Remdesivir Versus Sotrovimab in Coronavirus Disease 2019: A Systematic Review and Meta-Analysis
ABSTRACT
Background and Aim
Remdesivir and Sotrovimab have emerged as potential treatment options for patients diagnosed with coronavirus disease 2019 (COVID-19). This systematic review and meta-analysis sought to evaluate and compare the effectiveness and safety of these two drugs in the context of COVID-19 management.
Methods
A systematic search was conducted in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar up to July 2024. The effectiveness outcomes examined included mortality rate, hospitalization rate, emergency department visits, ICU admission, and adverse events. The risk of bias in nonrandomized studies of interventions was evaluated using a standardized tool, and data from the identified studies were meticulously analyzed using Comprehensive Meta-Analysis (CMA) software.
Results
The analysis incorporated a total of 9 studies involving 7841 patients. The meta-analysis findings indicated no significant disparity between the Remdesivir and Sotrovimab groups concerning mortality rate (odds ratio [OR] = 3.49, 95% confidence interval [CI]: 0.50–24.11, p = 0.20), hospitalization rate (OR = 2.11, 95% CI: 0.85–5.22, p = 0.10), emergency department visit (OR = 0.80, 95% CI: 0.11–5.62, p = 0.82), and intensive care unit (2.37, 95% CI: 0.18–29.90, p = 0.50). Moreover, comparable rates of adverse events were observed across both groups (OR = 0.98, 95% CI: 0.39–2.47, p = 0.97). The certainty of evidence for these findings was rated as low or moderate.
Conclusion
The study findings suggest that there is no significant difference in effectiveness between Remdesivir and Sotrovimab in the treatment of COVID-19 patients. Further research is needed to provide a more comprehensive comparison of these interventions for COVID-19.
1 Introduction
Despite the significant impact of coronavirus disease 2019 (COVID-19) vaccination in lowering the death toll associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, there are still vulnerable populations who lack adequate protection against the virus [1, 2]. Studies have highlighted that certain groups face a higher risk of mortality and morbidity from SARS-CoV-2, underscoring the need to explore alternative treatment options for these individuals [3-5]. The combination of vaccination and antiviral treatment has a synergistic effect on controlling SARS-CoV-2 transmission, with increasing vaccination rates amplifying the impact of antiviral treatment in reducing overall transmission [6]. This strategic approach can significantly lower COVID-19 hospitalizations and deaths in patients [6]. Various anti-SARS-CoV-2 agents have emerged as promising treatment options for managing COVID-19 patients, with evidence supporting the efficacy of antiviral agents and monoclonal antibodies in reducing death and disease progression [7-11]. Among the approved agents are Remdesivir, Paxlovid, Molnupiravir, and Sotrovimab, each contributing to improved patient outcomes [8, 12, 13]. Remdesivir, a potent antiviral medication, has demonstrated effectiveness against SARS-CoV-2, as evidenced by real-world data showcasing its benefits in enhancing clinical outcomes for COVID-19 patients [14]. Conversely, Sotrovimab, a monoclonal antibody, has also shown promise in improving patient outcomes in the treatment of COVID-19 [15]. Multiple real-world studies have directly compared the effectiveness of Remdesivir and Sotrovimab in treating COVID-19 patients [16-18]. While some studies suggest that hospitalization percentage of Sotrovimab is more effective than Remdesivir [19, 20], others have reported contradictory results [21, 22]. Due to the absence of a systematic review and meta-analysis directly comparing these agents in the treatment of COVID-19 patients, this study seeks to assess the effectiveness and safety of Remdesivir and Sotrovimab in individuals with COVID-19. By conducting a thorough analysis of the effectiveness and potential adverse effects of these drugs, this study aims to provide valuable insights for healthcare practitioners and researchers involved in the management of COVID-19 cases.
2 Methods
This study adhered to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for performing a systematic review and meta-analysis (Table S1) [23]. The protocol for this study was registered on PROSPERO with the registration number CRD42024569346.
2.1 Search Strategy
A comprehensive literature search was conducted by two independent researchers to identify relevant evidence up to July 2024. Various databases, including the Cochrane Library, Web of Science, PubMed, medRxiv, and Google Scholar, were utilized. In addition to database searches, references from systematic reviews and key studies were checked to identify any additional relevant records. The search terms used encompassed keywords such as “SARS-COV-2,” “COVID-19,” “Remdesivir,” “Sotrovimab,” “effectiveness,” and “safety” with no language restrictions. Detailed search strategies for each database can be found in the Supporting File.
2.2 Study Selection
Studies included in this systematic review and meta-analysis met specific criteria: patients with a confirmed positive polymerase chain reaction (PCR) COVID-19 test, treated with either Remdesivir or Sotrovimab as monotherapy, and reporting effectiveness and safety outcomes such as mortality rate, hospitalization rate, and adverse events. Studies reporting irrelevant outcomes, case reports, or involving healthy individuals were excluded from the analysis. The exclusion criteria were as follows: irrelevance to the research direction, lack of relevant data, and duplicate publications. Animal models, in vitro or in vivo studies, case reports, letters to the editors, and editorials.
2.3 Risk of Bias Assessment
Bias assessment in the included studies was independently conducted by two researchers using the risk of bias in nonrandomized studies of interventions (ROBINS-I) tool, which evaluates various domains including confounding, selection bias, and measurement biases [24]. Furthermore, the quality of evidence for each outcome was evaluated utilizing the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool, categorizing the evidence into four levels: very low, low, moderate, and high quality [25].
2.4 Data Extraction
Data extraction was carried out independently by two researchers using a standardized form, which included details on study characteristics (first author, year of publishing, place), patient demographics (number of participants, sex, mean age, COVID-19 vaccination rate, presence of comorbidities, and severity of COVID-19), treatment interventions (dosage and treatment duration), effectiveness outcomes (mortality rate, hospitalization rate, ICU admission, and emergency department visit), and safety outcomes (incidence of any adverse events). This encompassed information such as study location, patient demographics, treatment specifics, and the incidence of adverse events.
2.5 Evidence Synthesis
The Comprehensive Meta-Analysis (CMA) software was employed to compare the effectiveness and safety of Remdesivir and Sotrovimab in COVID-19 patients. For dichotomous variables, the odds ratio (OR) with a 95% confidence interval (CI) was calculated. Continuous data were analyzed using the standardized mean difference with a 95% CI. High heterogeneity, defined as I2 > 50% or p < 0.1, led to the application of a random-effect model. Conversely, studies with low heterogeneity utilized the fixed effects model. Leave-one-out meta-analyses were performed on all outcomes to evaluate the robustness of the findings. Propensity score matching was employed in our study to analyze the outcomes of mortality rate, hospitalization rate, and adverse events. This statistical technique allows the conditions of a randomized controlled trial to be mimicked by matching individuals with similar propensity scores, thereby reducing selection bias in observational data.
3 Results
3.1 Search Result
The search result, as shown in Figure 1, illustrates the process of selecting studies based on their title, abstract, and full-text review. After removing duplicate records, a total of 141 articles were examined against the inclusion criteria. Fifteen studies met the criteria for a full-text review, of which six were later excluded based on the criteria presented in Figure 1. Finally, 9 studies [17-22, 26-28] involving 7841 patients were included in the systematic review and meta-analysis. All the included studies had a retrospective design, with most comparing more than two intervention groups. Table 1 outlines the key characteristics of the included studies.

| References | Country | Severity of COVID-19 | Remdesivir | Molnupiravir | Follow-up (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age | N | Male (%) | SARS-COV-2 vaccination rateb | Comorbidity (%)a | Mean age | N | Male (%) | SARS-COV-2 vaccination rateb | Comorbidity (%)a | ||||
| Alonso et al. [16] | Spain | MM | 57.4 | 34 | 52.9 | NA | NA | 60.2 | 63 | 42.9 | NA | NA | NA |
| Pinargote-Celorio et al. [28] | Spain | MM | 62 | 124 | 54.8 | 64.5 | 83.9 | 53 | 35 | 48.6 | 42.9 | 85.7 | 30 |
| Chesdachai et al. [19] | USA | MM | 69 | 1099 | 48.4 | 67.3 | 90.1 | 60 | 2158 | 40 | 47.3 | 88 | 28 |
| Koh et al. [17] | Singapore | MM | 79 | 169 | 62.1 | 60.4 | 66.9 | 75.5 | 160 | 50 | 61.3 | 7.6 | NA |
| Lahouati et al. [26] | France | MM | 63 | 10 | 80 | 40 | 100 | 55 | 114 | 56 | 21 | 97 | 15 |
| Manciulli et al. [27] | Italy | MM | 67.4 | 142 | 41.6 | 55.6 | 88 | 64.7 | 314 | 52.9 | 50.6 | 79.6 | 28 |
| Piccicacco et al. [18] | USA | MM | 58 | 82 | 45.1 | 67.1 | 83 | 55.8 | 88 | 50 | 66 | 76.1 | 29 |
| Vora et al. [21] | USA | MM | 8 | 38 | NA | NA | NA | 16 | 16 | NA | NA | NA | 7 |
| Tibble et al. [20] | UK | MM | NA | 20 | NA | NA | NA | NA | 3229 | NA | NA | NA | 28 |
- Abbreviations: N, number; NA, not acquired.
- a Having at least one comorbidity,
- b Receipt of ≥ 1 dose SARS-CoV-2 vaccine.
3.2 Risk of Bias Assessment and Quality of Evidence
The evaluation of confounding risk across most studies was determined to be moderate. All studies exhibited a low risk concerning the classification of interventions and instances of missing data. The deviations from the intended interventions and the measurement of outcomes were assessed as moderate for all studies. The risk associated with other domains varied among the studies. Detailed findings from the risk of bias assessment, utilizing the ROBINS-I tool, can be found in Table S2. Additionally, the certainty of evidence for each outcome is outlined in Table S3.
3.3 Effectiveness Outcomes
3.3.1 Mortality Rate
A total of 5 studies [17, 19, 20, 26, 27], which included 7411 patients, reported cases of death in individuals who were administered either Remdesivir or Sotrovimab. The combined estimate of these studies revealed that there was no significant variance in mortality rate between the two groups (OR = 3.49, 95% CI: 0.50–24.11, p = 0.20) (Figure 2). The level of certainty regarding the evidence for this outcome was assessed as moderate.

3.3.2 Hospitalization Rate
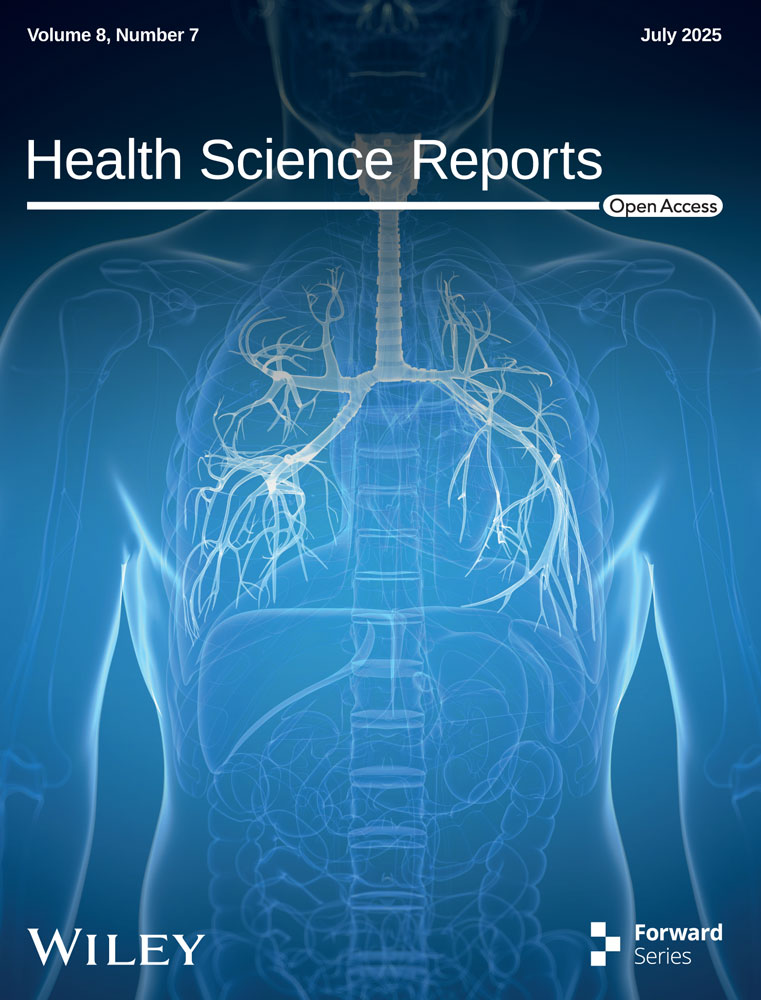
Across 8 studies [18-22, 26-28] involving 7566 patients, instances of hospital admission for individuals receiving Remdesivir or Sotrovimab were documented. A meta-analysis indicated that there was no notable distinction in the hospitalization rate between the two groups (OR= 1.90, 95% CI: 0.78–4.57, p = 0.15) (Figure 3). The level of certainty regarding the evidence for this outcome was assessed as moderate.
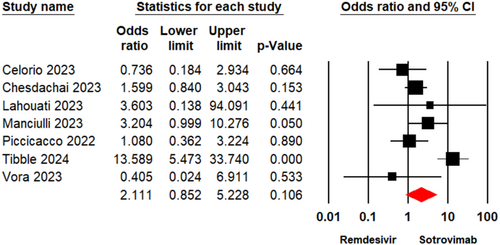
3.3.3 Emergency Department Visit
Two studies [18, 21] enrolling a total of 224 patients reported the viral clearance rate in individuals who underwent treatment with Remdesivir or Sotrovimab. The meta-analysis illustrated that there was no significant variation between the two groups (OR = 0.80, 95% CI: 0.11–5.62, p = 0.82) (Figure 4). The evidence certainty for this outcome was considered low.
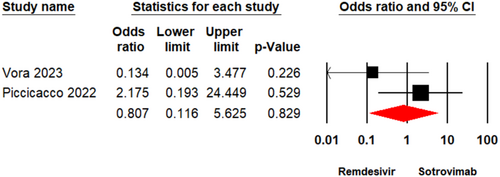
3.3.4 ICU Admission
Two studies [17, 26] enrolling a total of 379 patients reported ICU admission in individuals who underwent treatment with Remdesivir or Sotrovimab. The meta-analysis illustrated that there was no significant variation between the two groups (OR = 2.37, 95% CI: 0.18–29.90, p = 0.50) (Figure 5). The evidence certainty for this outcome was considered to be low.
3.4 Safety Outcomes
3.4.1 Any Adverse Events
Four studies [18, 21, 27, 28] including 2177 patients detailed the occurrence of any adverse events in the groups receiving Remdesivir or Sotrovimab. The combined estimate revealed there was no significant contrast between the two treatment groups regarding the incidence of any adverse events (OR = 0.98, 95% CI: 0.39–2.47; p = 0.97) (Figure 6). Evidence certainty for this outcome was classified as low.

3.4.2 Sensitivity and Subgroup Analyses
The sensitivity analysis conducted by leave-one-out method showed no significant change compared to the primary analysis for the outcomes of mortality rate, hospitalization rate, and adverse events (Figures S1–S3). Propensity score matching analysis revealed that there were no statistically significant differences in the outcomes of mortality rate and adverse events. However, a statistically significant difference was observed in the hospitalization rate (Figures S4–S6).
4 Discussion
The availability of effective and safe antiviral drugs is crucial for managing patients with SARS-CoV-2 infection, especially in high-risk individuals, as they can significantly impact clinical outcomes. This systematic review and meta-analysis focused on comparing the effectiveness and safety of Remdesivir and Sotrovimab, two potent treatment options for patients with COVID-19. The findings of this analysis revealed that there is no significant discrepancy in the ability of these interventions to enhance clinical outcomes for individuals with COVID-19.
Our analysis indicated that there was no significant difference in the mortality rate among patients receiving Remdesivir or Sotrovimab for the treatment of COVID-19. Individual study meta-analyses suggested that both Remdesivir and Sotrovimab were linked to a reduced risk of death in COVID-19 patients when compared to those not receiving these treatments [8, 29]. In addition, Sotrovimab has been shown to have comparable effectiveness to Paxlovid, which has established efficacy against SARS-CoV-2 infection [12]. However, outcomes from real-world studies showed varying results. For instance, Lahouati et al. [26] in 2023 reported no case of death in COVID-19 patients treated with Remdesivir, whereas four patients treated with Sotrovimab succumbed to the disease. On the other hand, according to Manciulli et al. [27] in 2023, 0.3% of COVID-19 patients treated with Sotrovimab died, while 1.4% of those in the Remdesivir group passed away. Overall, the percentage of COVID-19-related deaths was similar between the Remdesivir and Sotrovimab groups. This discrepancy in outcomes may be attributed to differences in patient populations, severity of illness at the time of treatment initiation, or variations in the timing and duration of therapy, which could influence the effectiveness of each treatment in real-world settings.
Our analysis also indicated that there was no significant difference between patients receiving Remdesivir or Sotrovimab in reducing the hospitalization rate among individuals with COVID-19. Current evidence suggests that both of these interventions may decrease the likelihood of hospital admissions among outpatient cases of COVID-19 [29-31]. A meta-analysis of real-world studies further emphasized that Sotrovimab infusion was notably linked to a lower rate of hospitalization in COVID-19 patients [29]. Several studies have also underscored the effectiveness of Remdesivir in mitigating the risk of hospitalizations among outpatients with COVID-19 [30, 31]. Gottlieb et al. [30] found that COVID-19-related hospitalization or death occurred in only 0.7% of patients receiving Remdesivir, compared to 5.3% in the placebo group. Additionally, a study by Rajme-López et al. [31] demonstrated that treatment with Remdesivir significantly reduced the risk of hospitalization or death.
Our findings indicated that there was no significant difference in reducing ICU admissions between patients treated with Remdesivir and Sotrovimab for COVID-19. Real-world data suggested that Remdesivir decreased the rate of ICU admissions compared to those not receiving Remdesivir [32, 33], while evidence also supported the effectiveness of Sotrovimab in reducing ICU admissions [34, 35]. A meta-analysis of 17 studies involving 27,429 COVID-19 patients indicated that Sotrovimab may significantly lower the admission rate to ICU compared to those not receiving the treatment [29]. However, results from studies comparing these interventions in treating COVID-19 patients varied. For example, a study conducted by Lahouati et al. [26] in 2023 found that the ICU admission rate was 10% in the Remdesivir group and 0.8% in the Sotrovimab group. On the other hand, the percentage of ICU admissions between the two groups was similar in the study by Koh et al. [17]. Our results also demonstrated no significant difference in reducing emergency department visits between patients treated with Remdesivir and Sotrovimab. Vora et al. [21] in 2023 reported no cases of emergency department visits in COVID-19 patients treated with Remdesivir, while one case was reported in the Sotrovimab group. In contrast, Piccicacco et al. [18] in 2022 reported emergency department visits for patients receiving Remdesivir and Sotrovimab at 2.4% and 1.1%, respectively.
Our meta-analysis revealed no significant difference in the incidence of adverse events between the two groups. Studies indicated that both interventions were generally safe and well-tolerated in COVID-19 patients. Meta-analyses of randomized clinical trials and real-world data showed no significant difference in adverse events between patients treated with Remdesivir or Sotrovimab compared to those who did not receive these treatments for COVID-19 [29, 36]. However, there are concerns regarding the use of Remdesivir and its potential for increasing liver enzymes in COVID-19 patients [37]. The most frequent adverse events associated with Remdesivir included hepatic injury and anemia, with the most common severe adverse events being hypokalemia, hepatic injury, and anemia [38]. Given the high incidence of severe adverse events with Remdesivir in COVID-19 patients, caution is warranted in its use [38]. The most common adverse events reported in COVID-19 patients receiving Sotrovimab included pyrexia, COVID-19 pneumonia, dyspnea, oropharyngeal pain, and eczema [39].
Sotrovimab, administered as a single 500 mg intravenous dose, received Emergency Use Authorization (EUA) from the FDA in May 2021. This authorization was granted for treating mild-to-moderate COVID-19 in adults and pediatric patients aged 12 and older who are at high risk of progressing to severe disease [40]. Sotrovimab is used in outpatients, whereas Remdesivir is primarily used in hospitalized patients with severe COVID-19. However, remdesivir also shows efficacy in mild to moderate cases [41, 42]. All included studies in the analysis focused on outpatients with mild to moderate COVID-19, which may introduce heterogeneity due to differences in target populations.
The results of the present meta-analysis study showed that there was no difference in the effectiveness of Remdesivir and Sotrovimab. Considering the similar effectiveness of the two drugs, cost can be a factor in choosing treatment. While Remdesivir and Sotrovimab are effective in reducing hospitalizations or mortality in high-risk patients during mild-to-moderate COVID-19, their high costs and complex administration limit their practicality compared to oral options like Paxlovid or Molnupiravir. Evidence showed that the price of a 5-day Remdesivir course in the United States is ~US $2335 [43]. Furthermore, results of a study showed that during 29 days of follow-up, mean costs across longer-than-24-h hospitalization were $5049 in the Sotrovimab group [44].
In our meta-analysis, viral clearance was not reported in the studies included in our meta-analysis, which limited our ability to assess this outcome. The role of viral clearance and its impact on reducing viral shedding time warrants further consideration. Recent evidence suggests that antiviral agents, such as Paxlovid and Molnupiravir, may outperform monoclonal antibodies like Sotrovimab in reducing viral shedding duration, potentially limiting transmission more effectively [45]. Moreover, it is important to consider the broader implications of relying extensively on antiviral drugs and monoclonal antibodies. Despite their safety and effectiveness, prolonged and widespread use of such therapies may carry the risk of inducing drug resistance, a phenomenon well-documented with antibiotics in bacterial infections. In this context, vaccination remains a cornerstone of prevention, particularly for high-risk populations, offering a proactive strategy that complements rather than replaces therapeutic interventions like Sotrovimab or antiviral agents. This underscores the need for a balanced approach, integrating vaccination with targeted pharmacotherapies, to optimize outcomes while mitigating the potential for resistance development [45].
This study has several limitations that should be considered. First, the inclusion of retrospective studies in our systematic review and meta-analysis may introduce biases that could affect the reliability of the results. Second, the heterogeneity of COVID-19 vaccination rates among the patients in the included studies may have impacted the outcomes, as the populations were not uniform. Additionally, the variability in the presence of comorbidities among the study populations could influence the comparison of interventions. Lastly, the inclusion of multiple groups in most studies analyzed in the meta-analysis may have added complexity to the data analysis process.
5 Conclusion
Our meta-analysis revealed no significant difference in the treatment outcomes of COVID-19 patients receiving either Remdesivir or Sotrovimab. Both interventions exhibited similar effectiveness in improving outcomes such as mortality rate and hospital admission, with comparable incidence of adverse events. However, it is crucial to note two key considerations in interpreting these results. First, the certainty of the evidence supporting these findings was rated as low to moderate. Second, these results may not be applicable to emerging SARS-CoV-2 variants. The potential impact of new variants on the effectiveness of these drugs should be carefully considered. Additionally, evaluating the cost-effectiveness and feasibility of these treatments in various healthcare settings is essential for optimizing patient care and resource utilization. By taking these factors into account, healthcare providers can make informed decisions to ensure effective and sustainable management of COVID-19 cases. While this study offers valuable insights into the comparative effectiveness and safety of these interventions, further high-quality research is necessary to corroborate or challenge these findings.
Author Contributions
Saeed Khorramnia: conceptualization, formal analysis, writing – review and editing, supervision, project administration. Zia Navidi: methodology. Ali Sarkoohi: methodology. Mojgan Mohajeri Iravani: methodology. Amirhossein Orandi: methodology. Amirali Orandi: methodology. Samrand Fattah Ghazi: methodology. Ehsan Fallah: investigation. Ebadallah Shiri Malekabad: investigation. Seyed Hamid Pakzad Moghadam: conceptualization, project administration, formal analysis, writing – original draft, writing – review and editing.
Acknowledgments
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Seyed Hamid Pakzad Moghadam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.