Relationship between advanced glycation end-products and advanced oxidation protein products in patients with type 2 diabetes with and without albuminuria: A cross-sectional survey
Abstract
Background and Aims
Literature suggests that oxidative stress plays a crucial role in the progression of diabetes. Since poor glycemic control enhances the formation of advanced glycation end-products (AGEs) and advanced oxidation protein products (AOPP) in individuals with diabetes, exploring the association between glycation and oxidative states in diabetes could also shed light on potential consequences. This study evaluated the effects of albuminuria on AGEs and AOPP levels and measured their relationship in participants with type 2 diabetes (T2D) with or without albuminuria.
Methods
A cross-sectional, matched case-control study was designed, including 38 T2D subjects with albuminuria and 38 matched T2D subjects with normoalbuminuria. Patients were matched by their body mass index (BMI), age, and duration of diabetes. The unadjusted and adjusted correlation between AGEs and AOPP in the studied groups were analyzed by multiple logistic regression. Using ggplot2, the ties between these two biochemical factors in cases and controls were plotted.
Results
This study elucidated a significant association between AGEs and AOPP in participants with normoalbuminuria (r = 0.331, p-value < 0.05), which continued to be significant after controlling for BMI, age, systolic blood pressure (SBP), and diastolic blood pressure (DBP) (r = 0.355, p-value < 0.05). However, there was no significant association between AGEs and AOPP in those with albuminuria in the unadjusted model (r = 0.034, p-value = 0.841) or after controlling for BMI, age, SBP, and DBP (r = 0.076, p-value = 0.685).
Conclusion
Oxidation and glycation molecular biomarkers were correlated in patients without albuminuria; however, this association was not observed in those with albuminuria.
1 INTRODUCTION
Diabetic nephropathy is a well-known microvascular diabetes complication.1 Accumulation of glycoxidation products, such as Nᵋ-carboxymethyl-lysine and pentosidine, is considered a pathogenic feature of diabetes.2, 3 Glycation and oxidation products can lead to endothelial and vascular impairments by triggering NF-κB and adapter protein complex-1 and nitric oxide resistance, respectively.4-7 Advanced thickening of the glomerular basement membrane and a consequent reduction in glomerular filtration function could result in diabetic nephropathy.8 Hyperglycemia, dyslipidemia, and glycation are the underlying mechanisms of susceptibility to diabetes-associated complications.2, 9-11 Earlier studies proposed that increased glycoxidation and insufficient counter-regulation of the antioxidant system are responsible for the formation of albuminuria in individuals with type 2 diabetes (T2D).12 Glycoxidation is a complex group of biochemical reactions and molecular interactions that results in structural damage to proteins, lipids, DNA, cell membranes, and endothelium.13-15 Oxidative and glycemic reactions increased in albuminuria status.16, 17 Prior literature suggested that advanced glycation end-products (AGEs) and advanced oxidation protein products (AOPP), as the parameters symbolizing glycemic and oxidative patterns in T2D, were correlated with urinary albumin-to-creatinine ratio and plasma creatinine levels, respectively.14
AGE formation is enhanced in an oxidative milieu.18 Metabolic and oxidative pathways produce reactive carbonyls in diabetes, which contribute to the AGE development.19 The formation of AGEs in hyperglycemic states is secondary to a covalent nonenzymatic biological reaction between reducing sugars and proteins or lipids.19, 20 AGEs interfere with the metabolisms of macromolecules through enzymatic dysfunction and cause subsequent diabetic vascular complications.12, 15, 21 AGEs can induce end-stage renal disease (ESRD) and renal damage in diabetes.22 As a result, AGEs-blockers have been introduced as a therapeutic option for ESRD.23 AGEs participate directly in the development of micro and macrovascular complications of diabetes.24 Studies have shown that methylglyoxal-derived substances could also have associations with diabetic nephropathy development.25, 26 AGEs and AOPP are different in several crucial aspects. AGEs are markers for oxidative and carbonyl stress processes, AOPP demonstrates a closer connection to inflammation and acute phase reaction compared to AGEs. AOPPs may serve as a superior acute marker, while AGEs could be considered as a marker of chronic injury.14, 27
Several lines of evidence suggest that AOPP produced by oxidative stress and inflammation may also participate in diabetic macrovascular complications.28, 29 AOPP is considered the remaining molecules from the interaction of reactive oxygen species (ROS) and chloramines with albumin and fibrinogen.16, 29 They comprise abundant dityrosines, which enable crosslinking, disulfide bridges, and carbonyl groups and are constructed particularly by chlorinated oxidants—hypochloric acid and chloramines produced from myeloperoxidase activity.30 ROS generation could result in inflammatory pathways activation and exacerbate diabetes progression.31 As a result, these products could be possible risk factors for diabetic vascular complications, especially diabetic nephropathy.12, 17, 32
The physiologic relationship between molecules could be disturbed in disease conditions. There have been earlier studies showing that in diabetic status, as an inflammatory state, the correlation between CRP and leptin disappears, whereas leptin and heat shock protein 70 (HSP 70) develop a pathological correlation. Additionally, HSP 70 forms an unfavorable association with asymmetric dimethylarginine (ADMA), but loses its ties with plasminogen activator inhibitor-1 (PAI-1).33-36 Evidence implies that oxidative stress plays a crucial role in diabetes development.37 Moreover, as poor glycemic control in patients with diabetes could enhance AGEs and AOPP formation, studying the relationship between glycation and oxidative states in diabetes could also provide further insight into any potential consequences.2, 20, 38 The current survey aimed to investigate AGEs and AOPP and their associations in patients with diabetes with or without albuminuria.
2 METHODS AND MATERIALS
2.1 Study design and population
This matched cross-sectional, case–control survey comprised 76 participants, including 38 individuals with T2D and albuminuria, as well as 38 control subjects with T2D and normoalbuminuria. There were close matches between cases and controls regarding sex, age, diabetes duration, as well as BMI for both groups. The subjects with diabetes were enrolled from a tertiary diabetic center affiliated with Tehran University of Medical Sciences.
Established albuminuria throughout multiple 24-h urine specimens was considered for case group selection. Individuals with established albuminuria who were confirmed on two consecutive measurements were selected for the study. Participants were not included in the case of (1) having any chronic disease except diabetes; (2) taking any vitamin supplements, oral contraceptive pill (OCP), or aspirin over the past year; (3) consuming alcoholic beverages in the month before the recruitment, and (4) being current smokers or having a history of smoking. In addition, exclusion criteria for patients with T2D were as follows; glomerulonephritis, dialysis, pregnancy, hormone replacement therapy, non-ketonic hyperosmolar diabetes, and diabetic ketoacidosis.
Before enrollment, all participants signed a written informed consent form. This study complied with the principles of the declaration of Helsinki. Protocol approval was obtained from Tehran University of Medical Sciences’ local ethics review committee.
2.2 Definition
A diagnosis of diabetes was made in accordance with the criteria established by the American Diabetes Association (ADA).39 A cutoff value of 30 mg/24-h albumin excretion was also taken from the ADA criteria for diagnosing albuminuria (defined as 30–299 mg/24-h excretion of albumin) and normoalbuminuria (defined as less than 30 mg/24-h excretion of albumin).40 The Quetelet formula was used to determine the body mass index (BMI; kg per square m).
2.3 Data collection
Demographic and anthropometric data, including age, sex, height, weight, and duration of diabetes, as well as patient's drug history, were recorded. A sitting position was chosen to obtain the blood pressure, which was checked twice after approximately 5 min. Following an overnight fast of 10 to 12 h, blood samples were drawn. Glucose assessments were conducted via the glucose oxidase method. HbA1c was quantified by high-pressure liquid chromatography (A1C, DS5; DREW). Total cholesterol, LDL-C, and triglyceride levels were assayed employing direct enzymatic methods (Parsazmoon, Karaj, Iran). HDL-C was also evaluated using a direct enzymatic method (Pishtazteb, Tehran, Iran).
The spectral features of AOPP are secondary to several chromophores, including pentosidine, dityrosine, and carbonyls.41 AOPP amounts were calculated with spectrophotometric methods (FLUOstar OPTIMA, BMG, Germany) in accordance with Kalousova et al.42 As part of this process, 200 μL of serum sample diluted with phosphate-buffered saline (PBS) at a ratio of one to five, 200 μL of chloramine T (0–100 μmol/L) as a calibration solution, and 200 μL of PBS as a blank were poured into each of the corresponding wells in the plate. Then, 10 μL of 1.16 M potassium iodide (KI) and 20 μL of acetic acid were added to each of the specified wells. Analyses were performed at an absorbing wavelength of 340 nm and reported in chloramine µmol/L units (intra-assay coefficient of variance [CV] = less than 5%).
The prominent feature of AGEs is the formation of cross-links in proteins, browning, and fluorescence emission.43 The process of AGE formation leads to the chemical and physical alteration of proteins. The changes are as follows: Changing the electrical charge status of the protein: by masking the positive charge of the amine group in the side chain of lysine, the overall charge of the protein becomes more negative. Consequently, glycated proteins can be separated by polyacrylamide gel electrophoresis using an electrical charge. In this method, proteins with higher levels of glycation and a more negative charge exhibit greater electrophoretic movement. With an increase in negative charge, the acidity of glycated protein increases, and its isoelectric pH decreases. Protein discoloration: Incubating a mixture of protein and sugar through nonenzymatic glycation results in the development of a distinctive yellow-brown color. AGE products with glycated protein cross-links become fluorescent and emit radiation at 440 nm when stimulated at 370 nm.44 AGEs were assessed by the spectrofluorimetric method of Kalousova et al.42 The serums of the patients were diluted in PBS by a factor of 50. Fluorescence intensity was documented at the emission maximum of 440 nm upon excitation at 350 nm and revealed in terms of fluorescent emission percentage (intra-assay CV = 5.1%).
Creatinine was quantified according to the calibrated Jaffe method (Parsazmoon, Karaj, Iran). Urea was evaluated via a colorimetric assay (Parsazmoon, Karaj, Iran). Analyses of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were measured following enzymatic photometry. An assessment of urinary protein excretion was administered to participants over a timed period of 24 h. Urine protein was assessed via immunoturbidimetry and according to the modification of diet in renal disease formula, the glomerular filtration rate (GFR) was assumed.
2.4 Statistical analysis
Means and standard errors of the means (SEM) were assessed for continuous variables. Numbers and percentages were used to represent categorical variables. The Chi-square test and independent sample t-test were employed for between-group comparisons as indicated. Multiple logistic regression analysis was utilized to assess the unadjusted and adjusted correlation between AGEs and AOPP in patients with normoalbuminuria and patients with albuminuria. The adjustment for confounding was applied for age (model 1), age and BMI (model 2), systolic blood pressure (SBP), and diastolic blood pressure (DBP) (model 3), and age, BMI, SBP, and DBP (model 4). A two-sided p-value < 0.05 was considered statistically significant. A statistical package for social science program (SPSS for Windows, version 19; Chicago, IL) was used for the analysis. R software (version 4.2.3, R Foundation for Statistical Computing, Vienna, Austria) was used to plot the figures showing the correlation between AGEs and AOPP in cases and controls.
3 RESULTS
3.1 Baseline characteristics
A total of 76 individuals with T2D enrolled in the current study. Of these participants, 38 were diagnosed with albuminuria (case), and the rest had normoalbuminuria. Women comprised 17 subjects in each group. Urine albumin excretion rate (mg/24 h) was 192.97 ± 21.00 in the case and 7.48 ± 1.11 in the control group (p-value = <0.001). The average age and the mean duration of diabetes were 58.02 ± 1.64 and 11.14 ± 1.53 years in the case group as well as 57.78 ± 1.51 and 9.18 ± 1.13 years in the control group, respectively. BMI (kg/m2) had a mean (± SE) value of 27.06 ± 0.74 in the case group and 26.30 ± 0.73 in the control group. Comparing cases and controls on matching variables (age, gender, diabetes duration, and BMI) did not reveal any significant differences. The mean (± SE) value of SBP and DBP (mmHg) in the case group was 124.08 ± 3.32 and 72.61 ± 1.78, whereas in the control group was 123.13 ± 3.84 and 72.84 ± 1.78. All participants had normal levels of liver biomarkers (AST, ALT, and ALP). Lipid profiles in each group were as follows: Total cholesterol (mg/dL) (181.90 ± 8.53 in case and 184.13 ± 8.85 in control), LDL-C (mg/dL) (105.36 ± 6.81 in case and 105.74 ± 6.79 in control), HDL-C(mg/dL) (46.43 ± 2.14 in case and 45.11 ± 2.54 in control), and triglyceride (mg/dL) (175.20 ± 16.81 in case and 163.42 ± 17.89 in control). A total of 66% and 61% were using lipid-lowering drugs in the case and control groups, respectively. Compared with patients with normoalbuminuria, patients with albuminuria were more likely to use oral antihypertensive drugs (61% vs. 29%, p-value < 0.01). eGFR (mL/min/1.73 m2) has been shown to have a mean (± SE) value of 66.24 ± 3.35 in the case and 77.67 ± 6.37 in the control group, respectively. Individuals in the case group were taking antidiabetic drugs, including OAD (74%) and insulin (31%). However, about 87% and 21% of adults in the control group were receiving OAD and insulin, respectively. In spite of being on antidiabetic drugs, the mean (± SE) levels of HbA1C (%) were 8.93 ± 0.45 in the case and 8.61 ± 0.39 in the control group. AGE (%) levels were about 61.05 ± 0.41 and 61.12 ± 0.38 in the case and control groups, respectively. The mean (± SE) value of AOPP (μmol/L) was 117.05 ± 2.37 in the case and 116.86 ± 2.44 in control participants. There was no significant difference between the levels of AGEs and AOPP in the group with normoalbuminuria and the group with albuminuria. All details of baseline characteristics of the study population are presented in Table 1.
| Patients with type 2 diabetes and albuminuria (cases) (n = 38) | Patients with type 2 diabetes without albuminuria (controls) (n = 38) | p-value | ||
|---|---|---|---|---|
| Female/male (n) | 17/21 | 17/21 | 1.000 | |
| Age (years) | 58.02 ± 1.64 | 57.78 ± 1.51 | 0.914 | |
| BMI (kg/m2) | 27.06 ± 0.74 | 26.30 ± 0.73 | 0.472 | |
| Urine albumin excretion rate (mg/24 h) | 192.97 ± 21.00 | 7.48 ± 1.11 | <0.001 | |
| Duration of diabetes (years) | 11.14 ± 1.53 | 9.18 ± 1.13 | 0.304 | |
| SBP (mmHg) | 124.08 ± 3.32 | 123.13 ± 3.84 | 0.853 | |
| DBP (mmHg) | 72.61 ± 1.78 | 72.84 ± 1.78 | 0.927 | |
| FBS (mg/dL) | 204.59 ± 14.14 | 196.58 ± 15.07 | 0.699 | |
| HbA1c (%) | 8.93 ± 0.45 | 8.61 ± 0.39 | 0.590 | |
| Creatinine (mg/dL) | 1.16 ± 0.05 | 1.07 ± 0.09 | 0.355 | |
| Urea (mg/dL) | 36.81 ± 2.72 | 33.36 ± 2.99 | 0.398 | |
| Total Cholesterol (mg/dL) | 181.90 ± 8.53 | 184.13 ± 8.85 | 0.145 | |
| HDL-C(mg/dL) | 46.43 ± 2.14 | 45.11 ± 2.54 | 0.692 | |
| LDL-C (mg/dL) | 105.36 ± 6.81 | 105.74 ± 6.79 | 0.969 | |
| Triglycerides (mg/dL) | 175.20 ± 16.81 | 163.42 ± 17.89 | 0.635 | |
| AST (U/L) | 21.39 ± 2.12 | 21.10 ± 1.60 | 0.915 | |
| ALT (U/L) | 26.17 ± 3.39 | 23.85 ± 2.52 | 0.595 | |
| ALP (U/L) | 314.80 ± 113.44 | 157.33 ± 27.87 | 0.342 | |
| GFR (mL/min/1.73 m2) | 66.24 ± 3.35 | 77.67 ± 6.37 | 0.099 | |
| AGE (%) | 61.05 ± 0.41 | 61.12 ± 0.38 | 0.905 | |
| AOPP (μmol/L) | 117.05 ± 2.37 | 116.86 ± 2.44 | 0.956 | |
| Antidiabetic drugs | OAD n (%) | 28 (74) | 33 (87) | 0.059 |
| Insulin n (%) | 12 (31) | 8 (21) | ||
| Antihypertensive drugs n (%) | 23 (61) | 11 (29) | 0.031 | |
| Lipid-lowering drugs n (%) | 25 (66) | 23 (61) | 0.453 | |
- Note: Data is presented as Mean ± Standard Error for continuous variables or frequency and percent for categorical variables. P-value < 0.05 is considered significant. Chi-square test and independent sample t-test were employed for between-group comparisons.
- Abbreviations: AGE, advanced glycation end products; ALP, alkaline phosphatase; ALT, alanine transaminase; AOPP, advanced oxidation protein products; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FBS, fasting blood sugar; GFR, glomerular filtration rate; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure.
3.2 Correlation between AGEs and AOPP in patients with albuminuria and patients with normoalbuminuria
Unadjusted analysis revealed a significant association between AGEs and AOPP in participants with normoalbuminuria (controls) (r = 0.331, p-value = 0.042), while a nonsignificant correlation was presented in those with albuminuria (cases) (r = 0.034, p-value = 0.841) (Table 2) (Figure 1).
| Patients with type 2 diabetes with albuminuria (cases) | Patients with type 2 diabetes without albuminuria (controls) | |||
|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | |
| Unadjusted analysis | 0.034 | 0.841 | 0.331 | 0.042 |
| Adjusted analysis | ||||
| Modela 1 | 0.052 | 0.761 | 0.368 | 0.027 |
| Model 2 | 0.080 | 0.653 | 0.340 | 0.046 |
| Model 3 | 0.017 | 0.923 | 0.367 | 0.028 |
| Model 4 | 0.076 | 0.685 | 0.355 | 0.043 |
- Note: Multiple logistic regression analysis was utilized to assess the unadjusted and adjusted correlation between AGEs and AOPP in patients with type 2 diabetes and albuminuria and those without albuminuria. p-value < 0.05 is considered significant.
- Abbreviations: DBP, diastolic blood pressure; BMI, body mass index; SBP, systolic blood pressure.
- a Analyses were adjusted for the following models:
- Model 1 was adjusted for age.
- Model 2 was adjusted for age and BMI.
- Model 3 was adjusted for SBP and DBP.
- Model 4 was adjusted for age, BMI, SBP, and DBP.
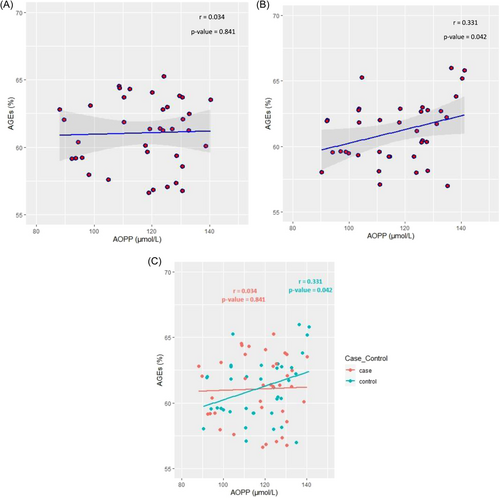
The adjusted correlation between these two variables was also assessed using multiple logistic regression analysis. This correlation continued to be non-significant among cases with diabetes and albuminuria after adjustment for age, BMI, SBP, and DBP.
Meanwhile, the correlation between AGEs and AOPP in controls with diabetes without albuminuria remained significant in all four models of adjustments. The correlation was changed to 0.368 (p-value = 0.027) in model 1, 0.340 (p-value = 0.046) in model 2, 0.367 (p-value = 0.028) in model 3, and 0.355 (p-value = 0.043) in model 4.
4 DISCUSSION
This matched case-control study showed that AGEs were positively correlated with AOPP in participants with T2D and normoalbuminuria. This association remained significant after adjustment for multiple comparisons, including age, BMI, SBP, and DBP. However, the analysis showed that the correlation between AGEs and AOP in the albuminuria group was insignificant. In addition, there was no difference in serum levels of AGEs and AOPP in patients with concurrent T2D and albuminuria compared with those with normoalbuminuria.
Glycation is a common pathway, which can occur in the protein translation process through enzymatic or nonenzymatic reactions. In enzymatic reactions, the intracellular sugar molecules could be added to the proteins via glycosyltransferases.45 However, in nonenzymatic reactions, a spontaneous reaction could happen mostly on Lysine amino acid. The initial reversible condensation between the carbonyl group and the amine group produces the Schiff base. This unstable molecule then rearranges to a stable ketoamide, called Amadori product. Then these glycated proteins change to the intermediate-glycosylation-products like 3-deoxyglucosone and finally form AGEs.46 Elevated serum glucose concentrations could increase the likelihood of this nonenzymatic reaction, and consequently, glycated product formation. The extent of Amadori product generation and the duration required to achieve equilibrium are influenced by the duration of interaction between glucose and protein. If the interaction time surpasses the equilibrium attainment period for Amadori products, the accumulation of AGE products on proteins is observed, particularly on those with a slower renewal rate.47 Therefore, proteins such as albumin and collagens could undergo architectural changes in high-glucose states in long-standing diabetes. Protein glycations could cause harmful alterations. Advanced glycation plays a crucial role in the onset and progression of various diabetic complications, such as nephropathy, retinopathy, and neuropathy.48 A considerable link between dicarbonyl glycation and diabetic nephropathy progression was previously established.49 Prior studies have shown associations between glycated proteins and diabetes.50-52 Kalosava et al. also reported that both AGEs and AOPP were higher in those with T2D compared to the healthy population. Moreover, a tight correlation between AGEs and AOPP (r = 0.47 in T2D and r = 0.75 in T1D) was manifested in those with diabetes.42 This was in line with the current study findings. Patients with T2D and normoalbuminuria had a significant positive association between the AGEs and AOPP levels in this survey. However, the above-mentioned relationship was not assessed in patients with diabetes and albuminuria. The chaotic milieu of inflammation and oxidative stress in diabetes may describe these findings. Nonlinear behavior of cytokine network and inflammatory response has been reported.53, 54 The chaotic theory is a mathematical theory that describes systems that have stable and unstable regions. In a chaotic region, a tiny change in one of the parameters may result in an unpredicted transition at any moment.55 Here, chaotic behavior in markers of oxidative stress in T2D was detected. A significant correlation between oxidative markers, including AGEs and AOPP, in patients with diabetes and normoalbuminuria, was assessed; however, this association was not observed in those with albuminuria. So far, the appearance and loss of correlation between biomarkers have been described in health and disease conditions. Prior studies documented the inexistence of a positive relationship between C-reactive protein (CRP) and leptin in rheumatoid arthritis as an inflammatory state and the lost correlation between leptin and CRP and also between HSP70 and PAI-1 in T2D. Others demonstrated an emerging pathologic correlation between HSP70 and asymmetric dimethylarginine (ADMA) and also between HSP70 and leptin in T2D.33-36, 56
The present work revealed that the serum concentrations of AGEs and AOPP did not show a significant difference in patients with normoalbuminuria compared with patients with albuminuria. In line with these results, Kalousova et al. demonstrated no significant differences in AGEs and AOPP levels in T2D cases without microvascular complications and those with associated complications.42 JAKUŠ et al. revealed that AOPP levels did not show a difference between patients with diabetic complications compared to those without complications. However, AGE levels were raised in the group with diabetes compared to controls and had higher values in patients with diabetic complications than those without such problems. The studied complications implied both microvascular and macrovascular ones.57 However, the other literature was controversial. AOPP levels were higher in patients with macrovascular than microvascular and mixed complications as shown by Piwowar et al.58 Pan et al. also described that higher levels of AOPP were detected in T2D compared to healthy controls and in patients with diabetic nephropathy in comparison with subjects without such ailments.59 AOPP was evaluated to be greater in patients with concurrent T2D and micro or macrovascular complications compared to those without according to Tabak et al. study.60 Chawla et al. also described higher serum concentrations of AOPP and AGEs in subjects with concurrent diabetes and microvascular or macrovascular complications.61 In a population with diabetic retinopathy also higher levels of AGEs and AOPP were detected based on the Rad et al. study.62 These results could be due to the longer diabetes duration in subjects with complications.59 Chronic kidney disease and diabetes could accelerate the mechanisms that lead to the formation of oxidized proteins, such as AOPP.63 Accordingly, AOPP is considered a reliable marker of oxidative stress and is associated with the progression of diabetic complications, especially nephropathy.18 Earlier, a documented link between AGEs and AOPP and diabetes duration has been delineated.64 However, the effect of the duration of diabetes was eliminated in the current analysis due to the close-matched duration of diabetes between cases and controls. Another explanation could be attributed to the filtration rates of these particles. As AOPP values are strongly correlated with creatinine clearance, they could be used as a valuable marker for the progression of kidney failure.41
The limitation of the current observation was its relatively small sample size. As the excitation wavelength for AGE fluorescence (350 nm) is close to the AOPP absorption wavelength (340 nm) and UV absorption measurement lacks specificity, other molecular entities with any conjugated molecular structure, such as those found in AGEs, could potentially interfere with AOPP measurements. Also, further research is recommended to assess this association considering other factors such as Nᵋ-carboxymethyl-lysine.
The novelty of the present study was evaluating the correlation between AGEs and AOPP in T2D subjects with and without albuminuria and explaining the missing correlation of these two biomarkers in the presence of nephropathy. This was the first time that the interrelationship of AGEs and AOPP was investigated in a matched case-control study with no difference in baseline characteristics between cases and controls except albuminuria.
5 CONCLUSION
Oxidative stress could contribute to diabetes development. The physiologic association between oxidative markers could be disturbed in disease conditions. AGEs and AOPP had a positive association in patients with concurrent T2D and normoalbuminuria. However, the mentioned association was not detected in patients with albuminuria.
AUTHOR CONTRIBUTIONS
Mehrdad Larry: Writing—original draft; Resources. Soghra Rabizadeh: Writing—review and editing; Validation; Supervision. Fatemeh Mohammadi: Formal analysis; Data curation; Writing—review and editing. Amirhossein Yadegar: Data curation; Writing—original draft; Formal analysis. Azadeh Jalalpour: Writing—original draft. Hossein Mirmiranpour: Investigation. Ghasem Farahmand: Resources. Alireza Esteghamati: Supervision; Methodology; Writing—review and editing. Manouchehr Nakhjavani: Project administration; Conceptualization; Supervision; Methodology; Writing—review and editing.
ACKNOWLEDGMENTS
The authors wish to thank the patients for their participation and kind cooperation.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHIC STATEMENT
The medical ethics council of Tehran University of medical sciences approved the study protocol and written informed consent was obtained from all participants. All authors have read and approved the final version of the manuscript. The corresponding author Manouchehr Nakhjavani had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
CONSENT FOR PUBLICATION
All authors and participants have consent for publication of this study.
TRANSPARENCY STATEMENT
The lead author Manouchehr Nakhjavani affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.