Pharmacogenomics revolutionizing cardiovascular therapeutics: A narrative review
Abstract
Background and Aim
Among the cardiovascular diseases (CVDs), heart failure, hypertension, and myocardial infarction are associated with the greatest number of disability-adjusted life years due to lifestyle changes and the failure of therapeutic approaches, especially the one-size-fits-all interventions. As a result, there has been advances in defining genetic variants responsible for different responses to cardiovascular drugs such as antiplatelets, anticoagulants, statins, and beta-blockers, which has led to their usage in guiding treatment plans. This study comprehensively reviews the current state-of-the-art potential of pharmacogenomics in dramatically altering CVD treatment. It stresses the applicability of pharmacogenomic technology, the threats associated with its adoption in the clinical setting, and proffers relevant solutions.
Methods
Literature search strategies were used to retrieve articles from various databases: PubMed, Google Scholar, and EBSCOhost. Articles with information relevant to pharmacogenomics, DNA variants, cardiovascular diseases, sequencing techniques, and drug responses were reviewed and analyzed.
Results
DNA-based technologies such as next generation sequencing, whole genome sequencing, whole exome sequencing, and targeted segment sequencing can identify variants in the human genome. This has played a substantial role in identifying different genetic variants governing the poor response and adverse effects associated with cardiovascular drugs. Thus, this has reduced patients' number of emergency visits and hospitalization.
Conclusion
Despite the emergence of pharmacogenomics, its implementation has been threatened by factors including patient compliance and a low adoption rate by clinicians. Education and training programs targeting both healthcare professionals and patients should be established to increase the acceptance and application of the emerging pharmacogenomic technologies in reducing the burden of CVDs.
1 INTRODUCTION
Tissues of the human body require a constant supply of blood from the heart through blood vessels, all of which comprise the cardiovascular system. Impairment in either of the two components can result in irreversible cell damage, culminating in different cardiovascular diseases (CVDs).1 As a result of modifiable factors such as high cholesterol, smoking, and diabetes mellitus as well as nonmodifiable etiologies such as sex, family history, and age, CVDs remain a leading cause of mortality globally, representing a significant burden on healthcare systems and communities.1-3 Its prevalence has been increasing tremendously in both developing and developed states. There is a record of about 17.9 million patients who die of cardiovascular diseases annually, and it is estimated that more than 23.6 million people will die of these conditions by 2030.2 Among the CVDs, heart failure, hypertension, and myocardial infarction (stroke) have been associated with the highest disability-adjusted life years (DALYs) due to various barriers such as less efficacious medications and lifestyle changes in the current ever-developing world.4
Medicine has evolved in the past century, and scientists have worked tirelessly to find medications for most illnesses.5 Traditional medicine focuses on a one-size-fits-all intervention model, which assumes that all individuals suffering from the same disease should be treated with the same drug at a standard dose. This intervention involves the administration of medicine on the basis of the pathological pathways of a disease, which is common in all individuals, instead of considering genetic variations.6 However, a study by the Center for Proteomic & Genomic Research (CPGR) in South Africa has shown that traditional medicine benefits only 20% of the target population, which is attributed to genetic variation. For instance, in hypertensive therapy, one-third of the patients show resistance, commonly owing to the inability to precisely predict the response to particular antihypertensive medicines.7 On the other hand, precision medicine for cardiovascular diseases utilizes individual and population genomics to guide the prediction and selection of effective interventions to optimize patient outcomes. It has enabled early diagnosis and targeted management and has lowered patients' exposure to the side effects of cardiovascular drugs.8
As a part of precision medicine, pharmacogenomics focuses on enhancing the use of available drugs by matching genetic variants responsible for the principles of pharmacokinetics and pharmacodynamics to interindividual variation in responses to drugs.9 Thus, there have been advancements such as defining the genetic determinants of the response to different drugs such as antiplatelets, anticoagulants, statins, calcium channel blockers (CCBs), and beta-blockers, among others, which led to its usage in guiding treatment plans.5 In practice, health professionals are noticing significant clinical improvement when treating cardiovascular diseases via pharmacogenomics and promise to experience even better outcomes in the near future.5 They also believe that conducting experiments will help in gaining advanced insights into the relationship between the medication response and genetic factors. This approach is thought to ultimately establish a more improved, specified targeted therapy for different diseases, including cardiovascular disorders.10 This study reviews the state-of-the-art potential of pharmacogenomic technology in dramatically altering the treatment of cardiovascular diseases, contributing to more impactful precision and personalized medicine.
1.1 Overview of the pharmacology of current cardiovascular drugs
This section highlights the mechanism of action, pharmacokinetics, indications, and drug–drug interactions associated with some of the commonly used cardiovascular drugs.
1.1.1 Aspirin
Aspirin at a low dose acts as an antiplatelet that is used for angina pectoris, myocardial infarction (MI), and as prophylaxis post-MI.11 It also plays an anti-inflammatory role at high doses (300 mg). It irreversibly inhibits cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) with a more potent effect on COX-1. Consequently, it achieves an antiplatelet effect at lower doses by decreasing thromboxane A2 (TXA2).12-14 It is mainly absorbed in the stomach (pka 3.5), its plasma level peaks within 40 min, and its oral bioavailability is approximately 40%–50%. Although its half-life is 20 min, its effects last for the lifetime of platelets (8–10 days).15-17 The hydrolysis of aspirin leads to salicylic acid, which is converted to salicyluric acid and salicyl phenolic glucuronide to be excreted via urine. Significant drug interactions can occur via different mechanisms of action and renal excretion is highly dependent on the urine pH. For instance, antacids raise the urinary clearance of aspirin by increasing the urinary pH.18 This in turn reduces the half-life of aspirin, leading to a lack of its therapeutic effects in the body. Moreover, metoclopramide, cholestyramine, and antacids affect aspirin absorption. However, ibuprofen and diclofenac compete with aspirin for serum protein binding.19
1.1.2 Clopidogrel
Clopidogrel is indicated for stable angina, acute coronary syndrome, percutaneous angioplasty, and for long-term prevention after CABG.20 In non ST segment elevation myocardial infarction, it is given as 300–600 mg loading dose followed by 75 mg per day for 1 year with aspirin.21 Additionally, clopidogrel is an irreversible P2Y12 receptor inhibitor, which inhibits ADP-mediated activation and aggregation of platelets via inactivation of GPIIb/IIIa receptors. After administration, only 50% is absorbed in the small intestines, from which 85%–90% is hydrolyzed in the liver by carboxylesterase 1 into inactive metabolite, clopidogrelic acid (SR26334). The rest of the drug particles are activated into clopidogrel active metabolite (clop-AM) through a two-step process by cytochrome P450 enzymes including CYP2C19, CYP1A2, CYP2B6, CYP2C9, and CYP3A4.22-24 Major interaction can occur with drugs that interfere with CYP450 enzymes. Statins enhance clopidogrel bioactivation via the induction of CYP2B6, CYP2C9, and CYP3A4.25, 26 However, calcium channel blockers (CCBs) and selective serotonin reuptake inhibitors (SSRIs) decrease clopidogrel action via CYP3A4 inhibition.27, 28
1.1.3 Amlodipine
Calcium channel blockers are among the first-line treatments for hypertension, after angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).29 As one of the commonly used drugs, amlodipine is a dihydropyridine calcium channel, particularly the L-type, blocker which induces smooth muscle relaxation and vasodilation. This mechanism eventually restores blood pressure due to a reduction in peripheral vascular resistance.30 Amlodipine reaches its peak level within 6–12 h with a bioavailability between 64% and 90% and it significantly binds plasma protein (93%). Furthermore, it is metabolized in the liver by CYP3A4 into inactive products that are mainly eliminated via urine. Moreover, it has the longest half-life among CCBs (30–50 h) so it is given once daily.30, 31 According to Courlet and colleagues, amlodipine bioavailability is decreased by CYP3A4 inducer (efavirenz) but increases with CYP3A4 inhibitors such as verapamil and diltiazem.32
There exist many challenges that need to overcome in cardiovascular therapeutics, mainly the challenge of side effects. For example, aspirin can cause major gastrointestinal bleeding, intracranial bleeding, and toxicity.33 Furthermore, drug–drug interactions can affect any step in the drug's pharmacokinetics or pharmacodynamics. For instance, digoxin in combination with beta blockers, verapamil, or diltiazem, can induce bradycardia or even AV block.34 In the era of personalized medicine, it is important to address each patient individually due to the variability in genetic factors, risk factors, comorbidities, lifestyle, and environment. This suggests the need for active research in individualized medicine to achieve the optimal results.35
1.2 Interpreting the genetic code of cardiovascular drugs response
According to clinical trials, not all patients respond to drug therapy in the same manner. Medications can induce considerable diversity in their effects, even when there is a minimal disparity in drug levels at intended sites of action, patient adherence, or compliance.36 This variability in pharmacodynamics tends to be specific to either the drug itself or the disease being treated, unlike pharmacokinetic variability, which in some cases, frequently applies across various medications and medical conditions.37 The identification of certain genomic markers, most commonly single nucleotide polymorphisms (SNPs), is associated with variability in different drug responses and outcomes or even adverse events.38
Antiplatelets such as clopidogrel, prasugrel, ticagrelor, and aspirin, are associated with genes such as CYP2C19, ABCB1, CYP3A4/3A5, and CES-1 which affect their efficacy.39 Notable variants include CYP2C19*2 and CYP2C19*17 that affect the response to prasugrel. However, no significant variations have been linked to ticagrelor.37, 38 Additionally, beta-blockers such as metoprolol and carvedilol as well as ACE inhibitors (e.g., enalapril, perindopril) are a subgroup of effective antihypertensive drugs that are known to be affected by ADRB1 variations.40-42 Notably, anticoagulants such as warfarin, heparin, dabigatran, and rivaroxaban share close genetic markers including VKORC1, CYP2C9, and CES1 whose variations affect their action especially for warfarin dosing.5, 40 Besides, variations in SLCO1B1, HMGCR, LPL, ABCB1, LDLR, and SREBF1 affect individual response to statins and cholesterol-lowering agents.43, 44 These drugs include lovastatin, atorvastatin, simvastatin, rosuvastatin, fluvastatin, and lomitapide.
Thus, multiple factors such as the presence of comorbidities, patient age and sex, principle components for ancestry, and other drug exposure, which has potential to affect the genes, should be considered when certain drugs are being prescribed.37 Further studies should also consider drug interactions, interactions between SNP genotype and drug exposure. In addition, distinguishing between monogenic and polygenic forms is necessary for patient-tailored cardiovascular assessment, counseling, and patient treatment.45, 46 By identifying and characterizing genes associated with cardiovascular disease, preventive measures along with treatments and quality of care could be improved and tailored for each case regardless of its specificity. Linkage studies and genome-based linked analysis have been useful in identifying genes related to cardiovascular diseases and targeting new causative genes to be focused on for molecular diagnosis and therapeutic interventions.47 DNA-based sequencing methodologies have made gene-based screening and diagnosis more feasible in routine clinical practice while maintaining clinical accuracy (Table 1).
| Class of cardiovascular drugs | Pathway/genetic marker | Examples of drug | Variant genetic determinants of drug responses | References |
|---|---|---|---|---|
| Antiplatelets | Thienopyridine inhibitor of platelet P2Y12 ADP receptor | Clopidogrel | CYP2C19 variation P2Y12 variation ABCB1 variation CYP3A4 and CYP3A5 CES-1 variation CYP2C9 variation |
[39, 48] |
| New oral thienopyridine | Prasugrel | CYP2C19*2 and CYP2C19*17 associated with attenuating and strengthening response to prasugrel | ||
| Nonthienopyridine P2Y12 receptor antagonist | Ticagrelol | No associated variations have been found | ||
| COX-1 and COX-2 inhibitor and inhibition of TXA2 | Aspirin | CYP2C9 (601130) PTGS1 (176805) |
[39, 43] | |
| Antihypertensive | Beta-blockers | Metoprolol Carvedilol Nebivolol Propranolol Alprenolol Atenolol |
ADRB1 (109630) | [40, 41] |
| Angiotensin-converting enzyme (ACE) inhibitors | Enalapril, Perindopril, Carpropril, Imidapril |
[42] | ||
| Anticoagulants | Blocking either action or synthesis of the coagulation factors via: | |||
| 1-inhibition of vitamin K-dependent activation of clotting factors II, VII, IX, and X | Warfarin | Variation of VKORC1 and CYP2C9 | [49] | |
| 2-catalysis of factor II and X inactivation | Heparin | CYP4F2 variation | ||
| 3-inhibition of factor X | Low molecular weight heparin | No variations have been identified regarding these drugs | ||
| 4-factor II inhibition | Dabigatran | CES1 (114835) | [5] | |
| 5-Factor Xa inhibition | Rivaroxaban | |||
| Statins | 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors | Lovastatin Atorvastatin Simvastatin Rosuvastatin Fluvastatin |
SLCO1B1 (604843) affects all drugs |
[43] |
| LPL (609708) acts on lovastatin moreHMGCR (142910) acts on pravastatin | [44] | |||
| CYP7A1 (118455); ABCB1 (171050); CETP (118470) affects mainly the action of atorvastatin | [50-52] | |||
| LDLR (606945); SREBF1 (184756) affects the action of fluvastatin | [53, 54] | |||
| Microsomal triglyceride transfer protein (MTP) inhibitor | Reduction of blood cholesterol by inhibiting microsomal triglyceride transfer protein | Lomitapide | LDLR (606945) | [49] |
- Note: Created by Nagham Ramadan.
Regulatory concerns encompass how genotyping tests are controlled, the degree to which pharmacogenetic studies should be integrated into the drug development process before or following extensive clinical trials and the methods for including pharmacogenetic data on product labels for the education of clinicians and patients. The US Food and Drug Administration (FDA) has initiated efforts to gather pharmacogenetic data throughout the drug development phase, potentially helping to resolve some of these challenges.55 Besides, incorporating genetic insights into clinical practice has been quite a challenge. Recent studies have shown that current healthcare workers have a low rate of adoption and comfort with ordering and interpreting pharmacogenomic test results.37, 56 Healthcare professionals encounter multiple obstacles in gaining pharmacogenomic knowledge, such as insufficient time, complex terminology, and constantly evolving evidence and guidelines.2, 12, 13, 57, 58 Another obstacle is the incorporation of pharmacogenomic education into the standard curricula of various medical fields to equip future healthcare practitioners.59
2 DISCUSSION
2.1 Advances in pharmacogenomic technology
Personalized medicine aims to achieve the optimal response with minimal adverse effects, thus improving the quality of care. Pharmacogenomics is a revolution in the era of personalized medicine because it reflects the effects of the genomic profile on an individual's response to a certain drug.60, 61 Currently, the Dutch Pharmacogenetics Working Group (DPWG) and the Clinical Pharmacogenomics Implementation Consortium (CPIC) aid in integrating pharmacogenomics into clinical management by establishing evidence-based guidelines.62, 63 Single nucleotide variants (SNV) detection and next-generation sequencing (NGS) are great technologies that have been used in the realm of pharmacogenomics (Figure 1).
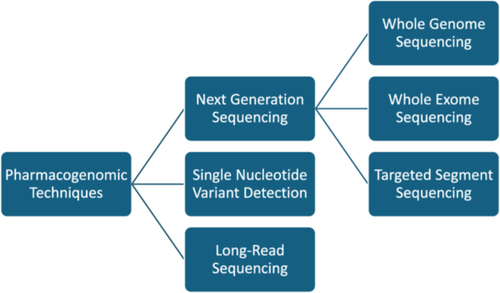
SNV panel testing is frequently used in pharmacogenomics, and it involves arrays that can be commercial or customized, limited to variants of a single gene or involving multiple variants of the whole genome. Additionally, these arrays may include either strongly associated variants or any variant that can be related to the drug in any other way.45 Most arrays use the PCR technique, in which the variant of interest is either detected by fluorescence or by mass spectroscopy.64, 65 Commercial panels such as VeraCode and Veridose, consist of 184 variants in 34 genes, and 68 variants in 20 genes, respectively, with 5 copy number targets for CYP2D6. Panels can even reach more than 4000 variants in more than 1000 genes (pharmacosan panel).45 Besides, next-generation sequencing (NGS) is widely used in research and diagnostics. However, it is still to be involved in clinical pharmacogenomics to personalize medication therapy. It can involve whole genome sequencing (WGS), only the coding part (whole exome sequencing: WES), or sequencing only the targeted segment of a certain gene.66 WES reflects only 1%–2% of the whole genome, and NGS is better than SNV because more variants can be identified. However, the implementation of NGS is challenging due to the large amount of data available for analysis. For instance, experiments with WGS have been noted to produce 3–4 million variants per person, which requires a high level of analytical skills.57 Long-read sequencing (LRS) is another method that can be used and involves two technologies: “Pacific Bioscience” technology and “Oxford Nanopore Technology.”67 It can be used in diagnosing diseases such as Parkinson's disease, for instance, through identifying ATXN10 repeats.68 Moreover, it can detect complex gene regions, such as those in CYP2D6, where there is SNV and structural variant.69 However, in pharmacogenomics, no large-scale studies have been performed via long-read sequencing.
SNV processing is quick and cheap due to the advanced selection of variants and a relatively small number of genes. However, not all arrays can detect complex regions that are abundant in pharmacogenes, such as repetitive sequences, copy number variants, and structural rearrangements.70 This kind of sequencing can detect more rare variants, but the effect of such variants are still ambiguous and thus cannot be applied in clinical practice.71 In practice, medical communities still lack knowledge of the importance of pharmacogenomics.37 Importantly, there is a challenge of being stigmatized and not receiving the right of care because the variant exists in the genome.72 In part, this has been associated with the lack of data on cost-effectiveness.37
Warfarin is a great example of how pharmacogenomics interferes with dosing and clinical response. It is an FDA-approved anticoagulant used for preventing and treating venous thromboembolism (VTE) and pulmonary embolism (PE). It inhibits vitamin K epoxide reductase, thus reducing the levels of clotting factors II, VII, IX, X, protein C, and S.73, 74 It is metabolized mainly in the liver by CYP2C9, so mutations in genes encoding CYP2C9 (*2, *3) can affect warfarin-S clearance. Polymorphisms that take the heterozygous form have a 37% clearance reduction while the homozygotes have a 70% reduction.75 According to Reider and colleagues, the vitamin K epoxide reductase complex subunit 1 (VKORC1) genotype exerts an effect that is triple that of CYP2C9, thus playing a major role in determining the warfarin dose.76 In addition, CYP4F2 can affect warfarin dosing because it codes for the CYP4F2 enzyme, which inactivates vitamin K. Initially, warfarin dosing was determined mainly by the fixed dosing technique, by which it was administered for 2 days, after which the international normalized ratio (INR) was monitored, and the dose was adjusted accordingly.77 However, in 2010, the FDA added the pharmacogenetic table to the warfarin label.78 Additionally, in 2011, the Clinical Pharmacogenomics Implementation Consortium established guidelines for warfarin dosing based on the CYP2C9 and VKORC1 (−1639 G > A) genotypes79 (Table 2).
| Dose (mg) | VKORC1 (−1639 G > A) | CYP2C9 | Reference |
|---|---|---|---|
| 0.5–2 | AA | *1/*3 | [78] |
| *2/*2 | |||
| *2/*3 | |||
| *3/*3 | |||
| AG | *2/*3 | ||
| *3/*3 | |||
| GG | *3/*3 | ||
| 3–4 | AA | *1/*1 | |
| *1/*2 | |||
| AG | *1/*2 | ||
| *1/*3 | |||
| *2/*2 | |||
| GG | *1/*3 | ||
| *2/*2 | |||
| *2/*3 | |||
| 5–7 | AG | *1/*1 | |
| GG | *1/*1 | ||
| *1/*2 |
- Note: Created by Rawane Abdul Razzak.
2.2 Future directions and emerging opportunities
As a leading cause of mortality worldwide, CVDs have pushed healthcare systems to rely on more patient-centered approaches via precision medicine. This significance is underscored in the present landscape of cardiovascular medicine, characterized by the production of vast and varied data types. This includes “omics” data (such as genomics and proteomics), high-definition medical imaging, biosensors, wearable technology, continuous physiological measurements, and electronic health records (EHR).80 Therefore, the use of artificial intelligence (AI) and machine learning (ML) has allowed more sophisticated data analysis.81 For clinicians, it offers the potential for enhanced accuracy, efficiency, and standardized interpretation of medical imaging, allowing improved diagnosis and risk assessment. AI also holds promise for optimizing workflow, minimizing medical errors, and ultimately improving patient outcomes. It also serves as a mediator to promote and foster education in terms of primary and secondary prevention for cardiovascular health.81, 82
Nevertheless, AI may necessitate the transformation of current clinical care models or the creation of new health models and services. These implementations might be costly, involving not only the initial expenses of integrating AI into clinical settings but also additional costs for personnel training.83 Obtaining high-quality data for training and validating AI also poses a significant challenge since AI outputs are solely based on the data it is trained on.84 Furthermore, ensuring patient privacy necessitates appropriately blinding or masking training data sets. High-security measures are essential to prevent breaches or data leaks. Additionally, AI systems may inadvertently perpetuate biases present in their training datasets.85, 86 Despite these challenges, blockchain technology has the potential to become an important aspect of healthcare infrastructure.87 Blockchains can offer strong data security due to their distinctive data storage methods and ensure the integrity of healthcare data because altering the blockchain is challenging, which support data access accountability and authentication (Table 3).
| Effectiveness of AI-based approaches | Patient benefit improvement | Improved decision making | Machine learning personalized cardiology | |
| Clinical utility | AI utilization allows improved imaging analysis, accuracy, and efficacy | Data analysis of factors such as age, race, medical history, and medications to improve clinical decision-making | Personalized treatments can be obtained through data analysis using machine learning | [85-87] |
| Improve patient safety if implemented in monitoring systems | Complication prediction and alert healthcare professionals to apply the necessary interventions | Patients risks of developing cardiovascular diseases and healthcare guidance for preventative medicine could be obtained through predictive models | ||
| Improve physician efficacy by spending more time with the patient |
- Note: Created by Nagham Ramadan and Samuel Inshutiyimana.
2.3 Threats to pharmacogenomics
Drug therapy and pharmacogenomics fields have been mostly unpredictable, providing a wide scope which allows the surge of multiple treatments. In addition, multiple obstacles including defining targeted genes and their pathways while addressing analytic, ethical, and technological issues have been areas of great concern.37 With an understanding of the human genome, educational efforts have failed to define clear-cut studies that show added value in understanding genetic information before any drug prescription.88 Another aspect to be considered is the quality of evidence and clinical relevance, in which evidence-reporting thresholds for gene-drug associations are often not transparent. For example, reports included only the number of studies that showed a gene-drug association and excluded important study details such as the number of patients, the number of independent population replications, and strength of the association.89 In addition, conflicting results from certain studies have been reported without adequate quality assessment, which limits test value. Cost-effectiveness is another major challenge that faces the field. Of course, cost-effectiveness depends on the country where economic evaluations of cost-effectiveness are considered on the basis of the number of patients with the relevant pharmacogenetics variant.90 However, the emergence of pharmacogenomic clinical trials that have been approved by the FDA has helped overcome the barrier of limited clinical information and its implementation. Evidence-based drugs and clinical practice guidelines have allowed doctors to make more informed patient-specific clinical decisions.88
3 CONCLUSION
Medications can induce considerable diversity in their effects, even when there is minimal disparity in drug levels at intended sites of action, patient adherence, or compliance. This variability in pharmacodynamics tends to be drug specific, unlike pharmacokinetic variability, which largely applies across various medications. Pharmacogenomics, as a revolution in the era of precision and personalized medicine, reflects the effects of the genomic profile on an individual's response to certain medications. DNA-based technologies such as next generation sequencing, whole genome sequencing, whole exome sequencing, and targeted segment sequencing have played substantial roles in identifying different genetic variants governing poor drug response and adverse effects. As a result, this has reduced patients' number of emergency visits and hospitalization. Nevertheless, similar to many emerging breakthroughs in treatment, several factors including patient compliance and a low adoption rate by clinicians, threaten pharmacogenomics. Furthermore, there have been challenges in the analysis of data generated by technologies in the field. In addition, the process of dosing based on genetic variation has not been exercised due to a lack of larger studies, which acknowledge the ability of genetic variants to influence drug responses or the existence of variants associated with the risk of developing cardiovascular diseases.
4 RECOMMENDATIONS
To realize pharmacogenomics as the cornerstone of clinical practice, sustainable approaches should be put in place by different stakeholders. The need for education and training programs, targeting healthcare professionals and patients, cannot be overemphasized. Notably, this could improve acceptance of emerging pharmacogenomic technologies for reducing the burden of cardiovascular diseases. In particular, clinicians should embrace the use of AI-based approaches to facilitate the analysis of large amount of data from pharmacogenomics. Moreover, higher-level institutions should consider the integration of pharmacogenomics courses in curricula for potential healthcare professionals. Besides, pharmacogenomic areas such as the mechanisms and pathways through which drug responses are affected by protein-coding and nonprotein-coding DNA remain fascinating and little-known. Governments and researchers should consider investing more in evidence-detection activities to realize advances in cardiovascular therapeutics through pharmacogenomics. As a result, this step is considered to pave the way for the development of newer guidelines for CVD treatment and ultimately lead to improved prognosis and optimal patient outcomes.
AUTHOR CONTRIBUTIONS
Olivier Uwishema contributed to conceptualization, project administration, writing, review, and designing; reviewed and edited the first draft; reviewed and edited the final draft. Magda Wojtara reviewed and edited the second draft. Samuel Inshutiyimana reviewed and edited the final draft. Figure 1 was drawn and analyzed by Rawane Abdul Razzak. Table 1 was created by Nagham Ramadan. Table 2 was created by Rawane Abdul Razzak. Table 3 was created by Nagham Ramadan and Samuel Inshutiyimana. All authors have read and approved the final version of the manuscript. All authors contributed to data collection and assembly, manuscript writing, and final approval of manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Olivier Uwishema affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.