Early prediction of gestational diabetes mellitus using first trimester maternal serum pregnancy-associated plasma protein-a: A cross-sectional study
Abstract
Background and Aims
The oral glucose tolerance test with 75 g glucose is commonly regarded as the gold standard (GS) for the detection of gestational diabetes mellitus (GDM). However, one limitation of this test is its administration in the late second trimester of pregnancy in some countries (e.g., Iran). This study aimed to evaluate the accuracy of pregnancy-associated plasma protein-A (PAPP-A) for predicting GDM in the early first trimester of pregnancy using a novel statistical modeling technique.
Methods
The study population consisted of 344 pregnant women who participated in the first trimester screening program for GDM. Maternal serum PAPP-A levels were measured between 11 and 13 gestational weeks for all participants. A Bayesian latent profile model (LPM) under the skew-t (ST) distribution was employed to estimate the diagnostic accuracy measures of PAPP-A in the absence of GS test outcomes.
Results
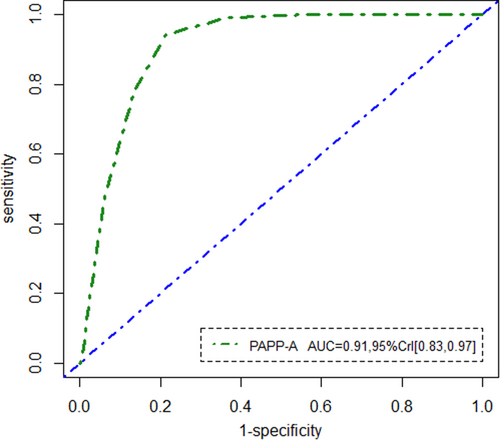
The mean (standard deviation) age of the participants was 28.87 ± 5.20 years. The median (interquartile range (IQR)) PAPP-A MoM was 0.91 (0.69-1.34). Utilizing the LPM under the ST distribution while adjusting for covariates, the sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) of PAPP-A were 92% (95% credible interval [CrI]: 0.89, 0.98), 81% (95% CrI: 0.76, 0.91), and 0.91 (95% CrI: 0.83, 0.97), respectively. Notably, the pregnant women with GDM had significantly lower PAPP-A values ( −0.52, 95% CrI [−0.61, −0.46]).
Conclusion
Generally, our findings confirmed that PAPP-A could serve as a potential screening tool for the identification of GDM in the early stages of pregnancy.
1 BACKGROUND
Gestational diabetes mellitus (GDM) is a condition in which a woman's pancreatic function fails to keep up with insulin production due to increased insulin resistance during pregnancy. Globally, GDM affects an estimated 4–18% of pregnant women, with a rate of 87.6% hyperglycemia in low- and middle-income countries.1 Over the past few years, GDM has emerged as a significant global public health concern, with its incidence increasing by 50%.2 GDM is associated with increased fetal-neonatal complications and has short- and long-term health consequences. These include fetal macrosomia, shoulder dystocia, neonatal hypoglycemia, hyperbilirubinemia, and respiratory distress syndrome.3 Additionally, GDM increases the risk of adverse pregnancy outcomes such as pre-eclampsia, urinary tract infection, pregnancy-induced hypertension, and a heightened risk of developing type 2 diabetes mellitus and metabolic syndrome later in life.4 Therefore, timely screening and adequate treatment of GDM are crucial to minimize its impact on adverse outcomes without causing harm to the mother or fetus.4-6
The current gold standard (GS) for diagnosing GDM is the oral glucose tolerance test (OGTT) with 75 g of glucose, typically conducted between 24 and 28 gestational weeks in many countries, including Iran.6-16 However, the OGTT has several limitations, such as being time-consuming, challenging for mass screening, and implementation challenges, which can contribute to diagnostic uncertainty.7, 17-19 Notably, one significant limitation is that the test is often conducted at the end of the second trimester, delaying diagnosis and intervention.9, 10, 13, 20-22 Some researchers advocate for identifying GDM earlier in pregnancy, as this could enhance antenatal care and improve clinical outcomes by reducing maternal and fetal exposure to metabolic alterations and potential epigenetic malprogramming.6, 23-25 Thereby, exploring a variety of screening and diagnostic methods to achieve early detection is desirable.
Several previous studies have investigated the use of first and early second trimester maternal serum markers to assess fetal risk for chromosomal aneuploidy and open neural tube defects.25, 26 One such marker is pregnancy-associated plasma protein A (PAPP-A), a macromolecular glycoprotein produced by the syncytiotrophoblasts, with a molecular weight of approximately 200 kDa, belonging to the metzincin superfamily of metalloproteinases. During early pregnancy, PAPP-A concentrations double approximately every 3 days, then the rate of increase becomes more gradual as the pregnancy progresses toward term. PAPP-A plays a crucial role in modulating the bioavailability of insulin-like growth factor (IGF) by cleaving IGF-binding proteins, thus promoting fetal growth and development.27-29 Abnormal levels of prenatal biomarkers such as PAPP-A have been linked to various adverse obstetric outcomes.25, 30 Reduced circulating PAPP-A concentrations early in pregnancy are associated with an increased risk of fetal growth restriction, pre-eclampsia, preterm birth, and GDM.21, 31-33 These associations suggest that low PAPP-A levels might reflect an early stage of glucose intolerance at the beginning of pregnancy.34 Elevated PAPP-A levels, although less commonly studied, are generally considered to fall within the normal range of pregnancy variations.35, 36
Although the relationship between first trimester PAPP-A levels and GDM detection has been studied retrospectively, no study to date has evaluated the predictive ability of PAPP-A for early pregnancy GDM risk before conducting the GS test at 24–28 weeks of gestation (i.e., in the absence of GS test outcomes). Hence, the objective of the present study was to examine the diagnostic power of PAPP-A in a sample of Iranian pregnant women during 11 and 13 gestational weeks using a novel Bayesian latent profile model (LPM) under skew-t (ST) distribution.
2 METHODS
2.1 Study design and sampling
For each woman, the maternal serum concentrations of PAPP-A were measured by the KRYPTOR Analyzer (Brahms AG, Hennigsdorf, Germany) using a rapid random-access immunoassay analyzer, which utilizes time-resolved amplified cryptate emission (TRACE) technology at 11–13 gestational weeks. The automatic system reports the result in immunofluorescence units (mIU/mL) and converts them to unit.25 PAPP-A values were available for all selected women due to standard early pregnancy screening practices. Between the 24th and 28th weeks of gestation, all pregnant women underwent an OGTT. A 75 g glucose load was administered after fasting, and glucose levels in plasma were measured after 1 and 2 h. The inclusion criteria were as follows: women with a singleton pregnancy, aged 20–40 years, with a current gestational age of 24–34 weeks, determined based on the first day of the last menstruation or confirmed by a first trimester ultrasound. The exclusion criteria were: type II diabetes in first-degree relatives, body mass index (BMI) ≥ 30 kg/m², habitual abortion, fetal anomalies and macrosomia, intake of medications affecting glucose metabolism, smoking, and drug use.
The covariates examined were maternal age and BMI, calculated as weight in kilograms divided by the square of height in meters. Pre-pregnancy BMI was categorized based on the World Health Organization (WHO) classification as underweight (BMI less than 18.5 kg/m2), normal-weight (18.5–24.9 kg/m2), and overweight (25–29.9 kg/m2).38
2.2 Data analysis
To evaluate the demographic characteristics of pregnant women, continuous variables were reported as the mean ± standard deviation (SD) or median (interquartile range [IQR]: Q1 [25%]– Q3 [75%]). These variables were compared between GDM groups using independent samples t-tests or Mann-Whitney U tests, depending on the normality assumption. In addition, categorical variables were presented as numbers and percentages and were compared using the chi-square test or Fisher's exact test if any expected value was 5 or less. The non-parametric Kolmogrov-Smirnov test was employed to check the normality of the PAPP-A distribution. Noticeably, the D'Agostino39 and Anscombe-Glynn40 tests were applied to examine the skewness and kurtosis in PAPP-A distribution, respectively. The statistical significance level for all the tests was set at a two-tailed p-value < 0.05. The preliminary analyses were carried out using R software, version 4.3.1.41
In practice, the true disease status is rarely known owing to the fact that obtaining GS test values may be difficult or even impossible. In such cases, classification error may occur, introducing serious bias in estimates of the accuracy measures of the diagnostic test [46]. Over the past thirty years, various studies have proposed the latent profile modeling approach as a solution to the problem of not having a GS assessment. LPM describes a statistical model that relates the observed results of diagnostic test performed on individuals to their latent disease status. Herein, it was provided a latent profile model under the Skewed-T distribution, considering covariates, to predict latent disease status. To evaluate the performance of the proposed models under skewed distributions and compare them with the Normal model, two simulation studies were implemented under different sample sizes and scenarios. After obtaining the estimation of LPM parameters, the diagnostic accuracy measures including sensitivity, specificity, and AUC for assessing the classification performance of PAPP-A in predicting GDM, were calculated. It is noteworthy that since skew-normal (SN) and ST distributions are complex, and computing the integrals of their probability density functions is often impossible, the sensitivity, specificity and AUC can be numerically approximated. Monte Carlo integration approximation was executed to calculate these diagnostic accuracy parameters. The 95% credible intervals (CrIs) were reported for the Bayesian inference. In this study, Bayesian models were fitted through a combination of R version 4.3.1 and OpenBugs software version 3.2.3 via the R package R2OpenBUGS.42 For more information about the statistical methods (including latent profile models and Bayesian inference) and simulation results, please refer to the Supplementary file.
2.3 Ethical considerations
This study was approved by the Ethics Committee of Tarbiat Modares University, Tehran, Iran, approved this study (approval numbers: IR. MODARES. REC.1398.061). Before any study-related procedures or measurements, written informed consent was obtained from all participants. Participants were informed about the purpose of the study, procedures involved, potential risks, and their right to withdraw at any time without any consequences.
3 RESULTS
3.1 Participant characteristics
A first-trimester screening for GDM was conducted among 344 pregnant women. Of these participants, 10 (2.9%) were underweighted, 151 (43.9%) had a normal-weight, and 183 (53.2%) were overweight. The mean (SD) maternal age of the women was 28.87 ± 5.20 years. Regarding the GS test outcomes, 123 women (35.8%) were diagnosed with GDM, while 221 women (64.2%) were not diagnosed during 24–28 gestational weeks. No statistically significant differences were found between women with GDM and those without GDM concerning education (p = 0.733), job (p = 0.663), BMI (p = 0.233), gravidity (p = 0.403), and history of abortion (p = 0.357). However, the mean age of pregnant women with GDM was significantly higher than that of women without GDM (29.34 ± 4.81 years vs. 28.87 ± 5.36 years, p = 0.015). Additionally, the median PAPP-A MoM was significantly lower in GDM cases compared to controls (0.85 vs. 0.99, p = 0.004) (Table 1).
| Characteristics | GDM | Total (N = 344) | P value | |
|---|---|---|---|---|
| No (n = 221) | Yes (n = 123) | |||
| Education, n (%) | 0.733a | |||
| Illiterate | 7 (3.2) | 3 (2.4) | 10 (2.9) | |
| Elementary | 24 (10.9) | 12 (9.8) | 36 (10.5) | |
| Guidance school | 14 (6.3) | 7 (5.7) | 21 (6.1) | |
| High school | 131 (59.3) | 82 (66.7) | 213 (61.9) | |
| College | 45 (20.4) | 19 (15.4) | 64 (18.6) | |
| Job, n (%) | 0.663a | |||
| Unemployed | 198 (89.6) | 112 (91.1) | 310 (90.1) | |
| Employed | 23 (10.4) | 11 (8.9) | 34 (9.9) | |
| BMI (Kg/m2), n (%) | 0.233a | |||
| Underweight | 7 (3.2) | 3 (2.4) | 10 (2.9) | |
| Normal-weight | 104 (47.1) | 47 (38.2) | 151 (43.9) | |
| Overweight | 110 (49.8) | 73 (59.3) | 183 (53.2) | |
| Gravidity, n (%) | 0.403a | |||
| 1 | 78 (35.3) | 49 (39.8) | 127 (36.9) | |
| 2 | 143 (64.7) | 74 (60.2) | 217 (63.1) | |
| History of abortion | 0.357a | |||
| No | 163 (73.8) | 85 (69.1) | 248 (72.1) | |
| Yes | 58 (26.2) | 38 (30.9) | 96 (27.9) | |
| Age (years), M ± SD | 28.87 ± 5.36 | 29.34 ± 4.81 | 28.87 ± 5.20 | 0.015b |
| PAPP-A (MoM), Median (IQR) | 0.99 (0.71–1.42) | 0.85 (0.65–1.15) | 0.91 (0.69–1.34) | 0.004c |
- Abbreviations: BMI, Body mass index; GDM, Gestational diabetes mellitus; IQR, Interquartile range; MoM, multiple of the median; PAPP-A, Pregnancy-associated plasma protein-A; SD, Standard deviation.
- a Chi-square test.
- b Independent samples t-test.
- c Mann–Whitney U test.
Remarkably, at the optimal threshold value of 2.21 MoM, PAPP-A exhibited corresponding sensitivity, specificity, positive predictive value, and negative predictive value of 64.3%, 56.8%, 67.5%, and 45.1%, respectively. Besides, PAPP-A yielded an AUC of 0.68 when considering the OGTT results. Importantly, the distribution of PAPP-A showed skewness and kurtosis values were 1.61 and 6.50, respectively. Meanwhile, statistical tests confirmed significant departure from normality: D'Agostino test (skewness: 9.12, p < 0.001) and Anscombe-Glynn test (kurtosis: 5.82, p < 0.001), alongside a non-normal distribution indicated by the KS test (p < 0.001). Histogram and normal QQ plots of PAPP-A are depicted in Figure 1. Graphically, it is clear that the skewness and long tailed behavior of the histogram make the use of the Normal distribution less optimal for the PAPP-A outcome. Additionally, the normal QQ plot indicated that the normality assumption is violated. The median (IQR) PAPP-A MoM among mothers was 0.91 (0.69–1.34). Consequently, in the absence of a GS test, the LPM with a skewed distribution remains a viable tool for assessing the diagnostic accuracy of PAPP-A.

3.2 Bayesian latent profile model under ST distribution
The Bayesian estimations of the regression coefficients for the logistic and linear regression models, along with sensitivity, specificity, and AUC, are summarized in Table 2. It can be seen that the odds of developing GDM among underweight mothers was about 0.04 lower than normal-weight mothers ( = −0.04, 95% CrI [−0.11, 0.06]). Additionally, the odds of GDM in overweight mothers was 0.16 times higher than in normal-weight mothers ( 0.15, 95% CrI [0.10, 0.22]). Moreover, age was positively and significantly associated with the risk of GDM, such that for each year of life, the chance of GDM increased by 0.08 ( 0.08, 95% CrI [0.03, 0.15]).
| Parameters | Mean | OR | Median | SD | MC error | 95% CrI |
|---|---|---|---|---|---|---|
| Logistic model | ||||||
| Intercept | −0.31 | 0.73 | −0.30 | 0.13 | 0.060 | (−0.42,0.19) |
| Age | 0.08 | 1.08 | 0.08 | 0.02 | 0.018 | (0.03,0.15) |
| Underweight | −0.04 | 0.96 | −0.05 | 0.01 | 0.014 | (−0.11,0.06) |
| Normal | Reference | |||||
| Overweight | 0.15 | 1.16 | 0.15 | 0.02 | 0.019 | (0.10,0.22) |
| Regression model with skew-t | ||||||
| Intercept | 1.23 | - | 1.22 | 0.19 | 0.010 | (1.11,1.34) |
| Age | −0.05 | - | −0.06 | 0.09 | 0.002 | (−0.09,0.13) |
| Underweight | 0.12 | - | 0.11 | 0.01 | 0.001 | (−0.08,0.19) |
| Normal-weight | Reference | |||||
| Overweight | −0.11 | - | −0.11 | 0.08 | 0.038 | (−0.19, −0.06) |
| Disease | −0.52 | - | −0.53 | 0.04 | 0.008 | (−0.61, −0.46) |
| No Disease | Reference | |||||
| Disease*Age | 0.06 | - | 0.06 | 0.01 | 0.002 | (0.02,0.13) |
| Disease*Underweight | −0.43 | - | −0.43 | 0.29 | 0.012 | (−0.49,0.06) |
| Disease*Overweight | 0.39 | - | 0.38 | 0.06 | 0.003 | (0.28,0.43) |
| 5.98 | - | 5.98 | 0.03 | 0.001 | (5.39,5.61) | |
| 8.86 | - | 8.86 | 0.03 | 0.001 | (8.28,8.95) | |
| 9.24 | - | 9.28 | 0.27 | 0.014 | (9.13,9.43) | |
| 11.22 | - | 11.23 | 0.12 | 0.012 | (10.96,11.38) | |
| Diagnostic accuracy indices | ||||||
| Sensitivity | 0.92 | - | 0.92 | 0.02 | 0.001 | (0.89,0.98) |
| Specificity | 0.81 | - | 0.79 | 0.06 | 0.09 | (0.76,0.91) |
| AUC | 0.91 | - | 0.90 | 0.09 | 0.012 | (0.83,0.97) |
- Abbreviations: AUC, area under receiver operating characteristic curve; CrI, Credible interval; MC, Monte Carlo; OR, Odds ratio; SD, Standard deviation.
According to the regression model, the PAPP-A value was higher for underweight women ( = 0.12, 95% CrI [−0.08, 0.19]) and significantly decreased by 0.05 as maternal age increased ( = −0.05, 95% CrI [−0.09, 0.13]). In contrast, the PAPP-A value was significantly lower for overweight women ( = −0.11, 95% CrI [−0.19, −0.06]). Likewise, pregnant women with GDM had significantly lower PAPP-A values ( −0.52, 95% CrI [−0.61, −0.46]). Further, our finding revealed a positive significant interaction between disease and maternal age, implying that the mean PAPP-A difference between diseased and non-diseased mothers was more pronounced for younger mothers ( 0.06, 95% CrI [0.02, 0.13]). However, there was no statistically significant interaction between disease and underweight ( −0.43, 95% CrI [−0.49, 0.06]). On the other hand, the negative significant interaction between overweight and disease indicates that the difference in PAPP-A measures between diseased and non-diseased groups was higher in overweight women compared to women with normal-weight ( 0.39, 95% CrI [0.28, 0.43]). The estimated (5.98, 95% CrI [5.39, 5.61]) and (8.86, 95% CrI [8.28, 8.95]) exhibit that the distributions of response data in diseased and non-diseased groups were right-skewed. On the other hand, the estimates of (9.24, 95% CrI [9.13, 9.43]) and (11.22, 95% CrI [10.96, 11.38]) suggest very thick tails.
Regarding the last row of the Table 2, based on an optimal cut-off value of 7 MoM, the sensitivity and specificity for early diagnosis of GDM were 92% (95% CrI [0.89, 0.97]) and 81% (95% CrI [0.72, 0.91]), respectively. Moreover, the AUC of PAPP-A was calculated as 0.91 (95% CrI [0.86, 0.98]). Ultimately, we would like to note that, according to the selected model (with ST distribution) and by estimating the latent status of the disease, out of the 344 pregnant women recruited at the time of screening, 111 (32%) were subsequently diagnosed with early GDM. In addition, about 233 (68%) of the 344 mothers did not have GDM between 11 and 13 gestational weeks. The ROC curve generated for the first trimester maternal serum PAPP-A is illustrated in Figure 2. As can be seen, PAPP-A had the best performance for predicting GDM.
4 DISCUSSION
To the best of the authors’ knowledge, this study is among the first to assess the ability of PAPP-A measured at 11–13 weeks of gestation for early prediction of GDM in the absence of GS test values, using an advanced statistical technique. The key finding from the LPM without covariate adjustment was that PAPP-A had a high AUC value of 0.85 in discriminating between women with and without GDM. Furthermore, 83% of pregnant women with GDM and 74% without GDM could be correctly diagnosed by measuring serum PAPP-A.
Several studies have investigated the predictive power of PAPP-A for GDM using ROC analysis in the presence of a GS test (i.e., OGTT). Kavak et al. found that first trimester serum PAPP-A had a low AUC of 0.46, indicating poor predictive capability.26 In another study, maternal serum PAPP-A at 15 weeks was not an acceptable predictor for GDM, with an AUC of 0.59.33 Similarly, Xiao et al. concluded that serum PAPP-A levels at 11–13 weeks had an AUC of 0.53, suggesting it was not a potential biomarker for GDM screening43 Visconti et al. assessed 2410 pregnant women and noted that serum PAPP-A was a weak predictor of GDM, with an AUC of 0.47 at 11–13 weeks of gestation.44
On the contrary, Farina et al. achieved an AUC of 0.68 for first-trimester serum PAPP-A, suggesting it could be a relatively useful tool for predicting GDM.45 The current findings, regardless of GS test values, align with Syngelaki et al., who confirmed that the MoM values of PAPP-A at 11–13 weeks could serve as an important antenatal screening biomarker for GDM, with an AUC of 0.86.46 However, it should be noted that unlike previous studies, this study considered the real data structure when estimating the accuracy measures of PAPP-A in GDM detection. Accounting for the true distribution shape can yield robust and reliable parameter estimates. Besides, all previous studies have estimated the power of PAPP-A to correctly identify healthy and diseased populations regarding OGTT outcomes between 24 and 28 weeks of gestation. It is noteworthy that their results might be affected by potential measurement errors in the GS, making the reported predictive values more questionable. Thus, calculating diagnostic accuracy parameters in the absence of a GS test might provide more reliable results.
Based on the results from LPM under a ST distribution, it was found a significant association between maternal age in early pregnancy and the risk of GDM. This aligns with established literature indicating that advanced maternal age is a well-established risk factor for GDM.47, 48 Studies by McFarland et al. and Abu-Heija et al. have underscored the increasing incidence of GDM and its adverse fetal outcomes with increasing maternal age.49, 50 Our findings are consistent with the existing body of evidence suggesting that older maternal age predisposes women to a higher risk of developing GDM. It is worth mentioning that advancing age can lead to insulin resistance, impaired lipid metabolism, and compromised glucose tolerance.
As expected, our study revealed that pregnant women with overweight status had a significantly elevated risk of GDM compared to those with normal-weight. Either overweight or obesity, combined with a sedentary lifestyle, led to obesity, affecting glucose metabolism in a cyclical manner. Obviously, this observation is supported by previous studies’ report.1, 51, 52 Namely, Cypryk et al. conducted a case-control study in pregnant women and proved the overweight increased the risk of GDM (odds ratio = 2.38).53 Similar to our study, Yen et al. identified significantly higher odds of GDM among overweight pregnant women compared to those with normal-weight.54 Originally, overweight during pregnancy emerges as a critical risk factor for GDM, particularly when accompanied by mild abnormalities in glucose metabolism.1, 55 Conversely, our study indicates that underweight women may have lower odds of developing GDM, consistent with findings from Sebire et al., who conducted a retrospective study involving 215,105 pregnant women in London.56
According to the findings of the regression model, a significant negative relationship between PAPP-A and GDM was observed, suggesting that PAPP-A may interfere with glucose homeostasis. Lower first trimester PAPPA-A levels in women destined to develop GDM could reflect an initial stage of glucose intolerance.30 This relationship between GDM and macrosomia underscores the role of PAPP-A within the IGF control system in trophoblasts as an IGF-binding protein (IGFBP-4). Decreased PAPP-A leads to reduced IGF and an increase in glucose and amino acids produced by trophoblasts.57 Reduced IGF levels lead to increased insulin, glucose clearance, and insulin resistance, potentially elucidating the relationship between abnormal PAPP-A and GDM. Recent studies have consistently shown that low PAPP-A levels during 10–14 gestational weeks are associated with GDM. For example, Lovati et al. reported a strong association between low maternal serum PAPP-A and subsequent GDM development in a study involving 307 diabetic and 366 nondiabetic pregnant women.30 Caliskan et al. similarly determined significantly lower serum PAPP-A concentrations in patients with GDM.34 Wells et al. further demonstrated that the association between low PAPP-A and early-onset GDM was stronger than with late-onset GDM.58 Meanwhile, some other studies have shown an association between PAPP-A levels and the need for insulin therapy.59-61
Based on the preliminary findings of the current study, measuring PAPP-A levels in the first trimester could help assess the risk of GDM. In addition, a significant negative association between PAPP-A and overweight status was found in this work. However, it failed to determine a significant difference in serum levels of PAPP-A between underweight and normal-weight groups. It should be emphasized that no papers on the relationship between PAPP-A and BMI groups have been published. Nevertheless, only three studies conducted by Varashree et al.,62 Gürbüz et al.,63 and Steinbrecher et al.64 have revealed a significant negative correlation between BMI and PAPP-A levels. Prospective studies would be warranted to confirm these observations. Another important consideration is that in this model, covariate adjustment resulted in slightly higher values of sensitivity, specificity, and AUC compared to the LPM without covariate adjustment. This suggests that the inclusion of covariates provided additional information that improved classification accuracy. Nonetheless, both models demonstrated sufficient predictive accuracy for PAPP-A.
This article introduces a new LPM under the ST distribution, which accommodates skewness and heavy tails. The methodology was validated and illustrated through simulations demonstrating the superior performance of the ST LPM compared to the Normal and SN models. It was also shown that the MSE of estimators decreases with increasing sample size. The ST distribution relaxes the symmetry assumption of the density function. In the data set related to GDM, which exhibited strong asymmetric behavior, the use of the ST distribution is appealing as it provides more appropriate density estimation compared to SN and Normal distributions based on the deviance information criterion (DIC). Prior studies have extensively developed LPMs under the normal distribution for modeling continuous test outcomes without a GS test.65-68 However, a limitation of these studies is their reliance on normality, which may overly restrict accurate representation of the data structure and lead to biased parameter estimates and accuracy measures. To address this limitation, given the skewed and heavy-tailed structure of our data, there is motivation to employ the ST distribution. Specifically, using the ST distribution for component density in the LPM enables flexible modeling of both skewness and long tails.
The present study has several significant strengths. A key advantage is the opportunity to assess the accuracy of PAPP-A in early detection of GDM without relying on OGTT outcomes. Previous research predominantly focused on evaluating PAPP-A in women diagnosed with GDM by the end of the second trimester, relying on OGTT results. Notably, existing literature did not explore PAPP-A's potential for early identification of GDM before OGTT administration. Early identification allows for timely interventions, potentially mitigating adverse maternal and fetal outcomes. Secondly, the study benefits from a large participant cohort. Thirdly, this study introduced an alternative LPM approach under the true distribution of PAPP-A to estimate diagnostic accuracy parameters (i.e., ST distribution). This approach enhances parameter estimation and offers flexibility by accommodating skewness and Bayesian approach was employed for parameter estimation, offering advantages over frequentist approaches, particularly in small sample sizes. We need to highlight that, to our knowledge; the current study represents the first reported use of Bayesian approach to evaluate PAPP-A's diagnostic performance in early pregnancy, acknowledging the absence of a perfect reference test. To this end, unlike recent studies that conducted ROC analysis without covariate adjustment, our research considers the influence of covariates on PAPP-A's discriminatory ability, enhancing its clinical relevance.
There are some limitations to this study. Firstly, defining disease explicitly in LPM models poses challenges. Secondly, generalizability may be limited as the study was restricted to Iranian pregnant women. Thirdly, the cross-sectional design precludes causal inferences. Missing information in patients’ records, such as blood pressure, pre-eclampsia history, familial diabetes, preterm delivery history, gestational weight gain, and pre-eclampsia development constitute another limitation. A notable limitation of our research is the lack of data on the severity levels of diabetes. Consequently, we were unable to investigate whether PAPP-A has predictive power for GDM regardless of the severity of diabetes. Such an analysis would provide deeper insights into the utility of PAPP-A in the management of GDM and potentially improve its clinical application. Lastly, our study did not incorporate results from the GS test, suggesting a direction for future research considering the true structure of data.
5 CONCLUSION
In this research, we proposed a novel Bayesian latent profile model under the ST distribution for early prediction and classification of GDM, with and without covariate adjustment. Based on results from our simulation study, we concluded that the presented model exhibited satisfactory performance. Furthermore, findings from the model applied to actual data confirmed that maternal serum PAPP-A levels effectively predict GDM during the early first trimester of pregnancy in the absence of GS test values. Consequently, we recommend the use of PAPP-A to identify high-risk pregnant women who can benefit from early intervention programs aimed at preventing and timely recognition of GDM.
AUTHOR CONTRIBUTIONS
Maedeh Amini: Conceptualization; Methodology; Software; Data curation; Supervision; Resources; Formal analysis; Project administration; Validation; Visualization; Writing—review and editing; Investigation; Writing—original draft. Anoshirvan Kazemnejad: Supervision. Aliakbar Rasekhi: Software; Resources. Azam Amirian: Resources. Nourossadat Kariman: Resources.
ACKNOWLEDGEMENTS
This paper is derived from a Ph.D. dissertation in Biostatistics submitted by the first author. Special thanks are extended to the respected editor and reviewers for providing us with their valuable and constructive comments. There was no source of funding for this research. No organization or institution provided a grant or financial support. Consequently, no funding sources had any role in the study design, data collection, analysis, interpretation, report writing, or the decision to submit the report for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Tarbiat Modares University, Tehran, Iran, approved this study (approval numbers: IR. MODARES. REC.1398.061). Before any study-related procedures or measurements, written informed consent was obtained from all participants. Participants were informed about the purpose of the study, procedures involved, potential risks, and their right to withdraw at any time without any consequences. All authors have read and approved the final version of the manuscript.
TRANSPARENCY STATEMENT
The lead author Maedeh Amini affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author [A.K.]. The data are not publicly available due to the data containing information that could compromise the privacy of research participants. Maedeh Amini had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.