Cholera rages in Africa and the Middle East: A narrative review on challenges and solutions
Abstract
Background and Aim
Cholera is a life-threatening infectious disease that is still one of the most common acute watery diarrheal diseases in the world today. Acute diarrhea and severe dehydration brought on by cholera can cause hypovolemic shock, which can be fatal in minutes. Without competent clinical therapy, the rate of case fatality surpasses 50%. The purpose of this review was to highlight cholera challenges in Africa and the Middle East and explain the reasons for why this region is currently a fertile environment for cholera. We investigated cholera serology, epidemiology, and the geographical distribution of cholera in Africa and the Middle East in 2022 and 2023. We reviewed detection methods, such as rapid diagnostic tests (RDTs), and treatments, such as antibiotics and phage therapy. Finally, this review explored oral cholera vaccines (OCVs), and the vaccine shortage crisis.
Methods
We carried out a systematic search in multiple databases, including PubMed, Web of Science, Google Scholar, Scopus, MEDLINE, and Embase, for studies on cholera using the following keywords: ((Cholera) OR (Vibrio cholera) and (Coronavirus) OR (COVID-19) OR (SARS-CoV2) OR (The Middle East) OR (Africa)).
Results and Conclusions
Cholera outbreaks have increased dramatically, mainly in Africa and many Middle Eastern countries. The COVID-19 pandemic has reduced the attention devoted to cholera and disrupted diagnosis and treatment services, as well as vaccination initiatives. Most of the cholera cases in Africa and the Middle East were reported in Malawi and Syria, respectively, in 2022. RDTs are effective in the early detection of cholera epidemics, especially with limited advanced resources, which is the case in much of Africa. By offering both direct and indirect protection, expanding the use of OCV will significantly reduce the burden of current cholera outbreaks in Africa and the Middle East.
1 INTRODUCTION
The comma-shaped bacteria Vibrio cholerae is the cause of cholera, an acute diarrheal disease.1 V. cholerae is a water-borne pathogen and can be transferred through vector and reservoir organisms such as flies, algae, and some crustaceans.2, 3 In coastal regions and estuaries, V. cholerae grows best in salt water.4-6 Fecal samples of human cholera carriers can also carry high concentrations of V. cholerae.7 Through such mechanisms, V. cholerae can contaminate water sources and food. Once ingested through the fecal–oral route, the disease can lead to symptoms such as diarrhea and dehydration.8 In 1817, cholera appeared in the Ganges Delta and expanded to seven pandemics that killed millions of people across the world.4-6 V. cholerae is constantly evolving, with novel phenotypes and genotypes arising as a consequence of outbreaks and growing drug resistance.9 In Africa, cholera occurs in two different ways: as an endemic condition that recurs every 2–3 years during specific seasons, or as acute epidemics that start suddenly, endanger thousands of lives, and strain the healthcare infrastructure.10 Cholera outbreaks are difficult to predict and therefore can occur in both endemic and nonendemic areas based on environmental changes, with refugee camps being especially vulnerable.11
Globalization and travel increase the spread of cholera, as was the case in Haiti following the 2010 earthquake, where imported cases from Nepal were the source of the infection. Cholera was brought to Haiti from a single source and then rapidly, widely, and continuously spread.12, 13 The spreading of cholera across countries' borders has been proposed between Iraq-Iran and Cameroon-Nigeria.14, 15 Since 2019, the COVID-19 pandemic has greatly increased mortality rates and put a burden on medical facilities all around the world. In Africa and the Middle East, COVID-19 has hindered the fight against infectious diseases that can be fatal, such as cholera, tuberculosis, HIV/AIDS, and several tropical diseases.16-18 Numerous nations in Asia, Africa, and Europe experienced cholera outbreaks during the pandemic,19-22 whereas no cases were recorded in the Americas in 2022. Due to inadequate monitoring and surveillance, it is anticipated that the actual number of cholera cases may be greater than those that have been released.19-22 In 2022, isolated cholera cases were associated with damage to the sewage and water systems due to the fighting in Mariupol, Ukraine, which resulted in several health issues.23-25
This study reviews recent cholera outbreaks in Africa and the Middle East, the geographical distribution of cholera cases in 2022 and 2023, the different serogroups and biotypes of V. cholerae causing pandemics and outbreaks, and the causes of cholera outbreaks raging in Africa and the Middle East. We also describe different manifestations and complications of cholera, methods of diagnosis, available oral cholera vaccines (OCVs), and antimicrobial drug resistance. Finally, we discuss management strategies and promising cholera treatments. We carried out a systematic search in multiple databases, including PubMed, Web of Science, Google Scholar, Scopus, MEDLINE, and Embase, for studies on cholera using the following keywords: ((Cholera) OR (Vibrio cholera) and (Coronavirus) OR (COVID-19) OR (SARS-CoV2) OR (The Middle East) OR (Africa)).
2 EPIDEMIOLOGY AND GEOGRAPHICAL DISTRIBUTION OF CHOLERA IN AFRICA AND THE MIDDLE EAST IN 2022 AND 2023
Since 2021, there has been an upsurge in cholera cases in Africa (e.g., Cameroon, the Democratic Republic of the Congo, Ethiopia, Kenya, Malawi, Mozambique, Nigeria, South Sudan, the United Republic of Tanzania, Zimbabwe, Niger, Burundi, and Somalia), as well as the Middle East (e.g., Syria, Lebanon, Yamen, and Iraq).26 Some countries fail to report cholera cases or deaths because of inadequate or nonexistent monitoring systems. Others delay reporting cholera outbreaks to reduce losses in commerce and tourism as well as societal hysteria. However, early detection of cholera outbreaks may lead to shorter epidemic durations and terminate any potential epidemics.27 Figure 1 shows the geographical distribution of cholera in Africa and the Middle East in 2022.
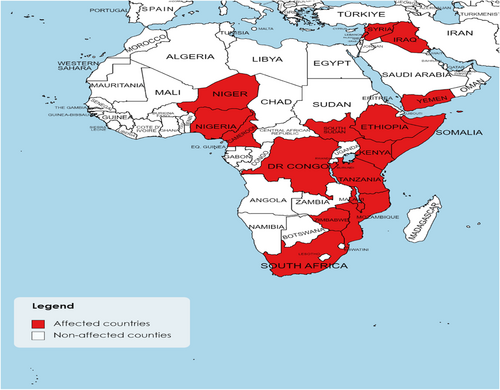
In Table 1, we collected the recently reported numbers of cholera cases, deaths, and case fatality ratios (CFRs) in Africa and the Middle East in 2022. According to our research, Malawi had the largest number of cholera cases reported in Africa in 2022, with 28,132 confirmed cases, 916 fatalities, and a CFR of 3.3%. While in the Middle East, Syria had the largest number of cases, with 77,561 suspected cases, 1893 confirmed cases, 100 deaths, and a CFR of 0.13%.
| Region | Country | Total cases (suspected and confirmed cases) | Confirmed cases | Deaths | CFR (%) | Reporting timeframe | Reference |
|---|---|---|---|---|---|---|---|
| Africa | Burundi | 81 | 66 | 1 | 1.2 | January 1, 2023 to January 15, 2023 | 28 |
| Cameroon | 15,117 | 1805 | 302 | 2 | October 25, 2021 to January 5, 2023 | ||
| Democratic Republic of the Congo | 14,290 | 1356 | 262 | 1.8 | January 3, 2022 to December 30, 2022 | ||
| Ethiopia | 911 | 27 | 27 | 3 | September 17, 2022 to January 15, 2023 | ||
| Kenya | 3939 | 142 | 70 | 1.8 | October 16, 2022 to January 8, 2023 | ||
| Malawi | 28,132 | 28,132 | 916 | 3.3 | March 3, 2022 to January 20, 2023 | ||
| Mozambique | 3930 | 16 | 21 | 0.5 | June 25, 2022 to December 18, 2022 | ||
| Nigeria | 20,768 | - | 498 | 2.4 | January 1, 2022 to November 27, 2022 | ||
| South Sudan | 424 | 56 | 1 | 0.2 | March 21, 2022 to November 20, 2022 | ||
| Zimbabwe | 2 | 2 | 0 | 0 | November 24, 2022 | ||
| United Republic of Tanzania | 24 | 3 | 1 | 4.2 | October 31, 2022 to December 11, 2022 | ||
| Niger | 72 | 14 | 1 | 1.4 | September 1, 2022 to November 14, 2022 | 29 | |
| Somalia | 15,653 | - | 88 | 0.6 | January 3, 2022 to January 1, 2023 | 30 | |
| South Africa | 10 | 10 | 1 | 10 | March 30, 2023 | 31 | |
| The Middle East | Syria | 77,561 | 1893 | 100 | 0.13 | August 25, 2022 to January 7, 2023 | 32 |
| Lebanon | 6158 | - | 23 | 0.37 | October 6, 2022 to January 17, 2023 | 33 | |
| Yemen | 556 | - | 0 | 0 | August 2022 | 34 | |
| Iraq | 3063 | 3063 | 19 | 0.6 | July 6, 2022 to November 29, 2022 | 35 |
- Abbreviation: CFR, case fatality ratio.
3 CHOLERA SEROLOGY
V. cholerae strains are defined by the structure of their O antigen, which has been classified into more than 200 O serogroups.36-39 Despite this large number of serogroups, only the V. cholerae serogroups O1 and O139 are toxin-producing and have been reported as the cause of disease in epidemics.39, 40 The O1 serogroup has three serotypes based on the antigenic factors A, B, and C: Inaba, Ogawa, and Hikojima, and two biotypes, classical and E1 Tor.39 99% of the worldwide cases are caused by O1 serogroup.41 Stool samples in the current outbreak in Yemen were positive for the O1 serogroup, Ogawa serotype.42, 43 In early October 2022, there was a rapid spread of cholera caused by Serotype O1 El-Tor Ogawa in Syria and Lebanon.44, 45
Usually, V. cholerae strains have a prophage, CTXФ, where the gene encoding V. cholerae toxins is present, the most important factor in its virulence. Atypical El Tor strains have the ctxB allele, which is a characteristic allele of the classical CTXФ strain (CTXClassФ).46 In the 2004 Mozambique outbreak, there were two types of prophages nearly identical to CTXClassФ, but they had the El Tor phenotype and were otherwise genotypically El Tor, so they were named Mozambique EI Tor.46-48 Other atypical El Tor strains are called hybrid EI Tor because they have classical ctxB and rstR genes. All of these variations are caused by the transfer of mobile genetic elements.46
Serogroups other than O1 and O139 arise less frequently and are likely under-reported due to the lack of diagnosis. Currently, 1%–3.4% of cases of acute diarrhea cases are due to the non-O1/non-O139 serogroup (NOVC).49 In a study of 58 hospitalized patients with acute diarrhea in Italy, 3.4% of the cases were due to NOVC.50 27.4% of hospitalized cholera patients with acute diarrhea at a hospital in India carried non-O1/non-O139 serogroup bacteria.51 The O5 serogroup was responsible for cases of cholera in Czechoslovakia in the 1960s; the O37 serogroup caused cholera infections in Sudan in 1968.52-54 After July 2015, there was a reported case of NOVC cholangitis and septicemia in Beirut, which was the third reported case of NOVC bacteremia in Lebanon and the first after the 2015 Lebanese waste crisis.55
Six pandemics caused by the O1 serogroup arising from India have spread worldwide from 1817 to 1923.56, 57 A seventh pandemic, which arose in Indonesia in 1961 due to the El Tor biotype, spread into Asia, Africa, and Latin America, including the devastating epidemic that occurred in Haiti in 2010. Other El Tor biotype outbreaks occurred in several African countries from 1997 to 1999, from 2012 to 2014 in Mozambique, and 2010 in Cameroon.39, 58-60 O139 epidemics have occurred less frequently but were responsible for infections among the elderly in Pakistan between 1995 and 2010,61 in Thailand,62 and sporadically in China63 and Bangladesh.64 Serotype O139 may be responsible for the probable eighth cholera pandemic, which emerged successfully between 1992 and 2015 before dying out, according to a study by Ramamurthy et al.65 They included 330 O139 isolates in their study and reported that two key genomic evolutionary changes are responsible for the eventual epidemiological decline of O139. First, there was a transformation from the homogenous toxin genotype in wave-A to heterogeneous genotypes, followed by gradual loss of antimicrobial resistance due to the progression of waves.65
Table 2 depicts the classification of V. cholerae into serogroups and biotypes, as well as major pandemics and outbreaks over the years.46, 54 Pulsed-field gel electrophoresis (PFGE), polymerase chain reaction (PCR)-based methods, ribotyping, and sequencing-based methods such as multi-locus sequence typing (MLST), variable number of tandem repeats (VNTR) analysis, and multi-locus variable tandem repeat analysis (MLVA) are molecular tools for studying V. cholerae.54
| Serogroup | Biotype | Country | Year | Reference | |
|---|---|---|---|---|---|
| O1 | Classical | From 1st to 6th pandemics | 1817–1923 | 66, 67 | |
| E1 Tor | 7th pandemic | 1961–1975 | 68 | ||
| Indonesia | 1961 | 69 | |||
| Lima and Peru | 1991 | 70 | |||
| Haiti | 2010 | 58 | |||
| Mozambique | 1997–1999 and 2012–2014 | 71 | |||
| Cameroon | 2010 | 60 | |||
| Yemen | 2016–2017 | 72, 73 | |||
| Syria and Lebanon | 2022 | 45 | |||
| Atypical E1 Tor | “MB El Tor” | Matlab in Bangladesh | 1991 and 1994 | 74 | |
| Altered El Tor | Bangladesh | 2001 | 74 | ||
| Mozambique El Tor | Mozambique | 2004 | 47, 48 | ||
| Hybrid El Tor | various African and Asian countries | 1991 and 2004 | 75, 76 | ||
| O139 | - | The 8th pandemic | 1992–2015 | 65 | |
| Pakistan | 1995–2010 | 61 | |||
| Thailand | 1983–2013 | 62 | |||
| Bangladesh | 2013–2014 | 64 | |||
| China | 1993–2013 | 63 | |||
| Non-O1/non-O139 | - | Czechoslovakia | 1960s | 53 | |
| Sudan | 1968 | 52 | |||
| Lebanon | 2015 | 55 | |||
4 WHY ARE AFRICA AND THE MIDDLE EAST FERTILE ENVIRONMENTS FOR CHOLERA?
The key factors contributing to Africa's ongoing and repeated cholera outbreaks include poverty, inadequate sanitation, poor water supply, and overpopulation. The situation is exacerbated by the human conflicts that occur throughout Africa as well as the Middle East.2, 8, 77, 78 Transmission becomes simpler in crowded environments like refugee camps, prisons, and war zones.10, 79 Additionally, climate-related factors such as rainfall, flooding, droughts, and strong winds also propagate cholera (Figure 2).2, 77
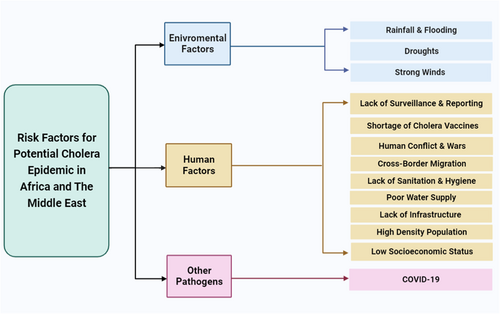
Several sociological factors have impacted cholera epidemics in Africa and the Middle East. In developing countries, high-density populations lead to sanitation issues such as unsuitable living conditions; clean water, sewage treatment, and clean, warm food may be limited or not available at all. These living conditions often do not allow for basic sanitation strategies with which the prevention of cholera can be achieved.80, 81 Water wells and rivers are common sources of water for displaced communities. Sewage treatment allows for the maintenance of a clean, non-contaminated water supply for the population and is lacking in many impoverished areas.78 Sanitation applies to not only water sources but nutrition as well. Cold meals, lack of nutrition, and roadside food vendors all increase the likelihood of contracting cholera.77 Helicobacter infection, which is common in young children and adolescents in developing countries in Africa and the Middle East, is another risk factor for cholera.82-84 Helicobacter pylori plays a significant role in the pathophysiology of diarrheal illnesses like cholera by causing hypochlorhydria, gastric ulcers, and gastric cancer.82, 84 Clemens et al. found that infection with H. pylori was related to a considerable increase in the probability of life-threatening cholera. However, this was only observed in people who lacked natural immunity against V. cholerae.85
As of 2023, there were about 5.4 million refugees from Syria in neighboring countries.8, 86 The prolonged civil war within Yemen has led to the immigration and emigration of individuals who may have been carriers of cholera.2 Due to the country's institutional gridlock and economic crisis, cholera affected Lebanon in 2022.78 In Ethiopia, the conflict in the Tigray region has led to nearly 53,000 refugees entering Sudan to be housed in refugee camps.8, 87 Political conflicts have not only displaced people but have also prevented vaccines from reaching the targeted host population.80 Cholera vaccinations are limited in impoverished countries due to factors such as political strife, vaccine costs, and the world's focus on other pathogens.80
Cases of cholera outbreaks brought on by climate change, such as those associated with droughts, heavy rainfall, and the propagation of V. cholerae reservoirs and vector organisms have occurred in Africa.46, 77 Climate change is likely to worsen cholera outbreaks in Africa and the Middle East. Increases in air temperature were linked to increases in cholera cases in Africa.88-93 Extreme weather events are predicted to increase with climate change94 and both abnormally wet and drought events have been associated with increased cholera cases in Africa95-98 and elsewhere.90, 91, 99 Droughts can lead people to use poor-quality water for drinking, whereas flooding can cause sewage systems to overflow into drinking water.92, 100 The obtainment of clean water can be expensive; water wells are often sought after, which may have cholera propagating within them.46, 77 Droughts are often accompanied by times of economic hardship for individuals who partake in agriculture. Obtaining vaccines, sanitation supplies, water, and food may be difficult, and cheaper alternatives may be sought, which may contain V. cholerae.77 Increased frequency of El Niño events can lead to more climatic extremes that increase cholera cases.96, 101-103 Changing ocean conditions favor the growth of V. cholerae bacteria through increased temperature92 and higher zooplankton populations that are fed by higher phytoplankton populations that benefit from nutrient-rich runoff from agriculture and human waste.88, 99, 100 Because V. cholerae is a temperature-sensitive pathogen, its populations grow within reservoirs at the ideal temperature. This in turn increases the likelihood of the pathogen increasing within the population.77 Droughts can allow brackish water up into streams allowing cholera carrying plankton inland.90, 91, 99
The COVID-19 pandemic exacerbated cholera outbreaks in different countries in Africa and the Middle East.16-22 As of February 2023, approximately 750 million confirmed cases and 6.7 million confirmed deaths have been attributed to COVID-19 globally.104 The pandemic caused a shift in the focus of governments, organizations, and the media away from cholera.81, 105 The worst consequences of the COVID-19 pandemic were suffered by many developing countries in Africa and the Middle East.106 The pandemic may negatively impact crucial cholera control measures like surveillance, a crucial defense against outbreaks and related mortality.16 However, an increase in hand washing and sanitization in response to the COVID-19 pandemic is expected to reduce food and waterborne infections. Researchers believe that the restricted availability of clean water and poor waste disposal in many rural and peri-urban slums in Sub-Saharan Africa make efficient hand hygiene implementation difficult.16
5 THE CHARACTERISTIC CLINICAL MANIFESTATIONS AND COMPLICATIONS OF CHOLERA
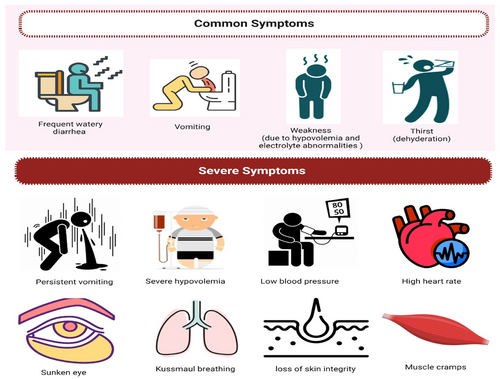
V. cholerae infection causes a range of symptoms, from asymptomatic intestinal colonization to severe diarrhea. Other typical symptoms include abdominal pain, borborygmi, and vomiting, especially in the early stages of the illness. The severe illness caused by cholera is attributed to the significant loss of electrolytes caused by diarrhea. The clinical signs of V. cholerae O1 versus O139 are similar.107 A normal incubation period for cholera is 1–2 days; however, the duration of cholera incubation varies depending on the host's susceptibility and the amount of the inoculum and can range from a few hours up to 3–5 days.108-110 While mild V. cholerae infection may be clinically indistinguishable from other causes of diarrhea, severe cholera is distinguished by rapid fluid and electrolyte loss. Cholera stools may contain fecal debris and bile in the early stages of the illness.6 Nevertheless, the passing of copious “rice-water” stool, a watery stool with mucous particles, is a defining feature of severe cholera (cholera gravis) and usually has a fishy odor. The diarrhea is normally painless and does not cause tenesmus. In the most severe instances, stool production in adults may approach 1 L per hour. The maximum rate of stool excretion in children with severe cholera is frequently between 10 and 20 cc/kg/h, and this rate of fluid loss is unusual in other types of diarrheal sickness.111 Additionally, cholera patients’ feces include a greater quantity of sodium, as well as considerable levels of potassium and bicarbonate, when compared to other causes of pediatric diarrheal disease.112, 113 Diarrhea is most severe in individuals treated with appropriate rehydration during the first 2 days and subsides after 4–6 days, with total volume loss of up to 100% of body weight occurring throughout the disease.114 Vomiting, which is sometimes accompanied by watery emesis, is common and can occur before or after the onset of diarrhea. Patients may experience abdominal cramps, but not the intense abdominal pain associated with dysentery.114
The most common sequelae of acute cholera diarrhea are hypovolemia and electrolyte abnormalities due to rapid fluid and electrolyte loss. Severe hypovolemia can develop within hours of the onset of symptoms. The median time between the onset of symptoms and death in those who died before being admitted to a cholera treatment facility during the early stages of the Haitian cholera outbreak was 12 h.115 Severe hypovolemia might cause sunken eyes, dry mouth, chilly, clammy skin, reduced skin turgor, or wrinkled hands and feet. The majority of patients exhibit apathy. Kussmaul breathing could develop from lactic acidosis brought on by inadequate perfusion in addition to acidosis from loss of stools' bicarbonate (Figure 3). Pneumonia is a common comorbidity in children with cholera, possibly as a result of aspiration during vomiting, and has been linked to death.116 Because fever is uncommon, the presence of a high temperature should raise the possibility of a concomitant infection or consequence. In the absence of diarrhea, “Cholera sicca” is a unique type of illness in which fluid collects in the intestinal lumen; circulatory collapse and even death may follow.117
Most cholera cases with neurologic complications involve infants and neonates and manifest as meningitis,118 but adults with a higher risk of intracranial infection have experienced neurological effects from cholera. A 58-year-old fisherman with left-sided paresis and a Babinski sign had an intracerebral abscess and bacteremia caused by V. cholerae that did not produce cholera toxin. He had a craniotomy 5 years before presentation for a subdural hematoma, which could have allowed the organism intracranial access.119 There were no risk factors for a 10-day-old boy with V. cholerae meningitis, but recent water studies in his rural Indian village found non-O1 non-O139 V. cholerae in 41% of drinking water samples and 100% of water, sediment, and plankton samples from three nearby lakes.118, 120
Cholera is particularly dangerous to pregnant women and their fetuses. Despite early research suggesting a high risk of fetal death linked to cholera during pregnancy (up to 50% during the third trimester), more recent research has revealed a decreased but still high risk (~8%).114, 121-123 According to one study from Guinea, most pregnant women affected by a 2012 cholera outbreak were in their third trimester. The classic clinical manifestations were associated with obstetric difficulties and maternal-fetal hazards. In addition to experiencing choleriform diarrhea in 100% of the women, dehydration was mild in 16%, moderate in 45%, and severe in 39% of the women. There were two threatened abortions, one preterm birth, four intrauterine deaths, and one maternal death.124
6 DIFFERENT METHODS OF CHOLERA DIAGNOSIS
In developing countries and poor settings where advanced laboratory diagnostic techniques are limited, cholera is diagnosed by clinical symptoms and manifestations. After the incubation period of V. cholerae (median = 1.4 days), outcomes range from symptomless intestinal colonization to mild or severe diarrhea.125 Identification of endemic species of V. cholerae O1 and O139 by isolation and culture of a stool specimen in alkaline peptone water (APW) then subcultured in thiosulfate-citrate-bile salts sucrose (TCBS) agar or taurocholate tellurite-gelatin (TTG) agar is the gold standard in the diagnoses with Cary Blair media as the ideal media for transport.126-128 Stool analysis is valuable for V. cholerae diagnosis as it enables further molecular phenotypic analysis of the specimen for antibiotic resistance and molecular characterization.128 External factors such as improper sampling and shipment can affect the efficacy of stool analysis as a primary diagnostic tool.128 Molecular detection and PCR have been developed for V. cholerae species identification and characterization. PCR requires a sample to be grown in pure culture without requiring viable strains. PCR can also detect the pathogen in food, water, and rectal swabs, it does not require a stool sample as most of the tests.129
Methods of diagnosis have been developed to replace complicated laboratory investigations in developing countries or endemic settings with poor healthcare quality.130 Rapid diagnostic tests (RDTs) are used directly on rectal swabs, fresh stool only, or after enrichment of the specimen by alkaline peptone water for (4–6 h).126, 131, 132 RDTs detect the pathogen qualitatively within a maximum of 30 min, which appears as a colored band that can be read with the naked eye.130 Almost all RDTs work by detection of antigens specific for lipopolysaccharide (LPS) of serotypes O1 and O139 of V. cholerae using monoclonal antibodies.133 These tests are developed to detect epidemics early to start cholera treatment for patients in their early stages to prevent the disease's progress. RDTs do not require experienced technicians to perform, are low-cost, and do not require cold storage.126, 134 These advantages of RDTs lead us to believe that they are more appropriate for use in refugee camps and impoverished regions of Africa and the Middle East. Table 3 shows the characteristics of the most common RDTs, including their sensitivity and specificity.135, 143
| Test | Sample in the test | Test duration | Vibrio cholerae species | Test assay | Target | Country of population | Cost | Sensitivity (%) | Specificity (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Cholkit | Stool | 15 min | O1 | Immunochromatographic lateral flow assay | LPS antigen | Bangladesh | ~$3 | 97.7 | 96.5 | 136, 137 |
| Crystal VC-O1 | Stool | 15–30 min | O1 | Immunochromatographic lateral flow assay | LPS antigen | Kenya | ~$2/kit | 97.5 | 100 | 138 |
| Crystal VC | Stool | 10–20 min | O1 and O139 | Immunochromatographic lateral flow assay | LPS antigen | Bangladesh | $1.9/test | 97.5 | 98.4 | 136 |
| SD Bioline | Stool | 10–20 min | O1 and O139 | Immunochromatographic assay | V. cholerae O1 and O139 antigens | Zambia | ~€2/test | 90.9 | 95.2 | 139 |
| Cholera DFA | Stool | Less than 30 min | O1 | Fluorescent monoclonal antibody staining kit | “A” factor of V. cholerae O1 LPS | - | - | 100 | 100 | 140 |
| IP dipstick | Stool | 3–58 min (In field practice) | O1 | Immunochromatographic dipstick assay | LPS antigen | Bangladesh | - | 94.2 | 84.0 | 141 |
| O139 | 100 | 92.5 | ||||||||
| Artron RDT | Stool | 15 min | O1 and O139 | Immunochromatographic assay | LPS antigen | Haiti | - | 98.6 | 69.1 | 142 |
- Abbreviations: LPS, lipopolysaccharide; RDT, rapid diagnostic test.
7 CURRENT AVAILABLE OCVS
There are four main vaccines for the prevention of cholera in the market. Vaxchora®, Dukoral®, Shanchol™, and Euvichol®/Euvichol-Plus have been prequalified by the WHO (Table 4).
| Vaccine name | Vaxchora® | Dukoral® | Shanchol™ and Euvichol®/Euvichol-Plus |
|---|---|---|---|
| Manufacturer | Emergent Travel Health144 | Valneva Canada Inc145 | Sanofi Healthcare India Private Limited (Shanchol™)146 |
| EuBiologics Co., Ltd. (Euvichol®/Euvichol-Plus)147 | |||
| Composition | Contains 4 × 108 to 2 × 109 CFU of live attenuated V. cholerae CVD 103-HgR148 | Contains inactivated WC V. cholerae O1 Inaba, Ogawa, classic and El Tor strains (31.25 × 109 vibrios of each), and rCTB 1 mg from classic biotype149 | Contains WC V. cholerae O1 and O139 without CTB150 |
| Age | 2–64 years150 | 2 years and older151 | 1 year and older151 |
| Regimen and dose | Single dose, 10 days before potential exposure to cholera144 | Adults and children >6 years old: 2 doses | Individuals older than 1 year old: 2 doses, 14 days apart152 |
| Children aged 2–6 years: 3 doses149 | |||
| Pharmaceutical form | Suspension151 | Suspension and granules for suspension151 | Suspension151 |
| Vaccination effectiveness duration | 3–6 months minimal153 | 2 years153 | 3 years minimal (for 2 doses) Short-term protection (for 1 dose)153 |
| Booster dose | No recommendation154 | Adults and children >6 years old: every 2 years | No recommendation155 |
| Children aged 2–6 years: every 6 months149 | |||
| Cost | $270/dose156 | ~$4.7–9.4/dose155 | Shanchol: $1.85/dose155 |
| Euvichol: $1.7/dose155 | |||
| Euvichol-Plus: $1.30/dose157 | |||
| Need for buffer | Yes27 | No27 | |
| Type of vaccine | Oral live attenuated151 | Oral killed151 | |
| Licensure | FDA 2016 (June)156 | WHO qualified152 | |
| Storage temperature | (−25°C to −15°C)150 | (2–8°C)149, 158 | |
- Abbreviations: CFU, colony-forming units; OCV, oral cholera vaccine.
7.1 Vaxchora
CVD 103-HgR marketed as Vaxchora is a recombinant live OCV vaccine. This strain comes originally from O1 classical Inaba strain 569B.151 This specific strain has been genetically modified by removing the gene encoding the cholera toxin A subunit to prevent the production of cholera toxin, which made the vaccine strain different from the wild type. This was achieved by the company Berne Biotech. Vaxchora is currently approved by the USD FDA for being used by individuals from 2 to 64 years old.150
A single dose of oral vaccination CVD 103-HgR offers quick protection, starting 10 days after administration.151 CVD 103-HgR vaccine can produce the immunogenic nontoxic cholera toxin B-subunit protein (CTB) in the gastrointestinal tract (GIT) and cause a rise in vibriocidal antibodies which help in the protection against V. cholerae.151 A human trial study conducted on 197 healthy adults ranging between 18 and 45 years old found that the vaccine prevents the symptoms of cholera in people who are in contact with this bacterium. Out of the total individuals enrolled, 95 received the vaccine, and 102 received a placebo. Out of the 95, 68 were introduced to the pathogen at different time points (35 subjects after 10 days of the vaccine dosage and 33 subjects after 3 months of vaccination). Only two subjects experienced moderate or severe diarrhea (5.7%) after 10 days of the dosage challenge, and four subjects (12.1%) experienced moderate or severe diarrhea after 3 months of vaccination. 59.1% of the individuals in the placebo group experienced moderate or severe diarrhea suggesting a strong protective effect of the vaccine against this symptom. The overall results suggest a 90.3% protection effect of the vaccine 10 days after the dosage, and 79.5% effectiveness 3 months postvaccination.150, 151, 159, 160 In individuals with blood group O, the vaccine efficacy was 78.4% in the 10-day challenge group, while it was 82.5% in the 3-month challenge group.159 This was a relevant finding because individuals with blood group O are more susceptible to developing severe symptoms from cholera.161
Another major study with a total of 3146 participants evaluated the levels of rapid serum vibriocidal antibody (SVA) and cholera toxin in individuals receiving the vaccine up to 181 days after vaccination. The results supported previous evidence with a cumulatively significant 96% SVA seroconversion rate in the postvaccination group compared to 4% in the placebo group.151, 162 Vaxchora protects against the serogroup O1, but not against O139, the strain found mainly in the Southeast area of Asia. Even though the evidence suggests that the vaccine is effective against the disease, it has an average cost of $270 per single dose.156 It also requires a cold chain to maintain the vaccine (between −25°C and −15°C). This limits the use of this vaccine to specific travelers from the United States to other countries.150
7.2 Dukoral
Dukoral was licensed internationally in 1991.152 Dukoral is a killed whole-cell (WC) monovalent (O1) vaccine that contains the recombinant cholera toxin B subunit (rCTB). This vaccine contains equal parts of four different strains of V. cholerae.150 It was licensed in Sweden, especially for travelers, and later used in other countries in Europe. To prevent the acid-labile CTB component from being degraded by gastric acid, the vaccination must be administered with a sodium bicarbonate buffer.152, 163 The population receiving the vaccine consists of children and people over 6 years old, receiving two doses in less than 6 weeks. Children between 2 and 6 years old receive three doses.149
The specific composition of this vaccine consists of the heat-inactivated V. cholerae O1 Inaba classic strain and the Ogawa classic strain. These two are combined with the formalin-inactivated V. cholerae El Tor strain and Ogawa classic strain, along with CTB.164 The vaccine induces antibodies for CTB and bacterial components simultaneously.149 The exact mechanism behind the immunologic system behind this compound is still unknown; however, a recent study suggested that the quality of the immunological response elicited after oral administration of the Dukoral vaccine may be regulated by toll-like receptor (TLR) pathways. Sirskyj et al. propose that MyD88/TLR signaling, rather than Trif signaling, is responsible for CD4+ T-cell proliferation in response to V. cholerae.164
The first challenge study for this vaccine was conducted at the University of Maryland. They enrolled healthy participants between the ages of 19 and 35 years old. The goal of the study was to compare the effectiveness of formulations of WC vaccines containing CTB (referred to as WC-CTB) to those without CTB (referred to as WC). Three doses, spaced by 2 weeks of either WC-CTB (containing 5 mg of CTB) or WC were administered to the participants. El Tor Inaba V. cholerae was given to the vaccinated participants and unvaccinated controls 4 weeks after the third dose of WC and 5 weeks after the third dose of WC-CTB. The WC and WC-CTB vaccines were shown to be 56% and 64% effective, respectively. Vaccinated individuals in both groups who contracted cholera experienced fewer severe symptoms than unvaccinated individuals and were completely protected from severe diarrhea.150, 165 In Bangladesh, 89,596 individuals, including women over the age of 15 and children aged 2–15, participated in a study of the original WC-CTB formulation. Three doses of the WC-CTB, the Killed WC vaccine, or a placebo were given to individuals at random. The protective effectiveness was maintained in adults and children older than 5 years of age for 2 years; however, protection was only maintained for 6 months in children 2–5 years of age, indicating that this age group needed to receive doses more frequently. Effectiveness against El Tor V. cholerae was shown to be significantly lower than against classical V. cholerae. Additionally, this study revealed that a two-dose regimen was just as efficient as a three-dose program given at intervals of 6 weeks.152, 166-168
In Peru, a study involving 1563 military personnel was done to evaluate the efficacy of WC-CTB. With an average follow-up of 5 months, the short-term preventive effectiveness in the Peruvian military study was 86% (95% confidence interval [CI] = 37–97, p < 0.01). In a high transmission environment, this study showed that an accelerated and shorter regimen (two doses given within 14 days) can induce protection within 2 weeks of immunization in a population that is primarily blood group O and is thought to be highly immunologically naïve.169 In Zanzibar, a longitudinal cohort study revealed that after a 15-month follow-up period, the protective efficacy of two doses of WC-CTB was 79% (95% Cl = 47–92). WC-CTB also offered significant indirect (herd) protection in the observed circumstance in addition to direct protection. As the neighborhood's vaccination rate rose, the risk of cholera among unvaccinated locals decreased, demonstrating the existence of herd protection.170 According to the findings, expanding the use of cholera vaccinations will significantly reduce the burden of cholera in endemic areas by offering both direct and indirect protection to those who receive them as well as indirect protection to those who do not receive them.
The WHO approved the use of Dukoral after the results of many field studies demonstrated its high efficacy and effectiveness against cholera. It has not been demonstrated that Dukoral protects against cholera caused by Vibrio species other than V. cholerae serogroup O139. Additionally, because of its expensive cost, short duration of protection, and logistical difficulties with vaccine delivery, it has not been regularly embraced for use in public health.152 The cost of a single dose of Dukoral is $6 to the public sector150; however, a cost-effectiveness study in Mozambique in 2017 revealed that the cost of a two-dose vaccine in an urban area was $2.09, without adding the manufacturing cost of the vaccine, which is relatively high.171, 172 The vaccine did not require a rigorous cold chain like others; it only needed to be refrigerated between (2–8°C).149
7.3 Shanchol and Euvichol/Euvichol-Plus
Shanchol and Euvichol/Euvichol-Plus are essentially the same vaccines but made by different providers. They are both currently available for vaccination and have been used in campaigns with more than 20 million doses administered.173 Shanchol is a killed WC vaccine consisting of killed WC O1 and O139 serogroups but without CTB. Shanchol is an oral vaccination that must be administered two times, at least 2 weeks apart. Immunity against cholera is anticipated to develop in 7–10 days following the second dose.150 This vaccine is intended for anyone aged more than 1 year with no current data for infants below a year of age.158 It has been demonstrated that giving the vaccine orally, which develops local immunity, is efficacious. A similar IgA antibody response (including memory) is elicited by the vaccination locally in the GIT to that induced by the cholera disease itself. The colonization of V. cholerae O1 and O139 is hampered by the intestinal antibacterial antibodies that stop the bacteria from adhering to the intestinal wall.158 Both Shanchol and Euvichol/Euvichol-Plus are stored at (2–8°C) and not frozen.158
A study was conducted in Kolkata, India to assess the effectiveness of Shanchol when administered in two doses separated by 2-week intervals. Twenty cholera incidents occurred throughout the 2 years of follow-up in the vaccine group, compared to 68 incidents in the placebo group, demonstrating a protective efficacy of 67% (one-tailed 99% CI, lower bound 35%, p < 0.0001). Furthermore, the vaccine protective effectiveness was 65% during the third year of follow-up (one-sided 95% CI, lower bound 44%, p < 0.001). At 5 years, the vaccine's cumulative protective effectiveness was 65% (95% CI = 52–74, p < 0.0001). Age-related differences have been noted: 1–4 years: 42% (95% CI = 5–64); 5–15 years: 68% (95% CI = 42-82); and 15 years and older: 74% (95% CI = 58-84).174-176
The catastrophic cholera outbreak in Haiti, which resulted in over 8000 deaths, and other significant cholera outbreaks in 2010 all helped to increase awareness of the OCV among the worldwide community and afflicted nations.177 The cooperation between the International Vaccines Institute (IVI) and EuBiologics Co., Ltd. was established in 2010 to conduct an OCV technology transfer and establish a second producer to accommodate the rising worldwide demand. Euvichol was licensed and subsequently WHO prequalified upon the results of a non-inferiority trial in 2016 since the production method and composition were identical to those of Shanchol. To further enhance Euvichol, EuBiologics switched the vaccine's packaging from traditional glass vials to plastic tubes (Euvichol-Plus), making it easier to administer in crises or during emergencies.177
A randomized, non-inferiority trial evaluating the safety and immunogenicity of two doses of either Shanchol or Euvichol/Euvichol-Plus was carried out in the Philippines on a total of 1263 healthy Filipino individuals (777 adults and 486 children). The results demonstrated that Vibriocidal antibody responses against V. cholerae O1 Inaba elicited by Euvichol/Euvichol-Plus were non-inferior to those generated by Shanchol in children (87% vs. 89%) and adults (82% vs. 76%). Comparable results were seen for V. cholerae O1 Ogawa in children (91% vs. 88%) and adults (80% vs. 74%). In addition, no significant adverse effects were recorded in either vaccination group.178
Since the creation of Euvichol, 100 million doses have been administered in over 20 countries.150 With the collaboration of the Pan American Health Organization (PAHO), 1.17 million doses of Euvichol arrived in Haiti on December 12, 2022. Over half a million doses were expected to arrive in the next several weeks to stop the spread of cholera.179 On November 7, 2022, Lebanon received 600,000 doses from the International Coordination Group (ICG), which manages the supplies of vaccines worldwide. The aim is to achieve a 70% vaccination by administering 200,000 doses in less than 2 weeks.180 The city of Damascus in Syria received two million doses of OCV on November 30, 2022.181 Both Shanchol and Euvichol/Euvichol-Plus are the selected vaccines for mass immunization since they are affordable and easy to distribute. The goal is to eliminate the transmission of cholera in 20 countries before 2030 arrives.182
8 CHOLERA VACCINE SHORTAGE CRISIS AND NEW PROMISING OCVS
The ICG managed the distribution of emergency vaccines and drugs to nations during significant outbreaks and received the global cholera vaccine stockpile from Shantha Biotechnics and EuBiologics. The WHO, UNICEF, and the International Federation of Red Cross all have representatives on the ICG. In 2022, outbreaks have been recorded from 30 countries, compared to an average of less than 20 during the past 5 years.183, 184 Cholera vaccines are in critically short supply, according to the WHO.183, 184 There is no short-term way to enhance production because vaccine manufacturers were already operating at full capacity. As of October 2022, a total of 24 million of the 36 million doses anticipated to be produced in 2022 have already been shipped for vaccination campaigns, and an additional 8 million doses have been approved by the ICG for a second round of emergency vaccination in four countries, highlighting the severe lack of the vaccine.185
The ICG had been forced to temporarily halt the standard two-dose vaccination regimen in cholera outbreak response campaigns in favor of a single-dose strategy that may allow the doses to be used in more countries, during a period of unprecedented increase in cholera outbreaks globally. Despite the paucity of data on the precise period of protection and the fact that protection seems to be substantially lower in children, the one-dose approach has been successful in controlling outbreaks. Despite the temporary interruption of the two-dose strategy causing a decrease and shortening of immunity, the advantage of administering one dosage still trumps administering none.186 A recent modeling study demonstrated that rapid vaccination campaigns with a single dose of OCV are likely to prevent more cases and fatalities than a two-dose pro-rata campaign in an epidemic environment with a limited vaccine supply.187
The COVID-19 pandemic caused collateral damage by affecting the production of Vaxchora. Due to a significant decline in international travel as a result of the COVID-19 pandemic, the vaccine manufacturer informed the FDA in May 2021 that they had decided to temporarily stop the production and distribution of Vaxchora in the United States.188 In addition, Shantha Biotechnics announced that the production of Shanchol, which comprises 15% of the global stockpile, would stop in 2022, and the distribution by the end of 2023.189, 190
A licensing and technology transfer agreement has been signed by the South African biopharmaceutical company Biovac and the IVI for the development and production of OCVs. This will help enable Biovac to create the antigen/raw materials needed for manufacturing vaccines. The goal of this collaboration is to expand the production rates to alleviate the severe worldwide vaccine shortage required to prevent cholera. This knowledge transfer will also create and show off capability for good manufacturing process scale-up, local manufacturing of clinical trial products, and end-to-end vaccine production in Africa as the production of the first trial batches is scheduled to begin in 2024.191
9 MANAGEMENT, ANTIMICROBIAL DRUG RESISTANCE, AND PROMISING CHOLERA TREATMENTS
Most cholera cases will be treated with oral rehydration therapy, which will be used to correct fluid and electrolyte imbalances192 (Figure 4). The primary treatment for cholera is rehydration therapy. Antibiotic therapy is also recommended for patients with severe illness and high-risk patients, such as those who are immunocompromised, pregnant, or have comorbid conditions (Table 5).193-195
| Patient group | Rehydration therapy | Antibiotic therapy | Zinc |
|---|---|---|---|
| Adults |
|
|
20 mg of zinc sulphate per day for 10 days |
| Children |
|
If the child is ≥12 years old: | |
|
|||
| Pregnant Women |
|
|
|
| Ref. | 193, 192, 195 | ||
- Abbreviations: ORS, oral rehydration salt; RL, Ringer lactate.
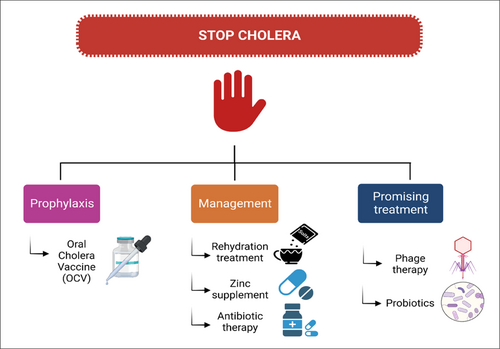
9.1 Rehydration treatment
One of the complications of cholera is dehydration. Adequate rehydration is critical for the patient's recovery. Depending on the patient, different modes of rehydration therapy can be administered.195 Oral rehydration salt (ORS) should be given immediately to patients with no or some dehydration. High-sugar drinks should be avoided in case of unavailability of ORS because they aggravate diarrhea.194 Intravenous rehydration is indicated in patients who have severe dehydration and conditions that interfere with the oral administration of rehydration solution such as loss of consciousness, and persistent vomiting.194 The fluid amount can be adjusted according to the hydration status of the patient. In the treatment of severe dehydration with IV rehydration, ringer lactate is the fluid of choice.195
In the case of patients with severe acute malnutrition, a rapid bolus infusion of 20 mL/kg Ringer lactate (RL) is given over 30 min, if the severity signs of dehydration are not resolved, the procedure can be repeated up to two times. Once severity signs have resolved, the patient should receive a continuous infusion of 70 mL/kg RL over 6 h, in addition to adding 100 mL of 50% glucose to each liter of ringer lactate. When the patient is stable and can drink in the absence of excessive vomiting, the patient can be started on standard hypo-osmolar ORS.193 Pregnant women do not bear any greater risk of being infected with cholera than the general population. Dehydration, on the other hand, can cause fetal complications. As such, treatment is given to correct the patient's dehydration and maintain the systolic blood pressure (SBP) >90 mmHg to ensure adequate perfusion of the fetus.193, 194
9.2 Zinc supplement
Zinc supplementation reduces the severity and duration of diarrhea in children aged 6 months to 5 years with watery diarrhea.193 20 mg of zinc sulphate P.O. per day for 10 days should be started immediately. Concurrent administration of zinc with antibiotics such as Ciprofloxacin can impair antibiotic absorption. As a result, the antibiotic should be given 2 h prior or 4–6 h after the zinc supplement.194, 195
9.3 Antibiotic therapy
Although oral and intravenous rehydration are the first-line treatments for cholera, antibiotic therapy is recommended in a variety of situations, including severely ill patients, patients with comorbid conditions, pregnant women, and patients with persistently high stool output despite adequate rehydration therapy.194, 195 The antibiotic of choice for all ages, including pregnant women, is Doxycycline.195 In the case of documented doxycycline resistance, Azithromycin and Ciprofloxacin can be considered. Antibiotic therapy should begin as soon as the patient is stable and capable of taking oral medication.195
9.4 V. cholerae's resistance to antimicrobial drugs
Antibiotic resistance is a growing concern in the treatment of infectious diseases. Cholera is no different, as several studies show the emergence of resistant variants of the pathogen for certain antibiotics. Resistance develops due to the misuse of antimicrobials, poor clinical care, poor water quality, and lack of proper regulations.196 Misuse includes suboptimal dose and frequency of medication, overuse, and non-adherence. The resistance profile of the pathogen appears to vary depending on the geographical location and year of the cholera outbreak.197 The pathogen develops resistance through mechanisms such as drug uptake limitation, drug inactivation, drug target modification, and efflux of drugs.1
Cholera antibiotic resistance in Mozambique from 2012 to 2015 showed an increase for Tetracycline, Nitrofurantoin, and Azithromycin. There was up to 100% resistance to Ampicillin and Nalidixic acid, but a decrease in resistance for doxycycline and no resistance for ciprofloxacin. They noticed that isolates that were 100% resistant to chloramphenicol in 2012 had a dramatic decline of approximately 50% in 2013 with a subsequent increase.198 In a recent meta-analysis, 20 studies that investigated 648 environmental V. cholerae O1/O139 isolates from Asia, Africa, America, and Europe were included. Fluoroquinolones, gentamicin, ceftriaxone, doxycycline, kanamycin, and cefotaxime were shown to have low resistance rates. Nevertheless, antimicrobial resistance is increasing, and the major focus is on the emergence of antimicrobial resistance in V. cholerae strains, particularly in low-income nations or endemic areas.199 Although the antimicrobial resistance profile of fluoroquinolones such as Ciprofloxacin is favorable, the risk of resistance development remains as the association between Nalidixic acid resistance and the reduced susceptibility profile of fluoroquinolones has been reported.200 Table 6 shows the characteristics of some studies' findings on the antimicrobial drug resistance to cholera.198-202
| Author(s), year | Study type | Country | Study subject | Outcome | Antimicrobial drugs with the highest resistance | Antimicrobial drugs with the least resistance | Reference |
|---|---|---|---|---|---|---|---|
| Yuan, X. H., et al., 2022 | Meta-analysis | Global | 648 Environmental V. cholerae O1/O139 isolates | Resistance rate of environmental V. cholerae to antibiotics | Nalidixic acid, Cotrimoxazole, Tetracycline, Furazolidone | Fluoroquinolones, gentamicin, ceftriaxone, doxycycline, kanamycin | 199 |
| Miwanda, B., et al., 2015 | Laboratory study | Democratic Republic of the Congo | 1093 isolates from major outbreaks in the DRC during 1997–2012 | Long-term evolution of antimicrobial drug susceptibility of V. cholerae isolates | Nalidixic acid, Tetracycline, Ampicillin, Chloramphenicol, Erythromycin | Fluoroquinolones | 200 |
| Cyclines | |||||||
| Dengo-Baloi, L. C., et al., 2017 | Laboratory study | Mozambique | 159 Rectal swabs from cholera cases during cholera outbreaks from 2012 to 2015 | Antibiotics resistance pattern of V. cholerae | Ampicillin, Nalidixic acid, Tetracycline, Cotrimoxazole | Ciprofloxacin | 198 |
| Azithromycin | |||||||
| Eibach, D., et al., 2016 | Laboratory study | Ghana | 92 V. cholerae isolates collected in 2011, 2012, and 2014 | Molecular Epidemiology and Antibiotic Susceptibility of V. cholerae | Sulfamethoxazole/trimethoprim | Chloramphenicol | 201 |
| Nalidixic acid | Gentamycin | ||||||
| Tetracycline | |||||||
| Ciprofloxacin | |||||||
| Ampicillin | |||||||
| Sambe-Ba, B., et al., 2017 | Laboratory study | Senegal | 50 V. cholerae serogroup O1 strains isolates | Genetic determinants of virulence and antibiotic-resistance in V. cholerae O1 isolated | Sulfamethoxazole/trimethoprim | Tetracycline | 202 |
| Streptomycin |
9.5 Promising methods for treating cholera
Phage therapy can play a critical role in cholera treatment. Bhandare et al. demonstrated that oral treatment of a single Podoviridae phage may prevent clinical cholera symptoms in infant rabbits with no development of resistance to the phage. The results showed that animals treated with phage therapy had shown no clinical signs of infection when compared to the 69% of untreated controls.203 From sewage water in Baghdad, Yassein et al. isolated virulent phages lytic for the isolate of V. cholerae O1. They then analyzed the spectrum of their activity and created phages in a phage cocktail to broaden the host range for the individual phages included in the cocktail. All of the phages identified in their research had a limited host range of 34%–65% against isolates of V. cholerae O1; however, a cocktail of eight phages had 100% activity against those isolates. Phage cocktail formulations may provide an alternative treatment for cholera since they are highly efficient against V. cholerae. Phage cocktails may be very useful in the field of water processing, cholera prevention, and treatment procedures.204
During a major outbreak of cholera in India, researchers isolated Vibrio phage VMJ710 from a sewage sample. This phage can lyse 46% of multidrug-resistant V. cholerae O1 strains. Within the first 4–6 h of treatment, the phage VMJ710 dramatically decreased the bacterial density in a mouse model. Additionally, they demonstrated that when given 6–12 h before the bacterial challenge, the phage VMJ710 can prevent the progression of V. cholerae infection and ailment. This phage could be a good choice for oral delivery and could help treat cholera infections brought on by pathogenic multidrug-resistant V. cholerae strains.205 Another promising cure for cholera is probiotics. The goal of probiotic treatment for cholera infection is to bring back balance to the gut bacteria and stop V. cholerae strains from taking over the digestive system.206 The conventional use of Rosaceae plant infusions for diarrhea seems applicable to cholera. The plant extracts impede the binding of the cholera toxin to receptors or prevent the internalization of the toxin, both of which reduce the development of pathogenic bacteria. While the plant extracts do not kill bacteria, they may slow it down, which may help other antibiotic treatments. The plant extracts may eventually be shown to be helpful additions to current ORS in the fight against cholera.207
10 CONCLUSION
V. cholerae is a complex bacterium that can persist in both human host populations and aquatic environments. In humans, the bacteria can cause severe diarrhea and death. The Middle East and Africa have suffered the brunt of the recent outbreaks of cholera and the purpose of this study was to review the causes and potential solutions for cholera in this region. The study's narrow temporal and geographical focus on cholera outbreaks in Africa and the Middle East may not provide a comprehensive understanding of cholera dynamics globally and limits the generalizability of findings. The impact of COVID-19 likely exacerbated local outbreaks of cholera in Africa and the Middle East as well as led to an undercounting of the number of cases. Going forward there are headwinds and promises toward combating cholera in Africa and the Middle East. Poverty, inadequate sanitation, inadequate water supply, overpopulation, and conflict continue to create environments susceptible to cholera. Conflicts in nations such as Syria, Ethiopia, and Sudan have led to crowded living conditions with reduced access to clean drinking water and cholera treatment. Climate change threatens to exacerbate cholera conditions by increasing the occurrence of flooding and drought in the region.
RDTs that do not rely on expensive laboratory materials will help detect outbreaks sooner, leading to more rapid population-level interventions. Vaccination efforts have been hindered by reduced production and increased demand for vaccines. Eubiologics' Euvichol/Euvichol-Plus will be increasingly relied upon as a relatively inexpensive and effective vaccine for Middle Eastern and African nations. As Africa and the Middle East emerge from the COVID-19 pandemic, renewed efforts will need to be made to address the increasing threat of cholera. Prevention and treatment strategies will likely require a multi-pronged combination of prevention and treatment strategies.
AUTHOR CONTRIBUTIONS
Abdulrahman K. Ahmed: Conceptualization; writing—original draft; validation; data curation. Victor Coll Sijercic: Investigation; writing—original draft; visualization; writing—review and editing; resources. Mahad S. Akhtar: Writing—original draft; methodology; visualization; software. Ahmed Elbayomy: Conceptualization; investigation; resources; data curation; formal analysis. Mohamed A. Marouf: Investigation; writing—original draft; data curation; methodology. Mahlet S. Zeleke: Conceptualization; writing—original draft; project administration; data curation; validation. Reem Sayad: Investigation; writing—original draft; formal analysis. Abdelrahman Abdelshafi: Writing—original draft; resources. Nicholas J. Laird: Writing—original draft; investigation; formal analysis; data curation. Mohamed A. El-Mokhtar: Supervision; project administration; writing—original draft. Gregory R. Ruthig: Conceptualization; writing—original draft; writing—review and editing; visualization; supervision; project administration. Helal F. Hetta: Writing—original draft; project administration; supervision.
ACKNOWLEDGMENTS
All figures were created with BioRender (https://biorender.com), except Figure 2 was created with MapChart 2023. https://www.mapchart.net/.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Mahlet S. Zeleke affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
All generated data are included in this manuscript.