Evaluation of the hematological inflammatory parameters in the patients with immune thrombocytopenic purpura: A case–control study
Abstract
Background and Aims
Inflammation is one of the immune thrombocytopenic purpura (ITP)'s aggravating elements due to inflammatory cells' function. This study aims to identify and evaluate hematological inflammatory parameters, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and hemoglobin-to-platelet ratio (HPR), in patients with ITP compared to the control group.
Methods
We retrospectively analyzed the profile of 190 ITP patients from August 2019 to January 2021 at Imam Reza Hospital of Mashhad, Iran, along with 100 healthy individuals who had no ITP-related clinical or laboratory symptoms. Immune cell counts, NLR, PLR, and HPR were calculated using the complete blood count at the time of diagnosis and after the treatment. The results were analyzed through MedCalc, SPSS software, and the receiver operating characteristic curve.
Results
The result showed that white blood cell (WBC) and neutrophil counts were higher in ITP patients (WBC: p: 0.001, neutrophil: p: 0.001), and conversely, platelet and lymphocyte counts were higher in the control group compared to ITP patients (platelets: p: 0.001, lymphocytes: p: 0.001). The indices analysis between the two groups revealed that NLR was significantly increased in ITP patients (p: 0.001), but PLR was significantly reduced in ITP patients (with the mean platelet count of 23.44 ± 35.26 × 109/L) compared to the control group (with the mean platelet count of 234.04 ± 55.88 × 109/L). The HPR index also significantly increased in ITP patients (p: 0.001).
Conclusion
An increase in NLR, PLR, and a decrease in HPR can be considered a valuable diagnostic algorithm in patients with ITP.
1 INTRODUCTION
Patients with immune thrombocytopenic purpura (ITP) experience hemostatic impairment and an elevated risk of bleeding caused by the circulating platelet count lower than 100 × 109 platelets/L.1 Although the exact pathophysiology of ITP is still not well known, the dysregulation of the immune system due to various reasons such as autoimmune conditions, infections, inflammations, and vaccinations as well as genetic background are involved in the occurrence of ITP.2 The immune reaction to platelet autoantigens causes the spleen to destroy premature platelets and the bone marrow megakaryocytes to produce fewer platelets.3 Autoantibodies that specifically target platelet glycoproteins (GPs), including GPIIb/IIIa and GPIb/IX, are regarded to be the main reason of platelet destruction and underproduction. In damaged blood vessels, the GP complexes operate as physiological receptors to mediate interactions between platelets and vascular subendothelium.4 Defects in membrane GPs in ITP can lead to platelet dysfunction and bleeding disorders through various mechanisms, including disruption of their adhesion and aggregation activities, and the platelets arrest hemorrhaging from lesions in the blood vessel wall.5
Numerous benign and malignant diseases have been shown to be significantly influenced by inflammation.6 Even though ITP is an autoimmune condition, the data regarding the relationship between ITP and inflammatory markers are inadequate. However, research in recent decades shows the prevalence of inflammation in ITP patients is high.7, 8 More importantly, it can exacerbate the ITP by increasing immune cells such as neutrophils, lymphocytes, platelets, and monocytes.9 The interaction of the immune cells spontaneously raises the levels of inflammatory and pro-inflammatory cytokines, including such as interleukin (IL-10), IL-18, interferon-alpha (IFN-α), tumor necrosis factor-alpha (TNF-α), and IL-17, which ultimately are associated with enhanced immune responses.10-12
According to previous studies, one of the leading immune mechanisms of autoantibody stimulation in ITP involves the interaction of immunomodulators, including regulatory T cells (Tregs), with inflammatory cells that direct immunostimulatory effects against autoantigens.13, 14 Inflammatory cells, especially CD8+ T cells, participate in immune response waves based on signals received from Tregs and each other. Neutrophils are involved in intensifying immune responses by secreting inflammatory cytokines and invoking other immune cells.15 On the other hand, in ITP, lymphocytes, unlike in normal conditions, target GPIbβ peptides presented through major histocompatibility complex-I on the platelet surface and destroy them by apoptosis; consequently, CD8+ T cells may attack platelets.16 Many investigations have demonstrated that ITP patients can have their CD8+ T cell abnormalities artificially stimulated to kill platelets, which causes adverse consequences by interacting with neutrophils, platelets, and other immune cells.17-19
Finding inflammatory risk factors is now crucial for developing customized treatments for ITP patients. In particular, the evaluation of cellular indexes has advantages over tracking the levels of inflammatory cytokines and other intracellular markers, including cost savings and reduced time to achieve results. The role of several cellular and noncellular mediators has been investigated in numerous studies, and the evaluation of changes in their levels is helpful in identifying patients. Therefore, evaluating the count of immune cells and comparing inflammatory cellular indices, including platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR), can be useful in diagnostic processes and selecting effective treatment methods. In this study, we assess and compare the values of PLR and NLR along with other hematological markers such as hemoglobin-to-platelet ratio (HPR) in patients with ITP compared to the control group and investigate the correlation of these parameters with the hospitalization period, therapeutic approaches, and clinical symptoms of ITP patients.
2 MATERIAL AND METHODS
2.1 Study subjects
In this study, we analyzed the data of 190 patients diagnosed with ITP from August 2019 to January 2021 at Imam Reza Hospital of Mashhad, Iran. The definition of ITP was adopted from the 2019 American Society of Hematology guideline.20 ITP patients were included, 130 females (68.4%) and 60 males (31.6%), and the average age of patients at diagnosis was 38.45 ± 17.96. The control group consisted of 100 healthy individuals (male: 35.4%, female: 64.6%) with a mean age of 45.33 ± 15.31 who were referred to the same hospital for their check-up tests. The control group consisted of people close to the patient group in age and gender (but without ITP disease), and to be close to the patient group, they had platelet counts in the low to mid-range of normal. All control group members had no history of chronic disease or ITP-related clinical or laboratory symptoms, including thrombocytopenia less than 100 × 109 platelets/L, bleeding, inflammatory illustrations, and thrombosis. All the procedures performed in the studies involving human participants were in accordance with the ethical standards of the local ethics committee of Mashhad University of Medical Sciences (IR. MUMS.FHMPM.REC.1400.047) as well as 1964 Helsinki Declaration. Also, informed consent was obtained from all participants joined into this study.
2.2 Inclusion and exclusion criteria
Inclusion criteria in this study are as follows: Thrombocytopenia less than 100 × 109 platelets/L (without any further bone marrow issues that might result in thrombocytopenia), bleeding signs, and the completeness of the profile and laboratory/clinical data. Exclusion criteria include the following: Patients with a previous history of systemic lupus erythematosus (SLE), Behcet's disease (BD), rheumatoid arthritis, ankylosing spondylitis (AS), thrombocytopenic thrombotic purpura, hemolysis, elevated liver enzymes and low platelets syndrome, smokers, patients taking immunosuppressive drugs, and patients with nonimmune thrombocythemia.
2.3 Inflammatory and hematological parameters
Patients' profile data and demographic, including age, gender, history, blood cell count (platelets, white blood cells, neutrophils, and lymphocytes), and other hematological parameters such as hematocrit (Hct) and hemoglobin (Hb) level, at the time of diagnosis and after the treatment, were extracted from the hospital archives department using predesigned checklists. Hematological inflammatory cellular indices, including PLR and NLR, were carefully calculated using the blood count of patients at the time of diagnosis and after the treatment. Similarly, other functional ratios, such as the combined cellular and noncellular index (i.e., HPR), were also calculated to be analyzed alongside other inflammatory indices. These findings were collected and analyzed for the control group under the same conditions.
2.4 Clinical findings and therapeutic approaches
All patients' clinical findings related to ITP (including petechiae, purpura, ecchymosis, bleeding, and fever) were evaluated and compared with hematological parameters. The medical procedures of ITP patients and therapeutic agents prescribed for them were extracted from the patients' profiles. These findings included prescription of Rituximab, Corton, tranexamic acid, prednisolone, intravenous immune globulin therapy, or surgical procedures such as splenectomy. Hematological inflammatory parameters, including NLR, PLR, and HPR, were also calculated and analyzed in two different periods, before and after the therapeutic approaches, to pursue their relationship with treatment methods and compare them with similar parameters after treatment. The patient's hospitalization period was scrutinized under the condition that it was the only cause of the hospitalization. The relationship between clinical symptoms in patients, treatment strategies, and the season of disease with the length of hospitalization was also explored.
3 STATISTICAL ANALYSIS
The data analysis was performed using SPSS software version 26. Analyses were performed based on two main groups: case and control. The case group was further subdivided into two subgroups: “before treatment (at the time of diagnosis)” and “after treatment.” Categorical variables (nominal or ordinal scale) were analyzed as the frequency with percentages, and to describe the continuous variables, mean ± standard deviation (mean ± SD) was reported. We assessed the normality of data using the Kolmogorov–Smirnov test. Associations between different indices with continuous scales were assessed through Pearson or Spearman correlation tests. The nonparametric Wilcoxon–Mann–Whitney and Kruskal–Wallis H tests were utilized to compare variables between the control group and ITP patients. All tests were two-sided. In all the analyses, the p value of <0.05 was considered statistically significant.
3.1 Assessment of sensitivity and specificity
Following the calculation of inflammatory indices (NLR, PLR, and HPR), the sensitivity and specificity of each index were evaluated by the receiver operating characteristic (ROC) curve. The cut-off and the area below the curve were analyzed separately to evaluate the sensitivity and specificity of different potential predictors of inflammatory indices. The ROC curve's results were analyzed through MedCalc 15.0. The optimal cut-off point values were obtained by maximizing Youden's index, so that for optimal point, sensitivity and specificity values were maximum. Following determining the predictive cut-off thresholds of hematological ratios, the sensitivity and specificity of each index were categorized into one of the following classes through the traditional academic point system; more than 90%: excellent (class I), 80%–90%: good (class II), 70-80%: acceptable (class III), 60%–70%: poor (class IV), 50%–60% or less than 50%: fail (class V).
4 RESULTS
Characteristics of ITP patients and the control group are summarized in Table 1. The median age of the patients with ITP at the diagnosis was 38.45 ± 17.96 years, and the mean age in the control group was 45.33 ± 15.31 years, which indicates a significantly lower age of ITP patients than the control group (p: 0.001). Patients included 130 females (68.4%) and 60 males (31.6%), and gender was homogeneous in both ITP patients and control groups (p: 0.51). The average duration of patients' hospitalization was 6.04 ± 5.39 days (range: 1–35). As shown in Tables 2 and 3, other single clinical symptoms have no significant relationship with the length of hospitalization except for the fever (the number of patients with fever: 23, p: 0.04). Although the results show that the number of patients with fever symptoms is less than those without this sign, the upward trend of the hospitalization period in symptomatic patients shows a direct and significant relationship between these patients and the increase in the hospitalization period. In other words, patients without fever were discharged from the hospital earlier. Among the clinical findings, ecchymosis and among the therapeutic agents, tranexamic acid, both had a statistically significant relationship with platelet count and HPR indices (Table 3). For example, the decrease in the platelet count (without ecchymosis: 26.938 ± 31.967 × 109/L; with ecchymosis: 18.197 ± 22.608 × 109/L) and the subsequent increase in HPR (from 17.00 to 22.93 ratio, respectively) were seen significantly in patients with ecchymosis, which can be considered in clinical examinations and practical applications. Also, the platelet count in patients who underwent splenectomy had increased significantly (p: 0.00), and the HPR index in these patients was significantly decreased (p: 0.00). The relationship between other clinical findings and treatment methods with inflammatory parameters can be seen in Table 3. Inflammatory cellular indices before and after treatment were also compared with each other, which are presented in Table 4. Among indices, NLR before treatment has a direct and significant relationship with the length of hospitalization, in such a way that reduction of NLR is associated with the reduction in the hospitalization of ITP patients (p: 0.001, correlation coefficient: 0.254).
| Characteristics | ITP patients | Control group | Effect size | p Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 38.45 ± 17.96 | 45.33 ± 15.31 | 0.214 | <0.001 |
| Gender | ||||
| Male, n (%) | 60 (31.6) | 35 (35.4) | 0.038 | 0.517 |
| Female, n (%) | 130 (68.4) | 64 (64.6) | ||
| Red blood cell × 1012/L | 4.41 ± 0.81 | 4.75 ± 0.60 | 0.173 | <0.01 |
| White blood cell × 109/L | 10.294 ± 6.693 | 6.389 ± 1.633 | 0.410 | 0.001 |
| Neutrophils × 109/L | 7.31 ± 6.11 | 3.77 ± 1.16 | 0.445 | <0.001 |
| Lymphocytes × 109/L | 1.97 ± 0.99 | 2.43 ± 0.70 | 0.321 | <0.001 |
| Platelet × 109/L | 23.44 ± 35.26 | 234.04 ± 55.88 | 0.818 | 0.001 |
| Hct, mean ± SD (%) | 37.62 ± 6.61 | 39.13 ± 3.87 | 0.074 | <0.05 |
| Hb, mean ± SD (g/dL) | 12.74 ± 3.14 | 12.99 ± 1.36 | 0.034 | 0.344 |
| HPR, mean ± SD | 19.17 ± 27.71 | 0.58 ± 0.15 | 0.797 | 0.001 |
| NLR, mean ± SD | 5.00 ± 5.99 | 1.62 ± 0.52 | 0.515 | 0.001 |
| PLR, mean ± SD | 14.25 ± 21.27 | 102.22 ± 35.19 | 0.789 | 0.001 |
- Note: Analysis in this table were performed using χ2 (for gender) and Wilcoxon–Mann–Whitney tests.
- Abbreviations: Hb, hemoglobin; Hct, hematocrit; HPR, hemoglobin-to-platelet ratio; ITP, immune thrombocytopenic purpura; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
| Clinical symptoms (at the time of diagnosis) | ITP patients, n (%) | |
|---|---|---|
| With symptom | Without symptom | |
| Bleeding | 73 (38.42) | 117 (61.58) |
| Purpura | 53 (27.9) | 137 (72.1) |
| Petechiae | 81 (42.63) | 109 (57.37) |
| Bruise | 8 (4.21) | 182 (95.79) |
| Ecchymosis | 76 (40) | 114 (60) |
| Fever | 23 (12.10) | 167 (87.9) |
| Therapeutic approaches | ITP patients, n (%) | |
|---|---|---|
| Treated with | Untreated with | |
| Corton | 148 (77.90) | 42 (22.10) |
| IVIg | 82 (43.16) | 108 (56.84) |
| Tranexamic-acid | 107 (56.31) | 83 (43.69) |
| Rituximab | 8 (4.21) | 182 (95.79) |
| Prednisolone | 86 (45.26) | 104 (54.74) |
| Splenectomy | 23 (12.10) | 167 (87.90) |
| Multiple symptoms (at the time of diagnosis) | ITP patients, n (%) | |
|---|---|---|
| Petechiae, purpura, and ecchymosis | 23 (12.10) | |
| Petechiae and purpura | 21 (11.05) | |
| Ecchymosis and petechiae | 18 (9.47) | |
| Epistaxis and ecchymosis | 6 (3.15) | |
| Epistaxis, bleeding, and petechiae | 8 (4.21) | |
| Bleeding and bruise | 2 (1.05) | |
| Multiple therapeutic | ||
|---|---|---|
| Prednisolone and IVIg | 9 (4.73) | |
| Prednisolone, IVIg, and corton | 29 (15.26) | |
| IVIG and corton | 40 (21.05) | |
| Seasons (at the time of diagnosis) | ||
|---|---|---|
| Spring | 45 (23.68) | |
| Summer | 62 (32.63) | |
| Fall | 46 (24.21) | |
| Winter | 37 (19.47) | |
| Hospitalization, mean ± SD (days) | 6.04 ± 5.39, range (1–35) | |
- Abbreviations: ITP, immune thrombocytopenic purpura; IVIg, intravenous immune globulin.
| Clinical symptoms, therapeutic methods, seasons | Effect size (p value)a | |||||
|---|---|---|---|---|---|---|
| Hospitalization | Plt | PLR | NLR | HPR | ||
| At the time of diagnosis | ||||||
| Clinical symptoms (at the time of diagnosis) | ||||||
| Purpura | 0.047 (0.51) | 0.340 (<0.001) | 0.295 (<0.001) | 0.015(0.84) | 0.356 (<0.001) | |
| Petechiae | 0.121 (0.09) | 0.433 (<0.001) | 0.401 (<0.001) | 0.065 (0.30) | 0.422 (<0.001) | |
| Bruise | 0.093 (0.19) | 0.048 (0.50) | 0.046 (0.53) | 0.122 (0.10) | 0.030 (0.68) | |
| Ecchymosis | 0.007 (0.91) | 0.141 (0.04) | 0.171 (0.02) | 0.223 (<0.001) | 0.174 (0.01) | |
| Bleeding | 0.045 (0.53) | 0.228 (<0.001) | 0.175 (0.01) | 0.022 (0.76) | 0.234 (<0.001) | |
| Fever | 0.149 (0.04) | 0.056 (0.43) | 0.010 (0.89) | 0.101 (0.18) | 0.005 (0.93) | |
| Hospitalization | Plt | PLR | NLR | HPR | ||
|---|---|---|---|---|---|---|
| After treatment | ||||||
| Therapeutic methods | ||||||
| Corton | 0.009 (0.90) | 0.022 (0.75) | 0.017 (0.056) | 0.377 (<0.001) | 0.017 (0.81) | |
| IVIg | 0.066 (0.35) | 0.086 (0.23) | 0.091 (0.057) | 0.179 (0.01) | 0.091 (0.20) | |
| Tranexamic acid | 0.091 (0.20) | 0.163 (0.02) | 0.203 (0.61) | 0.242 (<0.001) | 0.204 (<0.001) | |
| Rituximab | 0.113 (0.12) | 0.066 (0.35) | 0.082 (0.92) | 0.040 (0.60) | 0.082 (0.25) | |
| Prednisolone | 0.122 (0.09) | 0.005 (0.93) | 0.066 (0.89) | 0.001 (0.98) | 0.066 (0.36) | |
| Splenectomy | 0.113 (0.11) | 0.234 (<0.001) | 0.270 (0.32) | 0.026 (0.72) | 0.270 (<0.001) | |
| Hospitalization | Plt | PLR | NLR | HPR | |
|---|---|---|---|---|---|
| At the time of diagnosis | |||||
| Different seasons (at the time of diagnosis) | 0.011 (0.82) | 0.005 (0.57) | 0.001 (0.43) | 0.015 (0.93) | 0.004 (0.28) |
- Note: The analysis for clinical symptoms and therapeutic methods was performed based on the Wilcoxon–Mann–Whitney test. The analysis for seasons was performed based on the Kruskal–Wallis Test.
- Abbreviations: Hb, hemoglobin; Hct, hematocrit; HPR, hemoglobin-to-platelet ratio; ITP, immune thrombocytopenic purpura; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
- aData are reported as effect size (p value).
| Hospitalization/Indices | Before treatment | After treatment | Hospitalization | ||||
|---|---|---|---|---|---|---|---|
| PLR | NLR | HPR | PLR | NLR | HPR | ||
| After treatment | |||||||
| PLR | −0.292 (<0.001)a | −0.030 (0.692) | 0.292 (<0.001) | - | −0.061 (0.426) | 1.000 (<0.001) | −0.103 (0.159) |
| NLR | −0.216 (<0.01) | 0.281 (<0.001) | 0.294 (<0.001) | −0.061 (0.426) | - | −0.061 (0.431) | 0.013 (0.868) |
| HPR | −0.293 (<0.001) | −0.031 (0.687) | 0.293 (<0.001) | 1.000 (<0.001) | −0.061 (0.431) | - | −0.104 (0.155) |
| Before treatment | |||||||
| PLR | - | −0.277 (<0.001) | −0.951 (<0.001) | −0.292 (<0.001) | −0.216 (0.006) | −0.293 (<0.001) | −0.041 (0.582) |
| NLR | −0.277 (<0.001) | - | 0.389 (<0.001) | −0.030 (0.692) | 0.281 (<0.001) | −0.031 (0.687) | 0.254 (0.001) |
| HPR | −0.951 (<0.001) | 0.389 (<0.001) | - | 0.292 (<0.001) | 0.294 (<0.001) | 0.293 (<0.001) | 0.062 (0.407) |
| Hospitalization | −0.041 (0.582) | 0.254 (0.001) | 0.062 (0.407) | −0.103 (0.159) | 0.013 (0.868) | −0.104 (0.155) | - |
- The analysis in this table have been done using Spearman's ρ test.
- Abbreviations: HPR, hemoglobin-to-platelet ratio; ITP, immune thrombocytopenic purpura; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
- a Data are reported as effect size (p value).
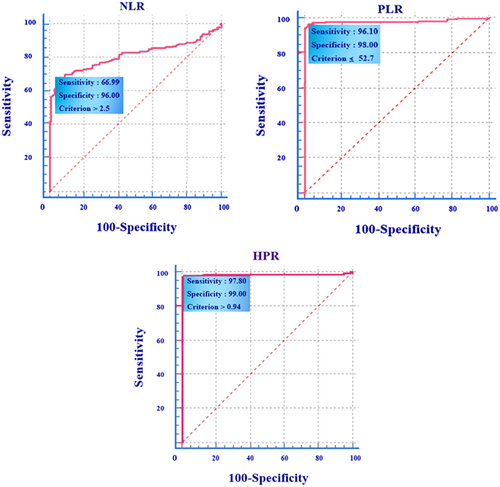
Clinical studies of patients showed that the prevalence of petechiae and ecchymosis was 42.63% and 40%, respectively, which was more common in ITP patients compared to other clinical symptoms. Analysis of the statistical results demonstrated significant differences in cell counts and other hematological markers between ITP patients and the control group. In the sense that white blood cell (WBC) and neutrophil counts were higher in ITP patients (WBC: p: 0.001, Neutrophil: p: 0.001), and conversely, platelet and lymphocyte counts were higher in the control group compared to ITP patients (platelets: p: 0.001, lymphocytes: p: 0.001). Red blood cells (RBCs) count and Hct were significantly lower in ITP patients (RBC: p: 0.002, Hct: P-value: 0.015); similarly, Hb in ITP patients was also lower compared to healthy individuals, but there was no significant relationship between it in the control and study groups (Hb: p: 0.34). The indices analysis between the two groups revealed that NLR was significantly increased in ITP patients (p: 0.001), but PLR was significantly reduced in ITP patients (with the mean platelet count of 23.44 ± 35.26 × 109/L) compared to the control group (with the mean platelet count of 234.04 ± 55.88 × 109/L). The HPR index also significantly increased in ITP patients (p: 0.001). Notably, there was a significant relationship between NLR and the duration of hospitalization, as mentioned above. The comparison of the area under the receiver operating characteristic curve indicated that the cutoff point to define the ITP risk was NLR > 2.5 (AUC: 0.809, 95% confidence interval [CI]: 0.757–0.854, sensitivity: 66.99%, specificity: of 96.00%), PLR ≤ 52.7 (AUC: 0.977, 95% CI: 0.952–0.991, p: 0.03, sensitivity: 96.1%, specificity: 98.0%), and HPR > 0.94 (AUC: 0.984, 95% CI: 0.961–0.995, sensitivity: 97.80%, specificity: of 99.00%) (Table 5 and Figure 1). Thus, all three indices NLR, PLR, and HPR were identified as valuable markers for better diagnosis of ITP patients.
| Indices | AUC | Cut-off (criterion) | Standard Errora | 95% CIb | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| NLR | 0.809 | >2.5 | 0.0262 | 0.757–0.854 | 66.99 | 96.00 |
| PLR | 0.977 | ≤52.7 | 0.0098 | 0.952–0.991 | 96.1 | 98.00 |
| HPR | 0.984 | >0.94 | 0.0090 | 0.961–0.995 | 97.80 | 99.00 |
- Abbreviations: AUC, area under the curve; CI, confidence interval; HPR, hemoglobin-to-platelet ratio; immune thrombocytopenic purpura; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
- a DeLong et al.21
- b Binomial exact.
5 DISCUSSION
ITP is a heterogeneous autoimmune condition that is brought on by a variety of factors, but the primary cause of ITP is the generation of abnormal autoantibodies developed against platelet membrane antigens.22 Autoantibody-bound platelets trigger macrophage and neutrophil-mediated phagocytosis via Fc receptors, especially in the spleen, which increases platelet apoptosis.23 The exact cause of the increase in antibody production is still unknown, although inflammatory cytokines and the direct contact of platelet, neutrophil, and monocyte subgroups with T helper/T regulatory cells have been suggested as potential explanations.24 The function of immunostimulants and inflammation in exacerbating ITP patients' conditions has received a lot of attention recently. Many molecular/cellular investigations have sought to discover effective diagnostic and therapeutic methods by examining various aspects of inflammation.25 The role of leukocyte counts and cellular indices, which can be a sign of the status of immune responses, has been investigated in autoimmune diseases, especially ITP.26 In this regard, the present study also evaluated the values of inflammatory parameters such as inflammatory cell count, PLR, NLR, and HPR in ITP patients compared to the control group. According to this study, the patients had fewer WBC and lymphocyte counts and higher neutrophil counts than the control group, which was aligned with the results of several previous studies. For instance, study conducted by Ahmed et al. shown that a low lymphocyte count at the time of diagnosis was a prognostic factor for persistence in children with ITP.27 Similarly, Deel et al. discovered that development of chronic ITP was associated with a low WBC count in the third month of the illness.28 On the other hand, other research has established that increased neutrophil counts and low levels of lymphocytes may be related to a worse prognosis in ITP patients. A study performed by Zahorec indicated that ITP patients who were severely critically ill, in addition to severe inflammation, had a significantly high level of neutrophil counts, while lymphocyte counts in these patients had reduced considerably.29 Considering the increase of neutrophils and their role in phagocytosis of antibody-sensitized platelets and the production of inflammatory cytokines (such as IL-17A) effective in the pathogenesis and development of ITP, the use of therapeutic strategies to block neutrophil FC receptors and suppress the production of neutrophil inflammatory cytokines in the future can be given more attention by researchers.30, 31
In recent years, cell indices' diagnostic and predictive value in inflammatory diseases has been identified. Nowadays, indices such as NLR and PLR, representing inflammatory status, are considered valuable cellular markers in many systemic inflammatory disorders. Tang et al.'s study on the acute exacerbation of chronic obstructive pulmonary disease patients announced that NLR and PLR levels were higher in nonsurvivors than survivors. Their findings also noted the relationship between NLR levels and C-reactive protein, a systemic inflammation marker in this inflammatory disease.32 The findings of Imtiaz et al. also reported that populations with hypertension or diabetes mellitus (as low-grade chronic inflammatory conditions) were likely to be at the highest levels of NLR compared to the patients who did not have hypertension or diabetes mellitus.33 Although the predictive value of NLR and PLR in various conditions, including myocardial infarction (MI), community-acquired pneumonia, malignant cancers, and acute pulmonary embolism (APE), has been proven, there is little information available on the connection between these indices and the clinical outcomes of ITP patients who are hospitalized.34-36 In our study, ITP patients were significantly associated with decreased PLR and increased levels of NLR and HPR. These indices' high sensitivity and specificity reinforced the diagnostic value of NLR, PLR, and HPR indices in ITP patients. Consistent with our results, the prior study in the field of ITP recurrence conducted by Song et al. has shown higher PLR associated with a decreased risk of ITP relapse. Their results suggested that PLR can be used as a valuable parameter to assess disease recurrence in ITP patients.37 Also, the significant correlation of increased NLR with extended patients' hospitalization period in our study highlights the importance of this index in predicting the course of the disease and hospital-related processes (p: 0.001, correlation coefficient: 0.254: 0.254). In other words, this marker can be a perspective of the active state of the immune system and its stimulating responses. In therapeutic processes, attention to this marker assists in regulating immune responses, using the correct dose of the pharmaceutical, predicting the duration of hospitalization, and prophylaxis procedures to reduce the recurrence of the disease. In line with our findings, Cavuş et al.'s study on APE patients showed a relationship between increased inflammatory markers and the severe course of the disease. Their finding has demonstrated the correlation of elevated NLR levels with increased 30-day mortality rates in hospitalized APE patients.38 Moreover, Horne et al.'s finding indicated the independent predictive value of the neutrophil, lymphocyte, and NLR for predicting the death of MI patients. Their research revealed that NLR compared to other parameters is a more powerful predictor of the mortality risk of patients with MI. On the contrary, some investigations violate the significant relationship between inflammatory cellular indices and autoimmune diseases. For example, the data concerning the role of NLR in some other autoimmune conditions, such as BD and psoriasis, are conflicting.39-41
In this examination, a significant relationship between clinical findings and treatment methods with hematological indices was observed. Our results depicted that ecchymosis, the second most common clinical symptom in ITP patients (after petechiae), has a significant relationship with platelet count, PLR, and HPR. In the present study, patients with ecchymosis were significantly associated with reduced platelet count, decreased PLR levels, and raised levels of HPR. The significant correlation of patients undergoing splenectomy with platelet count (increased) and HPR index (decreased) were other findings of this study concerning the therapeutic methods of ITP patients.
The use of indices can be effective for the treatment management of patients, either in terms of drug treatment or surgery such as splenectomy. Splenectomy is one of the treatments for ITP patients. Although this approach can be effective in increasing the platelet count and improving the bleeding of patients, however, it has a series of complications. An increase in platelet count can be accompanied by a decrease in HPR. On the other hand, splenectomy can lead to an increase in the number of leukocytes and finally an increase in the NLR parameter. The increase of these cells in patients can expose them to thrombosis. Therefore, the use of these indexes based on their availability, low cost, and noninvasiveness can be helpful in medical centers for the maintenance of patients and improvement of their clinical symptoms, as well as preventing the occurrence of thrombosis in them.42
In addition to the things mentioned earlier about the importance of each of the parameters used, it can be generally stated that based on the findings of this study, the investigated parameters, especially cellular and noncellular parameters such as white blood cell, neutrophil, and lymphocyte counts as well as PLR, NLR, and HPR, are useful parameters for better identification of ITP patients. In other words, each of these findings can be helpful in diagnostic and preventive processes based on clinical and laboratory interpretation. Although the role of these indices in autoimmune diseases and inflammatory diseases such as ITP cannot be determined with certainty, the importance of these indices in the processes of prognosis, diagnosis, and treatment of these diseases has been significant during the implementation of current knowledge.
There were some limitations in our study. First, investigating a single center in this study can affect its outcomes. Second, another limitation of this study is its limited statistical population, hence we advise additional investigation with a bigger target group.
6 CONCLUSION
This retrospective study demonstrated that NLR and HPR were significantly increased in ITP patients, and PLR was considerably reduced in these patients compared to healthy individuals. The incremental course of patients' hospitalization was directly related to NLR. The assessed sensitivity and specificity also revealed the diagnostic value of NLR, PLR, and HPR in better identification of ITP. Moreover, the relationship between clinical findings such as ecchymosis, as well as different therapeutic methods such as splenectomy, prescription of tranexamic acid with HPR, and platelet counts, was obtained in this study. Considering the significant relationship between inflammatory cellular indices and ITP patients, especially the relationship between NLR with the patients' hospitalization period, scientific investment and encouragement of more comprehensive research with the aim of optimizing prognostic and diagnostic methods seems logical and practical.
AUTHOR CONTRIBUTIONS
Mohammad Hossein Ahmadi: Conceptualization; formal analysis; project administration; supervision; writing—original draft; writing—review and editing. Mohsen Maleknia: Investigation; validation; writing—original draft; writing—review and editing. Reza Khoshbakht: Data curation; investigation; methodology; validation; writing—review and editing. Hadi Rezaeeyan: Conceptualization; formal analysis; project administration; supervision; writing—review and editing.
ACKNOWLEDGMENTS
We wish to thank all our colleagues in in Mashhad University of Medical Sciences.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Hadi Rezaeeyan affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Mashhad University of Medical Sciences. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from Hadi Rezaeeyan with the permission of Mashhad University of Medical Sciences.