Prevalence of Healthcare-Acquired Infections Among Adults in Intensive Care Units: A Systematic Review and Meta-Analysis
ABSTRACT
Background and Aim
Healthcare-acquired infections (HAIs) are a major cause of morbidity and mortality among critically ill adult patients admitted to intensive care units (ICUs) with underlying health conditions. Precisely estimating the burden of HAIs in adult ICUs is important for guiding prevention efforts and assessing the impact of intervention programs. This review aims to synthesize the available epidemiological data on the prevalence of different HAI types in adult ICUs.
Methods
A systematic search was conducted via the PubMed, Scopus, and Web of Science databases to identify prevalence studies of HAIs published up to August 14, 2024, involving adult ICU patients. Pooled prevalence estimates and 95% confidence intervals were calculated using random effects models. Heterogeneity was assessed using the I2 statistic.
Results
A total of 21 studies involving 15,966 patients with 4288 adult ICU HAI cases were included in the meta-analysis. Pneumonia, whether ventilator-associated (VAP) or non-VAP, demonstrated the highest prevalence. The pooled all-cause pneumonia and VAP prevalence were 51.0% (95% CI 40.0–63.0%, I2 = 98%, p < 0.01) and 54.0% (95% CI 31.0–76.0%, I2 = 98%, p < 0.01), respectively. The prevalence of bloodstream infections was 34.0% (95% CI 25.0–42.0%, I2 = 98%, p < 0.01), whereas those of urinary tract infections and surgical site infections were 26.0% (95% CI 17.0–35.0%, I2 = 98%, p < 0.01) and 12.0% (95% CI 7.0–17.0%, I2 = 92%, p < 0.01), respectively. Patient risk factors included chronic kidney disease (52%), malignancy (45%), immunosuppression (40%), and diabetes (35%). No significant differences in risk between sexes emerged. Invasive procedures, especially tracheostomy (74%), carried the highest risk.
Conclusion
This review revealed that BSIs, UTIs, pneumonia, and VAP constitute the most common HAIs in adults. Invasive devices, surgery, antimicrobial exposure, and underlying patient health conditions increase the risk. Targeting reductions in device usage durations and optimizing antibiotic stewardship may help lower infection rates in intensive care units.
1 Introduction
Healthcare-acquired infections (HAIs) remain a key patient safety challenge worldwide, contributing substantially to excess morbidity, costs, and antimicrobial resistance [1]. The available data indicate that approximately one in 31 hospitalized patients harbours at least one HAI on a given day [2]. The World Health Organization estimates that of the 421 million patients hospitalized annually worldwide, over 42.7 million acquire HAIs, representing a major patient safety issue faced internationally [3]. Each year in the European Union and European Economic Area, 4.3 million hospitalized patients contract an HAI [4], whereas in the United States, the Centers for Disease Control and Prevention reported nearly 687,000 HAIs in acute care facilities, resulting in 72,000 associated deaths [2].
HAIs also impose a significant economic burden, with direct and indirect medical expenditures in the United States estimated to be between $96–$147 billion annually [5] and $13 billion annually in Africa [6]. Surveillance data internationally demonstrate that intensive care unit patients disproportionately suffer from HAIs relative to other hospitalized populations in general wards [7, 8]. Approximately 5.7 million adults receive intensive care treatment in the United States alone each year [9]. Critically ill intensive care unit patients face elevated HAI risks due to compromised immunity combined with extensive medical intervention exposure [10, 11]. Undergoing multiple invasive procedures in intensive care units increases the risk of HAIs in adults [10, 12]. A prior 2003 systematic analysis approximated a pooled intensive care unit HAI prevalence exceeding 30% in this vulnerable group [13].
Previous reviews identified mechanical ventilation, clinical severity scoring, surgery, and prolonged antimicrobial usage as potential determinants [14, 15]. Understanding the associations between HAIs and specific risk factors could prioritize prevention efforts. However, knowledge gaps persist regarding the true HAI prevalence linked to these risk factors across diverse intensive care units. Given the considerable burden and evolving nature of HAIs, a comprehensive understanding of their prevalence and risk factor estimates across variable intensive care unit settings is urgently needed. These data can guide well-informed resource allocation, prevention guidelines, and performance monitoring globally. This review aims to comprehensively evaluate HAI prevalence and associated risk factors in adult intensive care unit patients.
2 Methodology
This systematic review strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16] to ensure methodological rigor and transparent reporting. Specifically, the 27-item PRISMA reporting checklist guided all stages of the review process from conceptualization through the conduct and dissemination of the results.
2.1 Search Strategy
A comprehensive literature search was conducted across major bibliographic databases from inception to obtain all relevant published studies. PubMed, Scopus, and Web of Science were systematically searched through August 14, 2024, via controlled vocabulary and keywords. The search paradigms incorporated both individual and paired combinations of the following terms: “risk factors”, “types”, “prevalence”, “intensive care”, “adult”, “ICU”, “healthcare-acquired infection”, and “hospital-acquired infection”. No restrictions were placed on the language or date of publication. Furthermore, reference lists of eligible manuscripts were manually searched to identify additional sources not indexed in the electronic databases. This rigorous search methodology aimed to gather all available evidence to conduct a robust systematic review and reduce the potential for reporting bias.
2.2 Study Selection
Study screening was conducted independently by two reviewers, who evaluated all titles and abstracts retrieved through the literature search to identify studies with potential relevance. The full texts of these provisionally eligible studies were then appraised by both reviewers in accordance with the predefined inclusion and exclusion criteria. Disagreements regarding the suitability of studies for inclusion were resolved through consultation with a third reviewer to arrive at a consensus decision. Adhering to this thorough multistage screening process helped ensure that only those publications meeting the review parameters were ultimately selected for inclusion and that selection biases were minimized.
2.3 Inclusion Criteria
- 1.
Observational epidemiological study designs (e.g., cohort, case-control) reporting quantitative prevalence data on risk factors and/or types of HAIs among adult ICU patients.
- 2.
Study populations exclusively comprising ICU-admitted patients aged 16 years or older.
2.4 Exclusion Criteria
- 1.
Review articles, editorials, letters, commentaries, case reports, and other publications lacking original quantitative outcome data.
- 2.
Investigations conducted fully or partially in non-ICU settings such as emergency departments or general medical/surgical wards.
- 3.
Studies exclusively recruiting pediatric populations (younger than 16 years).
- 4.
Analyses from specialized community/nonhospital ICU environments.
- 5.
Reports exclusively identifying/discussing risk factors without HAI prevalence quantification.
- 6.
Studies lacking the requisite statistical data for potential synthesis/aggregation in the meta-analyses.
2.5 Data Extraction
Data were extracted from the included studies in a standardized manner. A pre-established data collection form was employed to record the essential details. The extracted elements included the authors and year of publication, country, study design attributes, study population, HAI cases, risk factors, demographic profiles, and pertinent clinical profiles of the recruited participants. When disagreement arose between the two independent data extractors, it was resolved through consultation with a third researcher to arrive at a consensus. Adherence to this methodologically rigorous, replicable extraction process served to minimize potential errors during this pivotal stage of information retrieval and synthesis before analyses.
2.6 Quality Assessment
- 1.
Selection bias was assessed by assessing the representativeness of the sampled population.
- 2.
Bias in measurement by examining the consistency of the outcome/exposure identification.
- 3.
Bias due to missing data by scrutinizing the complete ascertainment of target outcomes.
- 4.
Outcome measurement bias by investigating the application of standardized measurement.
- 5.
Selective reporting bias through inspection for the potential omission of findings.
Each domain was objectively rated as low, some concerns, or high risk initially. Disagreements were resolved via consensus with a third reviewer. An overall judgment was derived from the highest-scoring domain. This quality information was then synthesized descriptively to holistically gauge the relative strengths and shortcomings across the primary investigations.
2.7 Ethical Considerations
As this was a systematic review and meta-analysis of the published literature, ethical approval was not required. However, all included primary studies had obtained the appropriate ethical approval and informed consent from participants, where applicable, before data collection.
2.8 Statistical Analysis
A random-effects model was employed for data pooling to account for between-study variability in addition to within-study variance [18]. The impact of regional disparities and income levels on prevalence estimates was examined through a meta-regression analysis. Heterogeneity was assessed using I2, which quantifies the percentage of total variation across studies beyond the sampling error [19]. Substantial heterogeneity was defined as an I2 value exceeding 50% [20]. Publication bias was explored through funnel plot inspection and statistically via Egger's test of asymmetry [21, 22]. Influential studies were identified by sequentially removing each from the meta-analysis and observing the impact on pooling and heterogeneity, informing stability. Statistical analyses were performed using R version 4.3.3 [23] and relevant packages, including “meta” and “metafor”, to generate the necessary plots and statistical outputs. Statistical significance was set at p < 0.05, and all tests were two-sided.
3 Results
3.1 Search Results
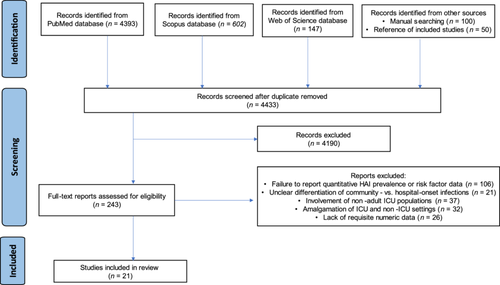
The literature search strategy involved querying major databases, including PubMed, Scopus, and Web of Science, resulting in 4393, 602, and 147 records, respectively, with a total of 5142 publications. Through manual search techniques and screening of the study reference lists, an additional 150 articles were identified, bringing the total to 5292 articles from the literature search. After removing duplicate reports (n = 859), 4433 studies were subjected to the title and abstract review. Most (n = 4190) did not meet the eligibility criteria and were excluded at this phase. The remaining 243 articles underwent full-text assessment, and 222 of these were excluded due to the following reasons: failure to report quantitative HAI prevalence or risk factor data (n = 106); unclear differentiation of community versus hospital-onset infections (n = 21); involvement of nonadult ICU populations (n = 37); amalgamation of ICU and non-ICU settings (n = 32); and lack of requisite numeric data (n = 26). Ultimately, 21 studies [24-44] satisfied all the inclusion criteria (Figure 1). Collectively encompassing 15,966 patients, these studies provided usable outcome data, enabling quantitative meta-analysis of infection prevalence estimates.
3.2 Study Characteristics
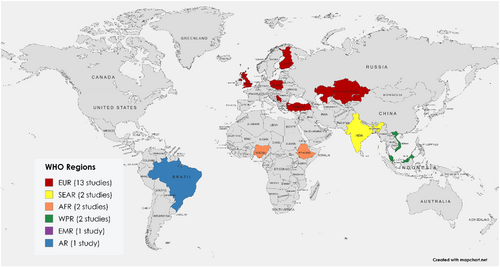
The key characteristics of the individual studies are summarized in Supporting Information: Table S1. The study population ranged from 71 to 3708 patients across the studies, with median patient ages reported between 34 and 66 years. The majority of studies were conducted in the European region (EUR) (n = 13, 61.9%), with the remaining studies from the African region (AFR) (n = 2, 9.5%), the Western Pacific region (WPR) (n = 2, 9.5%), the Southeast Asian region (SEAR) (n= 2, 9.5%), the Eastern Mediterranean region (EMR) (n = 1, 4.8%), and the American region (AR) (n = 1, 4.8%) (Figure 2).
In terms of study design, approximately one-third of the studies were multicenter studies (n = 6, 28.6%), whereas the remaining studies used single-center cohorts (n = 15, 71.4%). Consistent case definitions for identifying HAIs were applied in most studies, with 10 applying guidelines from the Centers for Disease Control and Prevention and seven using the European Centre for Disease Prevention and Control criteria. One study used recommendations from the Agência Nacional de Vigilância Sanitária (ANVISA) guidelines.
3.3 Pool Prevalence of HAIs in the ICUs
The random effects meta-analysis of 21 studies involving 15,966 adult ICU patients revealed that the overall pooled prevalence of HAIs was 32.0% (95% CI 26.0–38.0). However, significant heterogeneity was detected between studies, with I2 = 97% (p < 0.01). This finding indicates that over 97% of the variability in the observed prevalence was due to heterogeneity rather than chance. The subgroup analysis by region indicated varying HAI prevalence: 61% (95% CI: 52–68) in the Eastern Mediterranean region (EMR), 49% (95% CI: 44–53) in the African region (AFR), 41% (95% CI: 35–46) in the American region (AR), 29% (95% CI: 22–36) in the European region (EUR), 26% (95% CI: 18–34) in the Western Pacific region (WPR) and 20% (95% CI: 16–24) in the Southeast Asian region (SEAR) (Figure 3). In terms of income levels, lower middle-income countries exhibited a prevalence of 35% (95% CI: 19–50), while high-income countries had a lower prevalence of 26% (95% CI: 21–31) (Figure 4).
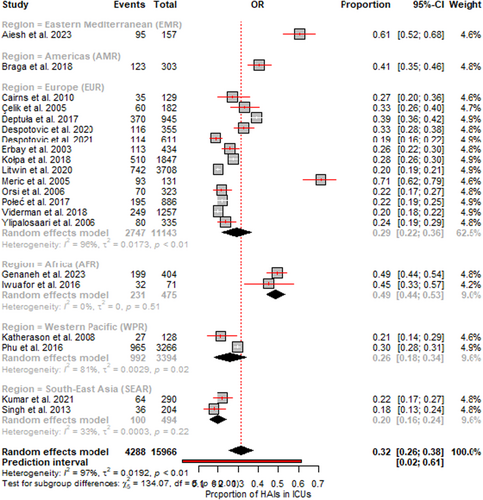
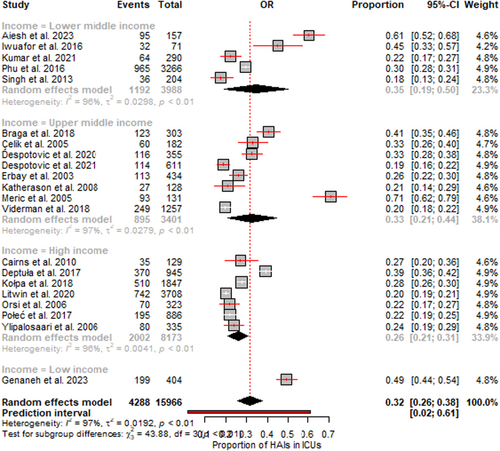
The forest plots (Figures 3 and 4) visually depict the individual prevalence reported in each study along with their 95% confidence intervals. The pooled prevalence is represented by the black diamond at the bottom. The widths of the confidence intervals correspond to each study's precision or sample size. Additionally, the funnel plot assessing publication bias is presented in Supporting Information S1: Figure S1.
3.4 HAI Types in the Adult ICUs
In adult ICUs, the prevalence of various HAIs was examined (Table 1, Supporting Information S1: Table S2). Ventilator-associated pneumonia (VAP) emerged as the most prevalent infection, with a prevalence of 54.0%, followed closely by pneumonia at 51.0% and bloodstream infections (BSIs) at 34.0%. The prevalence of urinary tract infections (UTIs) was reported to be 26.0%, whereas the prevalence of lower respiratory tract infections (LRTIs) was 14.0%. The prevalence of surgical site infections (SSIs) was 12.0%, that of skin and soft tissue infections (SSTIs) was 10.0%, that of gastrointestinal infections (GIs) was 6.0%, and that of central nervous system infections (CNSIs) was 2.0%. Forest plots illustrating these prevalence estimates are presented in Supporting Information S1: Figures S2–S19.
| HAI | No. of studies | No. of cases | Prevalence (95% CI) | I2 | p value |
|---|---|---|---|---|---|
| BSIs | 18 | 821 | 34.0% (25.0–42.0) | 98% | < 0.01 |
| UTIs | 20 | 874 | 26.0% (17.0–35.0) | 98% | < 0.01 |
| SSIs | 12 | 295 | 12.0% (7.0–17.0) | 92% | < 0.01 |
| LRTIs | 4 | 67 | 14.0% (2.0–27.0) | 96% | < 0.01 |
| Pneumonia | 13 | 1611 | 51.0% (40.0–63.0) | 98% | < 0.01 |
| SSTIs | 12 | 164 | 10.0% (4.0–15.0) | 91% | < 0.01 |
| GIs | 5 | 118 | 6.0% (4.0–9.0) | 74% | < 0.01 |
| CNSIs | 3 | 26 | 2.0% (1.0–3.0) | 0% | 0.65 |
| VAP | 6 | 716 | 54.0% (31.0–76.0) | 98% | < 0.01 |
- Abbreviations: BSIs, bloodstream infections; CNSIs, central nervous system infections; GIs, gastrointestinal infections; LRTIs, lower respiratory tract infections; SSIs, surgical site infections; SSTIs, skin and soft tissue infections; UTIs, urinary tract infections; VAP, ventilator-associated pneumonia.
3.5 Risk Factors
Risk factors identified in three or more studies were included in the meta-analysis. Seventeen risk factors met this requirement, including male sex, female sex, intubation, central vascular catheterization, urinary catheterization, malignancy, surgery after admission, exposure to antibiotics, diabetes mellitus, immunosuppression, blood transfusion, tracheostomy, chronic renal disease, nasogastric tube, endotracheal tube, proton pump inhibitors, and mechanical ventilation. In contrast, risk factors identified in fewer than three studies were excluded from the meta-analysis. The excluded risk factors included chest tube insertion, gastroscopy, bronchoscopy, alcohol abuse, smoking, urinary tract pathology, respiratory disease, chronic liver disease, cardiovascular disease, hypertension, family involvement in patient care, a peripheral vascular catheter, and a central venous line. Data used for the risk factor meta-analysis are summarized in Supporting Information S1: Tables S3–S33, and forest plots illustrating the meta-analysis results are presented in Supporting Information S1: Figures S20–S53.
The meta-analysis results from the studies on the risk factors for HAIs in ICUs are provided in Table 2. The risks were categorized into patient characteristics, medical interventions, and medication exposures.
| Risk | Prevalence (95% CI) | I2 | p value |
|---|---|---|---|
| Patient characteristics | |||
| Male | 36.0% (27.0–46.0) | 97% | < 0.01 |
| Female | 36.0% (23.0–49.0) | 97% | < 0.01 |
| Malignancy | 45.0% (18.0–72.0) | 84% | < 0.01 |
| Chronic renal disease | 52.0% (10.0–94.0) | 92% | < 0.01 |
| Diabetes mellitus | 35.0% (17.0–53.0) | 94% | < 0.01 |
| Immunosuppression | 40.0% (21.0–59.0) | 94% | < 0.01 |
| Medical interventions | |||
| Surgery after admission | 47.0% (37.0–57.0) | 93% | < 0.01 |
| Blood transfusion | 40.7% (30.0–50.0) | 77% | < 0.01 |
| Tracheostomy | 74.0% (69.0–80.0) | 26% | 0.25 |
| Nasogastric tube | 42.0% (31.0–53.0) | 94% | < 0.01 |
| Endotracheal tube | 56.0% (37.0–74.0) | 96% | < 0.01 |
| Mechanical ventilation | 49.0% (24.0–74.0) | 98% | < 0.01 |
| Intubation | 39.0% (28.0–49.0) | 96% | < 0.01 |
| Central vascular catheter | 41.0% (23.0–60.0) | 95% | < 0.01 |
| Urinary catheter | 39.0% (30.0–48.0) | 96% | < 0.01 |
| Medication exposure | |||
| Exposure to antibiotics | 52.0% (38.0–66.0) | 95% | < 0.01 |
| Proton pump inhibitors | 37.0% (16.0–58.0) | 97% | < 0.01 |
3.5.1 Patient Characteristics
The data indicate that patients with chronic kidney disease presented the highest risk of infection, with a prevalence of 52.0%. Those diagnosed with malignancies, including various cancer types, presented an elevated HAI incidence of 45.0%. Individuals receiving immunosuppressive cancer therapies also demonstrated high susceptibility, as evidenced by a prevalence of HAIs of 40.0%. Similarly, patients with diabetes presented an increased infection prevalence of 35.0%. Males and females in the ICUs presented a comparable HAI prevalence of 36.0%.
3.5.2 Medical Interventions
The medical intervention associated with the highest infection risk was tracheostomy, with an alarmingly high prevalence of 74.0%. Notably, the low I2 value indicates consistent findings across studies for this procedure. Endotracheal intubation conferred the second highest risk, with a prevalence of 56.0%. Mechanical ventilation posed the third greatest risk, as 49.0% of patients developed infections. Surgery following ICU admission and the use of nasogastric tubes increased the risk by 47.0% and 42.0%, respectively. Central vascular catheter devices and blood transfusions were associated with 41.0% and 40.7% infection prevalence, respectively. The lowest risk interventions were urinary catheters and intubation, both with a prevalence of 39.0%.
3.5.3 Medication Exposure
In terms of medication exposure, the meta-analysis revealed that antibiotics increased the infection risk the most, with a prevalence of 52.0%. The prevalence of HAI risk associated with proton pump inhibitors was 37.0%.
3.6 Statistical Analysis
The statistical outcomes of the meta-analysis demonstrated that almost all the investigated risk factors for HAIs in ICUs were significantly associated (p value < 0.01), indicating a robust relationship between each element and HAI risk. There were differences in the heterogeneity of the results, with high I2 values (e.g., 97% for males and females) denoting substantial variability in risk factors across diverse investigations. Moreover, lower I2 values (such as 26% for the tracheostomy procedure) suggest more uniform outcomes.
3.6.1 Meta-Regression
The meta-regression analysis offered valuable insights into the factors that may contribute to the variation in the prevalence of HAIs across the studies. It specifically examined the impact of regions (AMR, EMR, EUR, SEAR, and WPR) and income levels (low income, lower middle income, and upper middle income) on the reported prevalence of HAIs, as detailed in Table 3. The results indicate that there was no statistically significant association (p value > 0.05) between regions, income levels, and the reported prevalence of HAIs. This suggests that the differences in HAI prevalence may not be predominantly linked to the region or income level of the countries, underscoring the potential influence of other factors such as patient-specific risk factors, healthcare procedures, microbiological factors, and study design.
| Variable | Estimate | Standard error | Z-value | p value | Confidence interval (lower bound) | Confidence interval (upper bound) |
|---|---|---|---|---|---|---|
| Region | ||||||
| AMR | 7.7219 | 306.7015 | 0.0252 | 0.9799 | −593.4020 | 608.8457 |
| EMR | −20.2781 | 306.4634 | −0.0662 | 0.9472 | −620.9353 | 580.3790 |
| EUR | 93.1778 | 190.2641 | 0.4897 | 0.6243 | −279.7329 | 466.0885 |
| SEAR | −65.2878 | 250.3428 | −0.2608 | 0.7943 | −555.9506 | 425.3751 |
| WPR | 369.2467 | 251.7486 | 1.4667 | 0.1425 | −124.1714 | 862.6648 |
| Income level | ||||||
| Low income | −82.6930 | 266.5218 | −0.3103 | 0.7564 | −605.0661 | 439.6801 |
| Lower middle-income | −50.2728 | 146.4867 | −0.3432 | 0.7315 | −337.3815 | 236.8359 |
| Upper middle-income | −170.1299 | 129.3528 | −1.3152 | 0.1884 | −423.6568 | 83.3970 |
3.6.2 Sensitivity Analysis
The results of the leave-one-out sensitivity analysis, presented in Supporting Information S1: Table S34, revealed that the pooled prevalence estimate of HAI remained relatively stable, fluctuating between 26.08% and 28.93% with the omission of each individual study. This stability suggests that the original estimate of 32.0% (95% CI 26.0–38.0) was not significantly influenced by any single study. Notably, the study by Litwin et al. [37] had the most pronounced effect on the pooled estimate, as its exclusion resulted in an increase in prevalence to 28.93%. Nevertheless, the removal of most studies led to only minor changes in the pooled prevalence and its confidence interval, highlighting the robustness of the overall findings against the influence of individual studies.
3.7 Risk of Bias Assessment
Using the Robvis tool [45], the included studies (n = 21) were evaluated across five domains of bias, as shown in Figure 5.
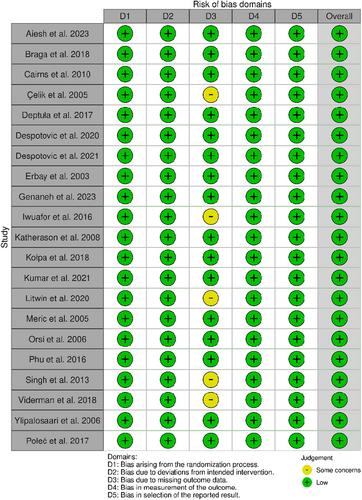
Selection of participants: All 21 studies (100%) had a low risk of bias, as they were recruited from representative populations and used objective inclusion criteria.
Departures from the intended exposure: All 21 studies (100%) adequately accounted for all patients exposed and unexposed to HAIs.
Missing data: Sixteen studies (76%) were at low risk, whereas five (24%) raised some concerns due to a lack of standard definitions for HAIs and incomplete study setting details.
Measurement of the outcomes: All 21 studies (100%) objectively measured outcomes in a standardized manner.
Selection of reported results: All 21 studies (100%) reported all prespecified outcomes and exposures.
Overall, the majority of the included studies were judged to have a low risk of bias across all domains, supporting the robust methodology and integrity of the results. Some areas with potential weaknesses were identified that warrant consideration during analysis and interpretation. However, the low-risk profile provides confidence in the conclusions drawn from this systematic review.
4 Discussion
This meta-analysis revealed an alarmingly elevated pooled prevalence of HAIs in adult intensive care units (ICUs), estimated at 32.0%, with a 95% confidence interval ranging from 26.0% to 38.0%. The prevalence was substantial, indicating that nearly one-third of adult ICU patients may experience at least one HAI during critical care treatment. Our findings align with those of a previous review, which reported that approximately 30% of ICU patients are affected by a minimum of one HAI [13]. The high degree of heterogeneity (I2 = 97%) signifies notable variances in methodologies, case definitions, and settings of individual studies in the analysis. However, the significant p value (< 0.01) suggests that the observed divergences are not due to chance alone. Standardizing surveillance protocols across ICUs could facilitate more consistent results.
Our subgroup analysis by geographical region revealed substantial variations in the prevalence of HAIs. A high prevalence was observed in the African, Eastern Mediterranean, and American regions, compared with the European, Western Pacific, and Southeast Asian regions. These regional differences may be attributed to a variety of factors, including variations in healthcare infrastructure, access to resources, adherence to infection control protocols, and antimicrobial stewardship practices. For instance, healthcare systems in low- and middle-income countries (LMICs) may face greater challenges in terms of resource constraints, inadequate staffing, and limited access to essential medical equipment and supplies [46, 47], all of which can contribute to the higher prevalence of HAIs observed in these regions. Furthermore, differences in the availability and implementation of evidence-based infection prevention and control measures, such as hand hygiene compliance, appropriate use of personal protective equipment, and the implementation of bundle-based interventions targeting specific types of HAIs, may also play a significant role in the observed regional variations.
Similarly, the subgroup analysis based on income levels revealed a higher prevalence of HAIs in lower middle-income countries than in high-income countries. This finding aligns with the regional variations, as many LMICs are located in the African, Southeast Asian, and Western Pacific regions, where the burden of HAIs appears to be more significant. The disparities in HAI prevalence between income levels underscore the importance of addressing the underlying socioeconomic and healthcare system-related factors that contribute to the higher risk of HAIs in resource-limited settings. These factors may include, but are not limited to, limited access to essential medical supplies and equipment, suboptimal infection control practices, inadequate staffing and training, and insufficient investment in healthcare infrastructure and surveillance systems. To effectively address the substantial burden of HAIs in ICUs, a multifaceted approach is required, involving a combination of evidence-based interventions and targeted strategies tailored to the specific needs and challenges of different regions and income levels. Improving adherence to evidence-based infection prevention and control (IPC) guidelines should be a priority in ICUs [48]. This includes ensuring consistent hand hygiene practices, appropriate use of personal protective equipment, and the implementation of bundle-based interventions targeting specific types of HAIs, such as central line-associated bloodstream infections, ventilator-associated pneumonia, and surgical site infections.
Our meta-analysis evaluated HAI incidence among various types of HAIs impacting adult patients in ICUs. Pneumonia posed the greatest risk among HAIs in adult ICUs, with an overall prevalence of 51.0%. The prevalence of VAP was even higher at 54.0%. As critically ill patients frequently necessitate mechanical ventilation support, this interrupts regular pulmonary clearance and defenses against respiratory pathogens [49], reflecting the elevated prevalence observed in our review. VAP is one of the leading causes of mortality among ICU patients. A recent systematic review revealed that mortality rates ranged from 6.3% to 66.9% [50]. However, higher mortality rates exist in surgical ICU patients [15]. On average, VAP can prolong ICU hospitalization by 7–9 days [51], underscoring the importance of prioritizing preventive strategies targeting mechanically ventilated patients.
We observed that bloodstream infections were the second most prevalent HAI in adults in ICUs, accounting for 34% of the identified cases. Intensive care patients frequently receive invasive vascular access devices that bypass skin and tissue barriers, increasing the risk of bacteremia in critically ill patients [52]. BSIs often lead to severe complications, including sepsis, which can be life-threatening [52]. Studies have shown that BSIs significantly increase ICU patient mortality, ranging from 35% to 50% [53]. Patients with BSIs often have prolonged ICU stays compared with those without BSIs [54]. Treating a BSI in an ICU patient is the costliest HAI, ranging from $30,919 to $65,245 per infection [55]. Therefore, measures shown to be effective for preventing central line-associated bloodstream infections, such as proper catheter insertion techniques, maximum sterile barrier precautions, site care maintenance according to best practices, and daily review of line necessity with prompt removal of nonessential lines, should be rigorously adhered to.
Our results indicate that UTIs occurred at a prevalence of 26%, which falls within the range of previous studies by López and Cortés [56], indicating that UTIs account for 20%–50% of ICU cases. Extended urinary catheter use enables uropathogens to migrate upwards and facilitate bladder colonization [57], potentially leading to UTIs. SSIs following operations accounted for 12% of the cases. This concurs with a meta-analysis that estimated that 11% of adult ICU surgery patients globally experienced SSIs [58]. Estimates indicate that between 187 and 281 million surgical procedures occur annually worldwide, resulting in at least 7 million annual surgical complications, of which over 1 million are fatal [59], indicating that surgery still results in high morbidity/mortality globally. However, up to 60% of reported SSIs are preventable through evidence-based guidelines [60]. We observed that GIs were less prevalent at 6.0%, which is relatively low compared with the relatively low prevalence of more common respiratory and bloodstream infections in ICU settings; however, this difference is still significant given patient vulnerability. CNSIs are the rarest, with a 2.0% prevalence, which is likely due to brain defenses [61]. Despite their lower prevalence, CNSIs can lead to severe outcomes, including long-term neurological deficits and high mortality, for example, 10%–30% for bacterial meningitis, depending on the pathogen/patient factors [62].
Several patient characteristics are associated with increased infection risk. Interestingly, our meta-analysis indicated that 36% of both male and female intensive care patients developed infections, suggesting that there was no statistically significant difference based on sex. However, having chronic conditions such as malignancy, chronic kidney disease, or diabetes mellitus substantially increases the likelihood of acquiring an infection. Patients with chronic kidney disease demonstrated the highest risk of HAIs at 52%, underscoring the serious peril imposed by renal failure and highlighting the need for additional precautions in this population. Similarly, cancer patients are more susceptible to HAIs, with a 45% risk, potentially due to compromised immunity and invasive medical procedures [63]. Other conditions, such as diabetes mellitus and immunosuppression, also increase the probability of infection due to immunocompromised phenotypes [64, 65].
Several medical interventions performed in ICUs have emerged as risk factors. We found that approximately 47% of patients who undergo surgery postadmission contract infections, potentially because invasive procedures can lead to complications and extended device exposure. Similarly, blood transfusions increased the risk by approximately 41%, possibly through contact with contaminated products. Invasive devices, such as tracheostomies, nasogastric tubes, endotracheal tubes, mechanical ventilation, intubation, central vascular catheters, and urinary catheters, also pose hazards. All of these methods introduce pathways for pathogens to breach sterile tissues. Our meta-analysis revealed a 74% risk of HAIs in tracheostomy patients, identifying this intervention as highly hazardous. Moreover, endotracheal tubes and mechanical ventilation are integral parts of critical care. For example, among the millions of patients admitted to ICUs globally each year, approximately one-third receive mechanical ventilation at some point during their stay [66], which may explain the high prevalence of pneumonia, as it contributes to the development of this infection. Likewise, central vascular catheter and urinary catheter insertion frequently cause bloodstream and urinary infections, respectively, rendering these common and prevalent infections after pneumonia. Ensuring a strict aseptic technique minimizes the contamination risk when accessing these vascular devices [67].
Exposure to medication also contributes to increased infection risk. We found that approximately 52% of the antibiotic-treated ICU patients developed infections. This finding indicates that necessary exposure to antibiotics can disrupt the natural flora and facilitate HAIs, especially those caused by resistant pathogens such as Clostridioides difficile [68]. Proton pump inhibitors increase the risk by approximately 37%, potentially by altering the gastric pH and impacting colonization [69]. These results highlight the importance of judicious antibiotic and PPI use.
The significant heterogeneity observed across most results suggests that risk may vary between populations and settings. However, certain factors, such as tracheostomies, mechanical ventilation, and device usage, yielded more consistent effects. Further research should explore how risk changes with the device insertion technique, infection control practices, and patient case-mix adjustments in the analyses. Studies could also integrate multiple risk factors into predictive mathematical models. Notably, while age is a pertinent factor when assessing hospital-acquired infection risk, a meta-analysis of age range was not feasible here because the included studies employed disparate age brackets, hampering the determination of risk associated with specific ages. Future research should aim to standardize age categories to facilitate more effective comparisons and analyses.
This systematic review provided insights into the significant risk determinants and trends for HAIs among adult intensive care patients. However, several limitations necessitate acknowledgement. Considerable heterogeneity between diverse clinical contexts and methodologies indicates that the prevalence estimates warrant cautious interpretation without adjustment. The lack of data on the causative pathogens, time of onset, and adjusted effect sizes constrains the clinical applicability of these methods. Variations in device utilization practices, antibiotics, and pathogens over time may not be fully accounted for. Most data originating from high-income settings restricts generalizability to resource-limited regions.
5 Conclusion
This systematic review and meta-analysis provided valuable insights into the epidemiology of HAIs among adult intensive care patients. The overall prevalence of HAIs was found to be high, with nearly one-third of adult ICU patients affected. Certain infection types, such as VAP and bloodstream infections, pose particularly substantial burdens. Several important risk factors for HAIs in the ICU setting were identified. Patient characteristics such as chronic conditions, immunosuppression, and invasive devices or procedures increase the risk of infection. Antibiotic exposure also contributes to disrupted microbiota and resistant pathogens. The considerable heterogeneity among studies indicates that these findings should be interpreted cautiously without standardized adjustments.
Author Contributions
Alex Odoom: conceptualization, methodology, software, data curation, formal analysis, validation, investigation, writing – original draft, writing – review and editing, visualization, resources, and project administration. Eric S. Donkor: conceptualization, resources, supervision, data curation, software, formal analysis, project administration, writing – review and editing, visualization, validation, methodology, writing – original draft, funding acquisition, and investigation.
Acknowledgments
The authors received no specific funding for this work.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The corresponding author, Eric S. Donkor, affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the supporting material of this article.