Understanding the Intersection Between Hormonal Dynamics and Brain Plasticity in Alzheimer's Disease: A Narrative Review for Implementing New Therapeutic Strategies
ABSTRACT
Background and Aims
Alzheimer's disease (AD), a chronic neurodegenerative disorder, is portrayed by neurocognitive decline in the structure and function of the human brain. Various factors are implicated in the pathogenesis to neuroplasticity alteration in the brain of an individual afflicted with AD. The subset of these elements known as “hormonal dynamics” is paramount in the pathophysiology of AD. This review dives into the complex relationship between hormonal dynamics and brain neuroplasticity with special handling of AD considering the impediments and opportunities for the implementation of therapeutic strategies.
Methods
A comprehensive review was conducted using online search databases PubMed/Medline and ScienceDirect, identifying—with a thematic approach—articles handling the interaction between the hormonal fluctuation and neuroplasticity in AD with special consideration sought from the emerging therapeutic strategies.
Results
This review reveals the influence of various hormonal fluctuations, including estrogens and androgens, on neuroplasticity alteration in the structure and function of the brain in AD. Furthermore, the forms of neuroplasticity and synaptic plasticity processes are significantly altered with underlying neuronal loss and cognitive impairment in AD. Therefore, pharmacological and nonpharmacological therapy approaches as virtual reality and repetitive transcranial magnetic stimulation (rTMS), that promote synaptic plasticity advancements, play a key role in decreasing the rate of deterioration and progression in AD.
Conclusion
Apprehending the intricate interactions between hormonal dynamics and neuroplasticity of the brain is necessary for advancing targeted therapeutics for AD. Upcoming studies should be directed toward the pathophysiological mechanism of hormonal neuroprotection and regeneration with the long-term effects of hormonal replacement therapies, advocating personalized management plans. It should also work on identifying specific imaging and biological markers for the monitoring of HRT. Furthermore, other influences such as environmental, epigenetic, physical, and psychological illness should be tackled.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- AHN
-
- adult hippocampal neurogenesis
-
- APP
-
- amyloid precursor protein
-
- AR
-
- augmented reality
-
- Aβ
-
- amyloid beta
-
- BDNF
-
- brain-derived neurotrophic factors
-
- GDNF
-
- glial cell-derived neurotrophic factor
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- HT
-
- hormonal therapy
-
- LOAD
-
- late-onset Alzheimer's disease
-
- LTD
-
- long-term depression
-
- LTP
-
- long-term potentiation
-
- LTSP
-
- long-term synaptic plasticity
-
- NFT
-
- neurofibrillary tangles
-
- NGF
-
- neuron growth factor
-
- NMDA
-
- N-methyl-d-aspartate
-
- PET
-
- positron emission tomography
-
- PSEN1
-
- presenilin 1
-
- PSEN2
-
- presenilin 2
-
- rTMS
-
- repetitive transcranial magnetic stimulation
-
- STSP
-
- short-term synaptic plasticity
-
- T4
-
- tetraiodothyronine
-
- TSH
-
- thyroid-stimulating hormone
-
- VR
-
- virtual reality
1 Methodology
The main objective of this study is to delve into the interconnection between hormonal dynamical changes and neuroplasticity in Alzheimer's disease (AD), aiming to identify novel preventive and therapeutic strategies. PubMed/Medline and ScienceDirect databases were utilized, and the focused search terminologies suggesting patterns included “Alzheimer's disease,” “hormonal dynamics,” “neuroplasticity,” and “therapeutic strategies in Alzheimer's disease.” Articles, that addressed or dealt with the interplay or interlink between hormonal dynamics and neuroplasticity in the setting for Alzheimer's disease as well as targeted therapies, were included in the study. In addition, papers focusing on the role of microbiota and the pathophysiological mechanism of the effect of several hormones on AD were also included. All articles not written in English were excluded. Further on, core ideas regarding identified recurring four themes—Hormonal changes and effects in the pathophysiology of AD, Neuroplasticity in AD, Interplay between hormonal dynamics and neuroplasticity, and Tailored therapeutic strategies—were extracted and analyzed. However, because of the nature of a narrative review that does not implicate a systemic review, some relevant papers may be missed or omitted with selection bias.
2 Introduction
AD, a chronic neurodegenerative disorder, is one of the most common causes of dementia worldwide surmounting 70% of cases [1]. It is characterized by the decline of patients' cognitive and functional abilities in a progressive manner [1]. For instance, the patient experiences dementia-like symptoms, including gradual deterioration in two or more of the following cognitive domains: behavior, personality, executive or visuospatial function, language, and memory [2].
Although minority of Alzheimer's cases, that exhibit early onset of symptoms (before age of 65 years), are linked to dominant genetic mutation of genes encoding for amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2), late-onset Alzheimer's disease (LOAD) is not hereditary but associated various environmental and metabolic risk factors instead [3]. For instance, those factors altering the vascular function, the brain-glucose metabolism and the mitochondrial activity as well as those elevating the level of oxidative stress and neuroinflammation are recognized as essential triggers of onset and progression of the disease [4]. However, the intricate molecular processes behind the development and pathogenesis of AD remains substantial [5].
It is well proposed that the accumulation of amyloid beta (Aβ) peptides, either due to a decrease in its clearance or an increase in its production, in the tissue of the brain and cerebral vessels forming insoluble intraneural neurofibrillary tangles (NFT) and promoting progressive loss of synapses, are the key hallmarks behind the neuropathology of AD [6]. This justifies the fact that the definitive diagnosis of AD necessitates a postmortem examination of the brain tissue providing a histopathological evidence of the disease, though a combination of cerebrospinal fluid biomarkers and positron emission tomography (PET) indicators in alliance with various element in a newly introduced clinical criteria can help in diagnosis non-deceased individuals [7]. However, in clinical practice, the diagnosis of AD is mainly based on symptomatology which lacks both sensitivity and specificity [8]. Actually, in the 2018 NIA-AA research framework, these hallmark cognitive symptomatology are not taken into account in the primary diagnosis of the disease but rather in the staging of its severity [9]. Despite being a major public health problem, there exist only two approved drug classes for managing symptoms comprising cholinesterase enzyme inhibitors and N-methyl-d-aspartate (NMDA) antagonists, both of which are not curative [10].
-
Functional plasticity: the capacity for some tasks formerly performed by a damaged portion of the brain to be performed by a healthy one [14].
-
Structural plasticity (or synaptic plasticity): the ability of the synapse to change with time [14], either chemically or electrically mediated, further subdivided into:
- ∘
Short-term synaptic plasticity (STSP): the temporary response to sensory inputs and transient change in behaviors and short-term memory [15].
- ∘
Long-term synaptic plasticity (LTSP): a phenomenon of reinforcing (long-term potentiation—LTP) or weakening (long-term depression—LTD) of the synaptic strength or signaling efficacy with corresponding changes lasting for hours, days, or lifelong [16].
- ∘
Neuroplasticity was found to be affected by various factors including sensory and motor experience, task learning, ageing, stress, diet, natural rewards, social play, electrical stimulation, neurotrophic factors, psychoactive drugs, anti-inflammatory drugs, and various hormones and hormonal variations [17]. Furthermore, hormonal substances contribute in populace adaption to novel environmental conditions as well as new-onset cognitive, emotional, and behavioral issues, previously inexperienced [18]. Apparently, neuroplasticity is influenced by hormones to be able to generate adaptive behavioral responses, including sexual maturity in both males and females [18]. Nevertheless, studies illustrate that the influence of hormones on brain plasticity remains operational throughout the lifetime of a human [18]. While aging, significant hormonal fluctuations take place, and this plays a role in the developmental coordination of the central nervous system probably altering both functional and structural plasticity of the brain [18]. To our knowledge, no study tackled the relation between hormones and neuroplasticity in the context of AD. Thereby, the purpose of this review is to explore thoroughly the interlink between hormonal fluctuations and neuroplasticity in AD to implement new preventive and therapeutic strategies in the field.
3 Hormonal Changes in Alzheimer's Disease
Hormones, the chemical regulators of the human body, play a pivotal role in the maintenance of many homeostatic processes such as growth and development, emotion control, and cognition [16]. Studies illustrate that these chemical messengers and their associated fluctuations are key factors that precipitate cognitive decline, given that they have an impact on various aspects of cognition involving thinking, problem-solving, spatial awareness, and memory [16].
Among the plethora of hormones that interfere in brain health, we will highlight the functionality of estrogen, progesterone, testosterone, cortisol, thyroid hormone, and insulin in AD [16, 18, 19].
3.1 Estrogen and Progesterone Effect on AD
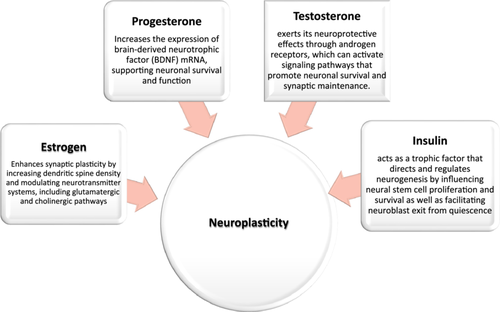
To begin, estrogen exhibits modest antioxidant effects by directly scavenging free radicals, contributing to neuroprotection [19]. It modulates the processing of APP, favoring the nonamyloidogenic pathway, which reduces the production of Aβ peptides [20]. This modulation is mediated through estrogen receptors that influence the activity of enzymes involved in APP cleavage [20]. Besides, estrogen improves cognitive functions by showing enhanced hippocampal and prefrontal cortex activity, which are crucial for memory and executive functions [21]. It enhances synaptic plasticity by increasing dendritic spine density and modulating neurotransmitter systems, including glutamatergic and cholinergic pathways [22]. This leads to improved cognitive functions and may counteract synaptic deficits observed in AD [22]. Estrogen's anti-inflammatory properties mitigate neuroinflammation, a factor implicated in AD progression [21].
Moreover, estrogen is shown to be able to alter human emotions, cognition, and other neuronal factors [23]. For instance, it is found to enhance tasks like inhibitory avoidance and object recognition, both of which decrease with age due to low levels of circulating estrogen affecting cognitive function, a finding that is beneficial in dementia and AD research [23]. Not only elucidated in females, the decrease in estrogen levels with ageing may be associated with the risk of cognitive decline and AD [24]. Besides, cognition is preserved and protected by estrogen, given that the latter promotes cholinergic activity, protects from toxic insults, increases neuronal synthesis, reduces the deposition of Aβ, and enhances its clearance [21].
Similarly, progesterone has been reported to be protective against various insults relevant to brain aging and neurodegenerative diseases, including AD [25]. It is proven that it possesses a neuroprotective role in AD since its neuroactive steroid properties reduce the process of neuroinflammation and autophagy dysfunction involved in the pathophysiology of AD [26]. Estrogen and progesterone can increase the expression of brain-derived neurotrophic factor (BDNF) mRNA, supporting neuronal survival and function [19]. Thus, targeting estrogen receptors may help prevent or control neurodegenerative processes characteristic of AD, suggesting a pathway for developing estrogen-based treatments [27].
As a result of the hormonal implications in AD, trials in hormonal therapy (HT) are on the rise [28]. These studies proved that HT preserves neuronal health by the corrective properties of estrogens, resulting in neuroprotective effects concerning dementia and cognition [28]. Recent literature indicates that early age at menopause is shown to be a risk factor for AD dementia, but women prescribed HT around menopause onset did not show increased risk [29]. The effects of HT, however, varied according to when it was initiated. Research indicates that starting any form of HT 5 years preceding the onset of menopause was associated with a lower risk of AD, whereas beginning HT 5 years or later did not result in a lower risk of AD [30]. However, the success of HT by itself is affected by predisposed risk factors—genetically predetermined—for other systemic diseases including cardiovascular risk factors [31]. Therefore, to recommend HT for an individual, the physician should consider the different aspects of the baseline characteristic including the genetic factors, cardiovascular risk, age, and the dose as well as the duration of this treatment till another effective modalities are available and switched to [28] (see Figure 1).
3.2 Testosterone Effect on AD
It has been demonstrated that the depletion of both estrogen and testosterone may be of great significance in AD development [35]. This is conceivable since these hormones are well-known regulators of several disease processes, including tau protein phosphorylation, reduced spine density, neuronal death, and Aβ accumulation [35].
It is noted that the neuroprotective effects of testosterone are thought to emerge from a decline in Aβ production, synaptic signaling refinement, and neuronal mortality prevention [36]. Testosterone has been shown to reduce Aβ levels and mitigate plaque formation, potentially preventing synaptic dysfunction [37]. Additionally, it exhibits anti-inflammatory properties by inhibiting pro-inflammatory cytokine production, contributing to neuroprotection in AD models [37]. Besides, it exerts its neuroprotective effects through androgen receptors, which can activate signaling pathways that promote neuronal survival and synaptic maintenance [22]. These pathways include the modulation of neurotrophic factors and inhibition of apoptotic processes [22].
However, studies conducted on testosterone and its effects on cognitive decline display conflicting results, some of which demonstrate cognition and testosterone to have a positive linear relationship while others showing an inversely proportional relationship [23]. Lower testosterone levels in men have been associated with an increased risk of developing AD [37]. Testosterone replacement therapy in hypogonadal men has shown improvements in cognitive functions, though results are mixed, indicating the need for further randomized controlled trials [37].
Although all sex hormones, estrogen, progesterone, and testosterone, have been shown to influence Aβ levels, a hallmark of AD pathology, research indicates that women may exhibit greater Aβ deposition than men, even at similar clinical stages of AD [38]. This suggests that sex hormones may differentially influence amyloid pathology [38]. Sex-based differences have also been observed in tau pathology, with some studies reporting more pronounced tau deposition in women [38]. The relation between sex hormones and tau pathology remains an area of active investigation [38]. The presence of the APOE ε4 allele is a known risk factor for AD [39]. faster cognitive decline compared to male carriers, indicating a potential interaction between sex, genetics, and hormone levels [39] (see Figure 1).
3.3 Glucocorticoids and Thyroid Hormones Effect on AD
Glucocorticoids are recognized as cofactors that cause memory impairment and increase the brain's susceptibility to various insults [23, 40]. Chronic activation of glucocorticoid receptors by cortisol can impair synaptic plasticity and potentiate Aβ toxicity, exacerbating AD pathology [41]. Higher levels of cortisol have been reported in individuals with AD and mild cognitive impairment, correlating with cognitive decline and disease progression [42]. Elevated cortisol levels, often resulting from chronic stress, can lead to hippocampal atrophy by promoting neuronal apoptosis and reducing neurogenesis [41]. This negatively affects memory formation and retrieval in AD patients [41]. Therefore, the chronic elevation of cortisol may exacerbate neurodegenerative processes, suggesting that managing stress and cortisol levels could be a potential therapeutic avenue [42].
Studies also illustrated that there appears to be an association between cognitive decline and disorders of thyroid hormone [43]. These hormones regulate the expression of genes involved in neuronal differentiation, myelination, and synaptic function, and thus alterations in their levels can disrupt these processes, leading to impaired neuroplasticity in AD [41]. Besides, thyroid hormones influence mitochondrial function and oxidative stress responses, so any dysregulation in their levels can lead to increased oxidative damage, a hallmark of AD pathology [41]. Literature has also demonstrated that in the context of AD, low free T4 and high TSH levels were associated with a higher cerebral Aβ burden [44]. For that, thyroid hormone supplementation has been shown to restore cognitive deficits and reduce neuroinflammation in the hippocampus of sporadic Alzheimer's-like disease models [45].
3.4 Insulin Effect on AD
Insulin has an essential role in the brain's neuroplasticity highlighted by its heightened effects on learning and memory that is associated with upregulation of insulin receptors in the hippocampus and entorhinal cortex [46, 47]. For instance, insulin—by itself—acts as a trophic factor that directs and regulates neurogenesis by influencing neural stem cell proliferation and survival (by AKT signaling pathway) as well as facilitating neuroblast exit from quiescence (by insulin/IGF-1 signaling pathway) [32-34]. Thereby, insulin is critical for the memory and cognitive brain function [48]. On the other hand, insulin resistance (IR) leads to alteration in neurotransmitter release and metabolism regulation eventually causing neurodegenerative changes and synaptic plasticity deterioration [49].
At the molecular level, insulin is responsible for the regulation of tau gene expression, tau protein phosphorylation, APP, and shapes the balance between amyloidogenic and nonamyloidogenic pathways of the APP processing [50, 51]. In AD, hyperphosphorylation of tau is a hallmark causing impairment in the binding to microtubule contributing to the formation of NFTs [52]. Furthermore, low levels of insulin, decreased insulin sensitivity or disruption of insulin/IGF-1 signaling pathway (resulting in inhibition of PI3K/AKT and increased GSK3-β activation) facilitate an additional increase in NFT production by enhancing tau hyperphosphorylation, resulting in oxidative damage at the cellular level and exacerbating AD [51, 53].
Additionally, insulin levels that are on the lower end of the spectrum or IR result in raised Aβ peptides levels by downregulating the activity of insulin degrading enzyme—that is responsible for the degradation of insulin and Aβ peptides—consequently forming cerebral amyloid plaques [53-55]. These plaques are responsible for neuroinflammatory reaction activation and synaptic plasticity disruption [56]. For instance, Aβ increase is the center of the neuroinflammatory hypothesis of the pathophysiology of AD, where neuroinflammation refers to recruitment of microglia and astrocytes—that release increasing amounts of cytokines, chemokines, and complements triggered by Aβ accumulation—and results in cell injury [57]. Furthermore, the accumulation of these peptides interferes with the insulin signaling pathway promoting the hyperphosphorylation of tau contributing to addition NFT formation [58].
Moreover, one of the pathways by which IR interferes with AD's pathophysiology is through peripheral hyperinsulinemia found due to oversecretion of insulin due to resistance [59, 60]. Insulin can cross the blood–brain barrier and reach the brain, where it is destroyed by the insulin-degrading enzyme that also acts on Aβ protein. The presence of excessive insulin competes with Aβ on the enzyme and leads to Aβ accumulation in the brain [59, 60].
Enhancing insulin sensitivity through lifestyle interventions or pharmacotherapy may offer neuroprotective benefits and mitigate AD progression [61-63]. In the light of what has been discovered in the field of IR and AD, studies showed that multiple antidiabetic drugs have an impact on AD [61-64]. A clinical trial showed that metformin, a first-line treatment for type 2 diabetes mellitus, acts on enhancing insulin peripheral sensitivity and decreasing hyperinsulinemia as a counter effect of IR, thus suggested to be effective in AD treatment [61]. Not only does metformin play a role in AD management, but also SGLT2 inhibitors do [64, 65]. Studies showed various mechanisms by which SGLT2 inhibitors affect AD; literature showed that it interferes with AD through the AD-IR link by decreasing peripheral hyperinsulinemia on the one hand and increasing the production of ketones as an alternative to glucose in certain areas of the brain [64, 65]. In addition, SGLT inhibitors play a pivotal role in AD management by suppressing acetylcholine esterase and increasing by that the presence of acetylcholine at the level of synapses [63]. Finally, recent clinical trials are held to investigate the beneficial effect of liraglutide, a GLP-1 agonist, in managing AD [62] (see Figure 1).
3.5 The Role of Microbiota in Alzheimer's Disease
The gut–brain axis facilitates the bidirectional communication between microbiota and AD [66]. Certain gut bacteria produce amyloid-like particles, which may seed amyloid plaque formation in the brain, a hallmark of AD [67]. Also, microbiota-derived bile acids have been shown to promote gamma-secretase activity through interactions with nicastrin subunits, influencing amyloid-beta production [68]. This highlights the role of gut metabolites in modulating AD-related enzymatic processes [68]. Besides, dysbiosis, or imbalance in gut microbiota, can lead to systemic inflammation, influencing neuroinflammatory processes that exacerbate AD pathology [66].
Research showed that estrogen, progesterone, and testosterone levels may influence gut microbiota composition, which in turn affects the neuroinflammatory processes linked to AD [69, 70]. These hormones have been shown to exert neuroprotective effects, potentially modulated by gut microbiota interactions; changes in gut microbiota composition can influence androgen and progesterone metabolism, affecting its availability and efficacy in neuroprotection related to AD [71]. Besides, studies showed that sex-specific differences in microbiome profiles have been observed, potentially explaining the higher prevalence of AD in women [69].
Not only do sex hormones affect gut microbiota, but so do glucocorticoids and thyroid hormones [72, 73]. It has been shown that chronic stress activates the hypothalamic–pituitary–adrenal (HPA) axis, increasing cortisol production [74]. This heightened cortisol can alter gut microbiota composition, which may further influence AD pathology through the gut–brain axis [74]. This imbalance may exacerbate neuroinflammation and cognitive decline in AD patients [72].
Moreover, dysregulation of thyroid function may alter gut microbiota, potentially affecting AD pathology through metabolic and inflammatory pathways [73]. Thyroid hormones are essential for neurodevelopment, and their interaction with gut microbiota may influence cognitive functions [73].
Furthermore, IR is a known risk factor for AD [75]. Emerging evidence suggests that gut dysbiosis may promote IR, thereby contributing to AD pathogenesis [75]. Modulating gut microbiota could potentially improve insulin sensitivity and mitigate AD progression [75]. Insulin plays a critical role in glucose metabolism, and its interaction with gut microbiota can influence cognitive functions [73]. Alterations in gut microbiota affecting insulin signaling may have implications for AD progression [73].
4 Neuroplasticity in Alzheimer's Disease
As highlighted earlier, neuroplasticity is the capacity of the human nervous system to alter and adapt its activity based on intrinsic and extrinsic conditions. This is via the rearrangement of cerebral structure, functionality, or neuronal connections in response to experience, activity, and injury [12, 13].
4.1 Synaptic Dysfunction in AD
As previously stated, AD is a net result of the coaccumulation of extracellular amyloid plaques, the Aβ peptide, and intraneuronal NFT, the tau protein [76]. These were found to occur years preceding neuropathological impairment, whereas the first signs implicated in AD cognitive deterioration begin when profound synaptic loss ensues [77].
Regarding synaptic dysfunction in the pathophysiological features of AD, studies suggested that it intercedes neuronal degeneration and is directly associated with cognition diminution [78]. Specifically the loss of synaptic density leads to functional impairment of synaptic plasticity, thus participating in AD pathogenesis [79].
Besides, synaptic density impairment in AD exhibits a strong relationship with the level of cognitive diminution. This proposes that impaired synaptic transmission resulting from toxic oligomeric species could estimate the disease severity better than neuronal loss at the disease's end stage. This is upheld by studies conducted on preclinical models where Aβ peptide and tau protein influence said synaptic plasticity by affecting the hippocampal LTP, responsible for memory and learning [80-82].
For instance, excitatory synaptic transmission is adherently controlled via the number of activated N-methyl-d-aspartate receptors (NMDARs) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) at the synapse, where the NMDARs activation plays a pivot role in synaptic plasticity (LTP/LTD) depending on the extent of the amount of intracellular Ca2+ concentration rise in the dendritic spines and the resultant further intracellular downstream activations [83]. Furthermore, for LTP to occur it requires the activation of synaptic NMDARs and an increase in intracellular {Ca2+}, which results in AMPARs activation and dendritic spines growth potentiation. While LTD requires the internalization of NMDARs, the activation of the perisynaptic NMDARs and the lesser increase in intracellular {Ca2+} leading to spinal shrinkage and synaptic loss [83]. Aβ oligomers effect on synaptic plasticity comes not only from its own intracellular accumulation and internalization but also from its influence over the NMDA and AMPA receptors [84, 85]. Whereby the excess Aβ acts on the synaptic cleft to block the glutamate uptake, decreasing its clearance and resulting in overstimulation of the NMDARs until a certain point where receptor desensitization occurs followed by these receptors internalization and thus synaptic response reduction and depression ensues [86].
Moreover as the glutamate spills over the synapse, it can activate the extra and perisynaptic R2B-enriched NMDARs as well as the metabotropic glutamate receptors (mGluRs) which are all involved in the facilitation of LTD by Aβ [87]. The excessive and inappropriate activation of NMDARs by the Aβ oligomers can lead to LTP suppression [88].
4.2 Neurogenesis
Neurogenesis, defined as the formation of new neurons from neuronal stem cells, has gained much interest in the research field [89]. It is apparent that the hippocampus, entorhinal cortex, and olfactory bulb harbor NFT tangles and Aβ plaques precortical progression. Since these cerebral areas, especially the hippocampus, are indicated in neurogenesis, they are most likely altered in AD [90, 91]. Recent research suggests that adult hippocampal neurogenesis (AHN) declines early in AD due to unknown pathophysiological mechanisms, contributing to cognitive diminution.
To illustrate, neurogenesis is reduced by ageing since progenitor cells and immature neurones progressively wane, stemming from inflammatory and hormonal modulations [92-94].
Additionally, ageing causes a decline in several hormones that have an impact on brain plasticity: for instance, insulin growth factor-1, vascular endothelial growth factor, and especially ghrelin. These have been demonstrated to promote hippocampal neurogenesis and synaptic plasticity, exhibiting neuroprotective effects [95, 96].
Neurogenesis is also affected by several other factors like that of nutrition, oxidative stress, neuroinflammation, brain injury, and opioid addiction [97, 98].
4.3 Glial Plasticity
Nonneuronal cells, such as glial cells, are essential for neuronal activity which was proposed by the quadripartite synapse model [99]. Glial cells are situated closely to the synapse and have a characteristic rapid alteration in response to environmental triggers, suggesting their critical position in synaptic manipulation [100, 101].
The role of glial cells is to be activated when neuronal injury ensues. In neuronal degeneration, while the ageing brain already stimulates gliosis and elevates pro-inflammatory precursors, neurodegenerative diseases also promote chronic activation of glial and astrocyte cells, leading to their degeneration [76, 102, 103].
According to studies, glial cells have a direct effect on the short-term neuroplasticity of synaptic clefts and exhibit sole plasticity when reacting promptly to environmental challenges in an effort to maintain network homeostasis [99, 100, 102].
This highlights the point of intersection between neuronal and glial plasticity in supporting neuronal health and activity, suggesting the contribution of chronic gliosis to memory and loss in learning skills in AD [100, 102].
4.4 Therapeutic Strategies Targeting Neuroplasticity to Decrease Cognitive Decline in AD Patients
Several proposed pharmacological and nonpharmacological strategies and therapies are currently being performed; however, we still lack defined standards to follow (Table 1).
| Therapies | Pharmacological | Nonpharmacological |
|---|---|---|
| Targeting neuroplasticity |
|
|
| Targeting hormonal changes |
|
|
| References | 28, 37, 45, 61, 62, 65, 104, 141 | 105-107, 142, 143 |
Of these pharmacological strategies, one targets neurotransmitters. For instance, cholinesterase enzyme inhibitors, such as Rivastigmine, Galantamine, and Donepezil, aim to enhance synaptic plasticity via increasing acetylcholine alongside NMDA antagonists like Memantine that affect glutamate [104]. In addition, neurotrophins like neuron growth factor (NGF), glial cell-derived neurotrophic growth factor (GDNF), and BDGF are recognized for their role in regulating the survival, growth, and development of the brain [104].
Some nonpharmacological strategies comprise physical exercise and aerobic training which is well-known for enhancing cognition as well as increasing neuroplasticity [105]. Another could be cognitive training and programs in brain fitness that focus on memory, attention, language, and problem-solving skills [106, 107].
Moreover, technology has marched its way in AD treatment strategies as virtual reality (VR) and augmented reality (AR), brain computer interfaces, wearable- and sensory-based technologies. For instance, a recent meta-analysis has found a significant improvement in cognitively impaired patients treated with VR compared to cognitive training programs [108].
VR, by providing a digital three-dimensional environment that allows the patient to interact, provide sensory inputs, and track changes, is helping in cognitive rehabilitation, for example, memory, visual attention, dual tasking, and treating psychological symptoms such as anxiety reduction and well-being elevation [108].
Nevertheless, such technologies should be used cautiously and relied on due to the fact of the presence of potential limitations regarding the device's availability as a therapy tool that is used under supervision in hospitals and other healthcare facilities due to safety concerns for patients [109]. Besides, the notable side effects discovered, which might hinder the patient from participation, such as negative emotions experienced by the patient when failing in specific activity demands by the technology, the simulator sickness, oculomotor dysfunction, and visual disturbances, in addition to neck pain and dizziness [109].
Furthermore, synaptic dysfunction is one of the first obvious pathophysiological traits of AD that is now widely prevalent, and it typically manifests before there is a notable neuronal loss [77, 110]. This further elucidates the hypothesis of synaptic plasticity deterioration as the pathological origin behind AD [78]. Therefore, stimulating enhancements in the synaptic plasticity provided by rTMS, at the neurobiological level, may implement a therapeutic strategy with long-lasting cognitive effects on the adjustment of neuroplasticity in AD [77, 111]. For instance, TMS is a noninvasive method that utilizes electromagnetic pulses to stimulate different cortical tissues in specific areas enhancing neuroplasticity by creating electrical current, stimulating action potential, and enhancing cortical reorganization [112]. Furthermore, rTMS—involving rhythmic electromagnetic pulse sequencing—alters neurotransmitter activity and produces effects resembling those of LTP or LTD promoting better neuroplasticity [113]. In fact, applied rTMS promises effective results declining the rate of cognitive deterioration and disease progression in patients with AD [114]. However, there are some safety concerns that go against the use of rTMS in some populations especially with the limited long-term data [115]. For example, using TMS in individuals with implantable devices (pacemakers, cochlear implants, etc.) or metals can produce unwanted effects ranging from heating up and causing tissue damage to malfunction, excessive stimulation, or demagnetization [115]. In addition, TMS can precipitate seizure which is considered the most severe acute side effect, and it can lead to hearing loss and psychiatric side effects [115].
5 Interplay Between Hormonal Dynamics and Neuroplasticity
Adult neurogenesis, the most enthralling research within the field of neuroplasticity, is defined as the generation of neuronal functioning in adulthood and is observed in multiple cerebral areas [116]. With age, hormonal action becomes evident on structural and functional cerebral plasticity [18]. Hormone-induced plasticity is not limited to the periods of hormonal spirts periadolescence, well-known for continual hormone-induced neuroplasticity throughout the lifespan of an individual [18]. According to the literature, alteration of distinct neurological phases—including cellular proliferation, differentiation, and survival—may be induced by numerous factors [117-119]. Of these, the hormonal influence of androgens and estrogens on the alteration of adult-generated functional neurones in neurogenesis implementing neuroplasticity is investigated thoroughly in previous studies [116]. Androgens, regulators of the male sexual function, and sociocognitive behavior resulting from the effects of testosterone and dihydrotestosterone alongside estrogens comprising estriol and oestradiol, are potent influencers of neuroplasticity affecting neurogenesis in stage-specific and in species-specific forms, respectively [116, 120, 121]. Actually, elevation of androgen levels is linked to the progression of hippocampal cellular survival implicated in neurogenesis [116]. On the contrary, the long-term effects of castration, in which androgen levels decline drastically, have a deleterious effect on adult neuroplasticity, influences appearing to be reversed with the administration of exogenous androgens [116]. In addition, the established role of estrogens in previous studies involves its impact on cerebral neuroplasticity alongside its contribution in synaptogenesis by the formation of dendritic spine synapses [122-124]. The effects of estrogen in neuroplasticity is species-related where elevated levels correlate to enhancement of adult neurogenesis in some animal species and reduction of it in others [116]. Furthermore, the effect of estrogens on the proliferation of neural cells depends on the estrogen type and dose [116]. For instance, 7β-oestradiol (E2), produced in the forebrain from androgen under the influence of local aromatase, is responsible for the regulation of synaptic plasticity and development of cognitive function in mice hippocampi [125].
Influenced by external and internal factors, neurogenesis is generally inhibited by stress. However, the differentiation between positive and negative stress is essential despite both activating the HPA axis promoting the release of glucocorticoids [126]. However, negative stress may implement opposite influence on the hippocampus, suppressing its neuroplasticity especially with traumatic or chronic stress leading to cognitive impairment [127, 128]. Moreover, positive stress, such as physical exercise, is associated with the promotion of hippocampal plasticity by enhancing adult neurogenesis [127]. Furthermore, the hippocampus exhibits potential for HPA axis discontinuation due to the presence of glucocorticoid receptors advancing the feedback loop [127]. For instance, HPA axis activation due to postnatal maternal separation increases glucocorticoid release, whereas chronic stress implements termination of HPA axis regulation that is induced by acute stress [129, 130].
Correlating with various stages of life, neuroplasticity is established to be enhanced by pregnancy due to the effects of estrogen, where parity is found to decrease the degree of significant brain aging among humans [18, 131, 132]. Thereby, multiparous populations are found to possess more “youthful” brains in the middle-age group via neuroplasticity inclination [18]. However, this fact stands against studies that demonstrate the development of AD being correlated with increased parity [133]. This inconsistency is explained by the benefits of estrogen on female neuroplasticity depending on health status, particularly bringing advantages to a healthy individual [134]. Therefore, although it has been proven by existing literature that estrogen protects women from the risk of AD by enhancing neuroplasticity, it only does so in individuals with no risk for AD in their genotype [134]. On the contrary, androgens are potent enhancers of cognitive functionality in various aspects [135]. Therefore, androgen decline may increase cerebral vulnerability to neurodegeneration and dysfunction [135]. For instance, lower testosterone levels are found in patients with AD in comparison to their counterparts without AD [136]. Thus, the employment of androgen therapy provides a safe therapeutic modality and a mode of prevention against the development and progression of AD in ageing individuals with lower testosterone levels [137].
6 Methodological Challenges and Future Directions
The limitations of the articles reviewed pertain to the abundance of ongoing preclinical trials, with fewer human studies that precisely prove this theorem. As for hormonal studies, first, there is no standardized methodologies relied on for measurement of hormonal levels and no attention given to hormonal fluctuations, differences with ageing, gender, or ethnic concerns. Also, endocrinological diseases, with their impact on hormonal levels aside, alongside extrinsic hormonal therapies, should gain more attention.
In addition, conflicting data suggest that older men with low testosterone and age-related memory impairment treated with testosterone did not show the expected improvement in memory or other cognitive functions [138, 139]. Another article concludes that estrogen-only therapies have different effects based on the age at which they're prescribed, where women under 60 years old who started on estrogen therapy early after menopause experienced reduced AD risk, while those in menopause and above 60 years old on such therapy may have elevated AD risk within the first 5 years of treatment, and that combination menopausal hormone therapy (MHT) may increase AD risk regardless of the age of the women and especially after prolonged use [139, 140].
Although recent studies suggest a role for microbiota in AD, one should not generalize such facts since individual variability may highly influence these findings, as an individual's microbiota composition varies widely between people due to various factors, such as diet, genetics, lifestyle, medications such as antibiotics or probiotics, and diseases.
Given the bidirectional correspondence between neuronal plasticity and hormones, it is onerous to determine their causality. Moreover, the scarcity of diagnostic tools that would differentiate between neuroplasticity subtypes is another challenge to overcome.
Future studies could focus on longitudinal tracking of hormonal fluctuations and neuroplasticity to better comprehend their causality. Gender-specific investigations as well as those concentrating on cognition and substance misuse would further aid in etiology classification for AD. Dynamic collaboration between multidisciplinary teams in the field such as neurologists, psychiatrists, endocrinologists, radiologists, and geneticists would yield more collective action and potentially better results in the future.
7 Conclusion
Studying the intricate interaction between hormonal fluctuations and brain plasticity with its evident effect on the progression and development of AD and its role in the development of therapeutic strategies is fundamental. This article highlights the effects of various hormones on cerebral plasticity and their substantial role in AD—where estrogen and progesterone diminish, androgen regression, thyroid decline, insulin decrease/resistance, and glucocorticoid elevation impair synaptic ad neuroplasticity and are associated with increased risk and progression of AD—alongside highlighting the importance of microbiota and the implementation of novel HRT in its prevention and control. Moreover, several forms of neurogenesis, neuroplasticity, and synaptic and glial plasticity deterioration in AD were deliberated at the molecular level—highlighting the vital role of NMDA receptor activation in the advancement of brain neuroplasticity and the receptor desensitization effect of Aβ—together with information on the hormonal dynamics underlying each and proof of their dysfunction in AD. Addressing these impairments in the neuroplastic mechanisms enforces the utilization of pharmacological—as cholinesterase enzyme inhibitors and NMDA antagonists—and nonpharmacological treatments like VR and rTMS as that may be limited by specific challenges and side effects in special population especially psychiatric. The apprehension of mechanisms underlying neuroplasticity and of hormonal neuroprotection characteristics may be advanced therapeutically to mitigate neurocognitive decline, providing a better quality of life for populations with AD.
Instigating the results of this review, policymakers are advised to support research diving into the hormonal effect on neuroplasticity. This is subject to the provision of appropriate funding and infrastructure, thereby commending guidelines for the employment of novel HRT based on such achievements in research. Furthermore, physicians are advised to reconsider the hormonal status of individuals diagnosed with AD and personalizing interventions advocating HRT to control disease progression. In addition, future research should tackle the dearth in literation, investigating the mechanisms behind hormone-induced neuroprotection and neuroregeneration implicated in AD. This will allow long-term patient outcomes to be elucidated, where therapeutic potential toward personalized prevention may be a future prospect in the management of AD. For instance, the conduction of longitudinal studies to test for the efficacy as well as safety index of each hormone in the replacement therapy and the role of prophylactic replacement for delay onset of the disease should be emphasized. Furthermore, future research should delve into the identification and utilization of specific biomarkers and imaging finding leveraging advanced neuroplasticity models to monitor for results of hormonal therapies on the advancement of neuroplasticity in patients with AD. Also, studies on other influences including environmental and epigenetic influences should be tackled more deeply in the setting of hormonal changes in AD, and future research should focus on unusual populations of specific ethnic group or those with associated specific comorbidities of psychiatric illness.
Author Contributions
Abir Ghosson: writing – original draft, methodology, formal analysis, data curation. Fatima Soufan: data curation, formal analysis, methodology, writing – original draft. Hussein Kaddoura: data curation, formal analysis, methodology, writing – original draft. Elissa Fares: writing – original draft, methodology, formal analysis, data curation. Olivier Uwishema: supervision, formal analysis, project administration, writing – review and editing, methodology, writing – original draft, conceptualization. All authors have read and approved the final version of the manuscript.
Acknowledgments
We would like to thank Oli Health Magazine Organization (OHMO)'s members for their contributions and support for this manuscript.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Abir Ghosson affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
Olivier Uwishema had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.