Medizintechnik). Fforced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and mean expiratory flow at 25%, 50%, and 75% of vital capacity (MEF25/50/75) were calculated using the software provided with the spirometry device. The best of three consecutive measurements was used for statistics.
2.4 Intervention
ACT using the BDD Simeox® (Physio-Assist S.A.S.) was performed according to the manufacturers’ instructions and supervised by an experienced respiratory physiotherapist. Briefly, participants inhaled via the nose and exhaled via a mouthpiece connected with a tube to the study device. During expiration, the device generated a series of ultrashort negative pressure impulses, set at 12 and 6 Hz (personal communication with Physio Assist GmbH, December 12, 2021). Three cycles of 10 breaths were conducted with 45 s break in between the cycles. The intensity was set to 100% at the beginning of each expiration and continuously downregulated, reaching 25% at the end of the expiration. The manufacturer recommends the approach to lower the POWER setting in his guide “How to carry out a Simeox session—For healthcare professionals” (page 2) to enable a more effective therapy and to reach distal parts of the lung by addressing expiratory reserve volume. The POWER setting allows the alteration of the amplitude of the pneumatic vibration signal and the degree of applied negative pressure. To start with 100% of POWER allows a fast build-up of a negative pressure level (20 cmH2O, measured at the mouthpiece), which is needed to reach the distal parts of the lung. As the volume of lung air decreases during exhalation, reducing the POWER level to 25% during exhalation allows the maintenance of this negative pressure below 40 cmH2O. BDD treatment lasted 20 min.
2.5 Sputum properties
Spontaneously expectorated sputum was collected before and after the use of the BDD. FRAP was used to assess microstructure as described previously by others.16, 17 Briefly, the diffusion of fluorescein isothiocyanate (FITC) dextranes is assessed by using an IMIC digital microscope (Till Photonics). A part of the freshly collected sample was stained with 20 kDa dextrans conjugated to fluorescein (Merck KGa). FRAP of fluorescein was measured in a single plane 20 µm below the surface. After the acquisition of two baseline images, a 10 × 10 um area was photobleached with a 488 nm laser for 1000 ms and time series images were captured every 400 ms for a 20 s period. FRAP was measured at eight different locations of each sputum sample. Recovery curves were fitted using Live Acquisition Software (Till Photonics) according to f(t) = A × (1−e−t−τ) (A: final recovered intensity; t: time; τ: recovery time constant). The halftime of recovery was calculated as T1/2 = ln0.5/−τ and normalized to T1/2 of 0.9% sodium chloride solution. Bleaching correction was performed from the fluorescence decline of the entire image over time.
For quantification of total DNA concentration, a part of the mucus sample was treated with 1 mM β-mercaptoethanol and diluted 1:20 in PBS containing 10 mM Tris-EDTA and 1% Triton-X100 with pH 7.2. Samples were stained with 10 uM SYTOX™ Green (all from Thermo Fisher Scientific Inc.) and fluorescence was measured using an Infinite 200 plate reader with iControl software package (Tecan). From each sample, 10 replicates were measured and averaged. DNA concentrations were calculated from a calibration series within the respective concentration range.
2.6 Statistical analysis
For statistical analysis, we used the statistical software GraphPad Prism version 9.1.2 (GraphPad Software Inc.). The normal distribution of data sets was tested with the Anderson–Darling test. Differences between data sets before and after intervention were statistically analyzed using Student's paired t test for normally distributed data and Wilcoxon matched-pairs signed rank test for not normally distributed data, respectively. A priori levels of significance were *p < 0.05, **p < 0.01, and ***p <0.001.
3 RESULTS
3.1 Study population
Twenty-one adult subjects (11 male and 10 female) with CF, between 21 and 53 years of age, were included. Baseline spirometry parameters assessed at the first study session (Table 1) ranged from severely impaired to normal lung function parameters (minimum and maximum FEV1: 35%–106% of predicted value). Eighty percent of patients had moderate to severe, 20% mild bronchiectasis, but even in the latter, FEV1 ranged from 64% to 104%. Forty-five percent of male and 30% of female participants had started a CFTR modulator (Supporting Information: Table 1). All participants were able to perform two study sessions. No complications or adverse events occurred related to the intervention.
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 30.10 ± 9.26 | 21–53 |
| Male/female (number) | 11/10 | |
| BMI (kg/m2) | 21.62 ± 2.07 | 18.0–25.1 |
| FEV1 (L) | 2.75 ± 1.20 | 1.23–6.29 |
| FEV1 (% predicted) | 71.55 ± 20.47 | 35–106 |
| FVC (L) | 3.921 ± 1.23 | 2.33–6.44 |
| FVC (% predicted) | 88.55 ± 21.96 | 54–126 |
- Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity.
3.2 Diaphragm ultrasound
Improvement of diaphragm mobility upon chest physiotherapy has been demonstrated in subjects with COPD.22 We, therefore, hypothesized enhancement of diaphragm movement upon the intervention using the BDD in our CF cohort. Ultrasound-based functional diaphragm analysis was performed as demonstrated in Figure 2A–C. Maximum diaphragm excursion and slopes during inspiration and expiration (excmax, slopeins, and slopeexp) were assessed from M-mode measurements, reflecting the extent of diaphragm mobility and the velocity of diaphragm movement during inspiration and expiration, respectively. Comparative analysis of the parameters assessed before and after the intervention revealed slight elevation of excmax from 2.27 ± 0.74 cm (mean ± SD) baseline to 2.44 ± 0.85 cm (p = 0.02). after using the BDD (Figure 2D). Slopeins was not affected, slopeexp showed a tendency towards faster diaphragm movements during expiration without reaching statistical significance (Figure 2E,F).

3.3 Spirometry
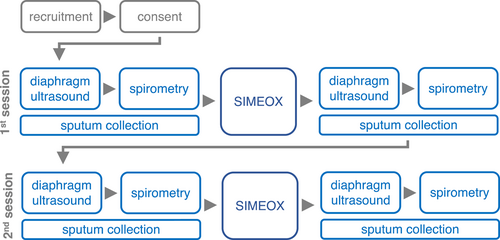
To evaluate the effect of the study device on pulmonary function tests (PFT), spirometry was performed before and after therapy with the BDD (Figure 3). FVC, FEV1, MEF75, MEF50, and MEF25 before and after the intervention were compared and analyzed using Student's paired t test (FVC, FEV1, MEF75) and accordingly Wilcoxon matched-pairs signed rank test for not normally distributed data sets (MEF50 and MEF25). No differences in the above-listed parameters were found between the two time points for FVC, FEV1, MEF25, MEF50, and MEF75, respectively.
3.4 Mucus properties
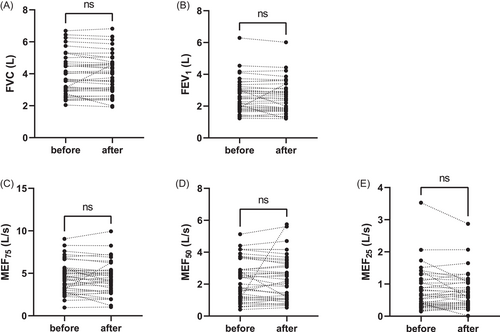
Spontaneously expectorated mucus samples both before and after the intervention could be collected in 55% of all study sessions. Forty-three percent of patients with normal baseline lung function (FEV1 > 80%) and 60% of patients with decreased baseline lung function (FEV1 < 80%) provided sputum. Halftime of FRAP recovery of mucus samples (T1/2) normalized to the halftime of NaCl 0.9% solution (T1/2[NaCl]) was assessed as a surrogate measure of sputum microstructure as demonstrated in Figure 4A for a representative individual data set of samples before and after using the BDD. T1/2/T1/2(NaCl) markedly increased from 1.93 ± 0.37 s (mean±SD) before to 2.24 ± 0.49 s after the intervention (p < 0.001), indicating a change of sputum properties in the sputum portion after ACT with the BDD (Figure 4B). In the airway of subjects with CF and other inflammatory lung diseases, the accumulation of neutrophils leads to high levels of DNA in the airway mucus, which substantially contributes to mucus hyperviscosity.18 In this study, we investigated mucus DNA levels as they represent a major determining factor of mucus properties in CF. Quantification of total mucus DNA concentrations revealed elevated DNA levels in samples expectorated after using the BDD compared to samples collected before, as DNA concentrations increased from 11.43 ± 4.7 to 12.29 ± 4.4 mg/ml (p = 0.02) (Figure 4C). To test for a potential correlation between elevated DNA concentrations and alteration of mucus properties, the differences of FRAP T1/2/T1/2(NaCl) between the samples before and after the intervention were plotted against the differences of DNA concentrations before and after the using the BDD of the respective pair of sputum samples of each individuum (Figure 4D). As data sets were normally distributed we applied Pearson correlation analysis23 that showed a relevant dependency between changes of FRAP T1/2/T1/2(NaCl) and DNA concentrations (Pearson correlation coefficient: 0.59, p = 0.007). Those results indicate the contribution of DNA concentration and point to changes in sputum properties upon therapy with the BDD.
4 DISCUSSION
In this study, we show the first data set on the clinical effects of a novel airway clearance device (Simeox®) in subjects with stable CF. We demonstrate that therapy with the investigated BDD can improve diaphragm mobility and promotes the expectoration of mucus with altered properties.
In contrast to our initial hypothesis, we found no effect of BDD use on spirometry parameters (FCV, FEV1, MEF25, MEF50, MEF75). In line with our results, a randomized trial previously demonstrated, that neither different modes of physical exercise nor chest physiotherapy had short-term effects on lung function parameters in subjects with CF, although some of the interventions significantly increased sputum expectoration.24 Probably, acute effects of airway clearance may be too subtle for detection via spirometry. It can be hypothesized that regular therapy with the device over a longer time period might also positively affect lung function as this is well known from chest physiotherapy in general.6, 8 So far, only a published trial concerning the Simeox® device, found beneficial effects in a cohort of pediatric subjects with exacerbated CF on most lung function parameters after regular use of the BDD over 2 weeks. However, the level of improvement was comparable to a control group treated with conventional physiotherapy only. As MEF25 improved only in the BDD and not in the control group, the authors assumed the superiority of the BDD regarding the effect on small airways.10 The potential of spirometry parameter changes also depends on their baseline level. In the present study, we investigated a heterogenous study cohort as subjects were included independently from the severity of lung disease, and baseline values ranged from 35% of predicted FEV1 to participants with normal lung function parameters. As the latter group will hardly show any further improvement in spirometry values, cohort composition may mask potential differences in subjects with more severe lung disease. Due to the small cohort size, we could not address this question in subgroup analyses.
Spirometry represents the standard diagnostic method to determine lung function in chronic lung diseases. Ultrasound-based diaphragm analysis provides an additional tool that allows noninvasive monitoring of respiratory function. This technique has been frequently used in intensive care medicine to assess diaphragm dysfunction in critically ill patients 20, 25 and patients with suspected neuromuscular diaphragmatic dysfunction.26 In the study reported here, we found a detectable increase in diaphragm mobility after using the novel BDD. In recent years, there is an increasing number of studies concerning COPD using ultrasound-based diaphragm analysis.11-14 In this group, impaired diaphragm mobility seems to correlate with pulmonary function parameters that reflect air trapping and airway obstruction 14, 27 and is inversely correlated with disease severity.13, 27 Interestingly, impaired diaphragm mobility can be partially reversed by chest physiotherapy training and rehabilitation. Moreover, a recent publication by Grabrysz-Forget et al.15 described that diaphragm ultrasound has been used in CF patients for disease severity assessment. Of note, Chun et al.12 found that pulmonary rehabilitation improved diaphragm mobility in subjects with COPD without affecting spirometry parameters. Though using a slightly different ultrasound analysis, this is in accordance with our findings and suggests that diaphragm mobility analysis is a sensitive method to detect subtle changes in respiratory function upon physiotherapy. Although measurement techniques and readouts sometimes considerably differ between studies, maximum diaphragm excursion either during tidal breathing or forced inspiration is one of the most frequently used parameters reflecting diaphragm mobility14, 22, 27, 28 and was therefore measured in the present study together with the slopes during inspiration and expiration, which reflect the respective velocity of diaphragm excursion. Although established for COPD, diaphragm ultrasound has been only occasionally used in subjects with CF to monitor therapy effects, mostly using parameters aiming at diaphragm strength rather than mobility. For instance, the diaphragm thickening ratio increased upon inspiratory muscle training in subjects with CF.29 Recently, an ultrasound study in children with chronic pulmonary disease including CF, demonstrated reduced diaphragm excursion in this group compared to healthy children.30 As indicated in this study, diaphragm mobility is most likely also impaired in advanced CF lung disease as it is known from COPD. Therefore, ultrasound-assessed diaphragm excursion seems to be a suitable readout for the monitoring of ACT effects in subjects with CF as we demonstrate in this study.
Oscillatory devices, for example, flutter, cornet, or high-frequency chest wall oscillators are commonly used alone or in combination with manual physiotherapy in CF care to facilitate mucus expectoration. In this context, mechanical reduction of viscoelasticity of airway mucus is postulated as a putative mechanism.6 We investigated a BDD providing a novel oscillatory technology. According to the device's developers, the applied vibrating signals are supposed to liquefy mucus and thereby promote its removal. Previous trials analyzing sputum properties upon ACT with oscillatory devices were inconsistent regarding the effects on sputum viscosity. Dwyer et al.31 found a reduction of mechanical impedance upon both the use of flutter and treadmill exercise. In contrast, in another study aiming at the effect of flutter and cycling exercise, sputum viscoelasticity was not affected by any intervention.32 It should be noted that flutter or OPEP apply positive expiratory pressure at variable frequencies,32 whereas the BDD used in our study applies negative pressure impulses, set at 12 and 6 Hz (personal communication with Physio Assist GmbH). In the present study, we quantified the diffusion of FITC-dextrans in mucus samples using FRAP. This parameter has been shown to correlate with increased airway surface liquid viscosity of CF airways,16, 17 even though it does not directly measure viscosity. We observed reduced FITC-dextran diffusion in samples after the use of the BDD compared to samples collected before. This result may appear contradictory to the mucus liquefying effect, that is claimed by the device's developers and generally expected from oscillatory devices. In this context, it is noteworthy, that in this study two separate sputum samples were analyzed. Mucus samples expectorated at different time points can differ regarding their origin and composition. Such changes will interfere with the impact of mechanical forces and may explain contradictory results. As FRAP measurements do not comprehensively assess complex sputum rheology, but rather nanoscopic particle diffusion, we additionally measured DNA concentrations. High levels of DNA concentrations, mainly arising from neutrophil granulocytes, markedly contribute to thickened mucus in subjects with CF as well as other inflammatory lung diseases.18, 19 We found a correlation between increased FRAP and elevated total DNA concentrations of sputum samples and therefore could reproduce this relationship within our pilot study cohort. This indicates that varying DNA concentrations at least partially account for altered mucus properties. As high DNA concentrations point to the high abundance of neutrophil granulocytes, we suppose that mucus expectorated before and after BDD may derive from different regions with presumably different levels of inflammation within the airways, respectively.
The strength of our study is the first evaluation of a potential short-term effect of a novel BDD by concomitant investigation of three different parameters: lung function, diaphragm mobility, and sputum properties. Even though we aimed at fulfilling the STROBE guidelines (http://www.strobe-statement.org). our study has some limitations. As we carried out a pilot study to evaluate the basic effects of the BDD, the study design was monocentric and a relatively low number of cases was targeted, which additionally resulted from personal and technical capacities but also from the availability of appointments for our patients. With regard to the pilot study design, the case number was approved by the Institute for Epidemiology and Medical Biometry of Ulm University.
Based on the results of our pilot study, further trials are required to evaluate the beneficial effects of the novel BDD compared to established bronchial drainage methods and to identify patient groups, particularly benefitting from the novel airway clearance therapy.
5 CONCLUSION
Our study demonstrated that the investigated novel BDD improves diaphragm mobility and changes sputum properties, namely, molecular composition (DNA concentration) and microstructure. Whether the sputum macrorheology is also changed would be a possible subject of further studies in subjects with CF. The novel BDD with unique properties should be further evaluated in CF-specific physiotherapy and could complement the physiotherapy methods for CF patients suffering from severe mucus retention.
ACKNOWLEDGMENTS
We thank Physio-Assist S.A.S. (Aix-en-Provence, France) for providing the Simeox® device for use in CF patient care. PhysioAssist was not involved in the design, data acquisition, data analysis, or manuscript preparation of the present study. This study did not receive any specific grant from agencies in the public, commercial, or not-for-profit sectors. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Dorit Fabricius affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Data, that is not already included in the manuscript or appendix, is available from the corresponding author upon reasonable request.