Direct oral anticoagulants compared to warfarin in long-term management of cerebral venous thrombosis: A comprehensive meta-analysis
Abstract
Objectives
We compared the safety and efficacy of direct oral anticoagulants (DOACs) with those of warfarin in the long-term (≥6 months) treatment of cerebral venous thrombosis (CVT).
Methods
We searched electronic databases up to November 2023 to compare the use of DOACs and warfarin in CVT management. Modified Rankin scores (mRS), new intracranial hemorrhage, all-cause mortality, recurrence and nonrecanalisation events were used to assess outcome. RevMan v5.4 software and the Cochran-Mantel-Haenszel method were utilized to analyse data.
Results
A total of 25 studies involving 2301 patients were identified as having treated CVT with either DOACs or warfarin. Good long-term mRS scores 0–2 (risk ratio [RR] = 1.01, 95% CI = 0.98–1.03; p = 0.61), new intracranial hemorrhage (RR = 1.00, 95% CI = 0.48–2.08; p = 0.99), all-cause mortality (RR = 1.00, 95% CI = 0.50–1.98; p = 0.99), nonrecanalisation (RR = 0.95, 95% CI = 0.77–1.18; p = 0.65) and recurrence venous thrombosis events (RR = 0.63, 95% CI = 0.33–1.22; p = 0.17) were similar between the two treatment arms. Subgroup analysis found recurrence of venous thrombosis was lower in the rivaroxaban group compared to warfarin (2.2% vs. 8.5%, RR = 0.33, 95% CI = 0.11–0.98; p = 0.05).
Conclusion
DOACs and warfarin provide comparable long-term safety and efficacy profiles. DOACs may be preferred over warfarin due to their ease of clinical management.
1 INTRODUCTION
Cerebral venous thrombosis (CVT) is a rare form of stroke, accounting for approximately 0.5% of all strokes and is caused by a thrombus in the dural venous sinuses or cerebral veins.1 CVT requires rapid treatment to prevent significant neurological disability and death due to hemorrhagic complications and venous infarction.1, 2 All-cause mortality in CVT is found to diminish to 5%–15% with early diagnosis and prompt management, but morbidity rates remain high at 20%–30%.2, 3
Current guidelines suggest using systemic anticoagulant therapy with unfractionated or low molecular weight heparin followed by long-term warfarin to improve recanalisation and prevent a recurrence.3-5 Small numbers of randomized controlled trials and observational studies have suggested that direct oral anticoagulants (DOACs) are potentially a better alternative to warfarin, but most of these studies have been underpowered.6-30 However, DOACs have several benefits, including improved patient compliance, ease of clinical management, lower risk of bleeding and fewer interactions.2-5 Nevertheless, considering the rare occurrence of CVT, undertaking large-scale RCTs comparing the safety and efficacy of DOACs and warfarin is challenging. Meta-analysis potentially offers a method to overcome these challenges by increasing the sample size in a timely manner and reducing the effects of bias.
This comprehensive meta-analysis evaluated the safety and efficacy of DOACs compared to warfarin for long-term (≥6 months) anticoagulation management of CVT patients.
2 METHODS
The study was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines (PRISMA checklist supplied as a supplementary file).31 The primary study objective was to explore the efficacy of the DOACs compared to warfarin measured with good clinical outcomes based on the modified Rankin scale (mRS) score. Further, the secondary objectives were to compare the all-cause mortality, new onset of intracranial hemorrhage (ICH), nonrecanalisation, and recurrence of thrombosis events between the DOACs and warfarin arms.
3 SEARCH STRATEGY
We searched all published original research articles in English for the safety and efficacy of DOACs versus warfarin in long-term anticoagulation for cerebral venous thrombosis in MEDLINE, EMBASE, CINAHL via EBSCO host, and the Web of Science Collection up to November 2023. Boolean search operators “AND” and “OR” were used to link search terms. Search terms for the disease were “cerebral venous thrombosis” OR “Cerebro-venous thrombosis” OR “cerebral venous sinus thrombosis” OR “venous sinus thrombosis” OR “cortical vein thrombosis” OR “dural venous sinus thrombosis” OR “deep cerebral vein thrombosis” OR “intracranial venous sinus thrombosis” OR “CVT” OR “CVST” AND treatment “direct oral anticoagulant” OR “direct oral anticoagulation” OR “novel oral anticoagulant” OR “novel oral anticoagulation” OR “new oral anticoagulant” OR “DOACs” OR “NOAC” OR “rivaroxaban” OR “dabigatran” OR “apixaban” OR “edoxaban” OR “warfarin” OR “vitamin K antagonists” OR “factor Xa inhibitor” OR “direct thrombin inhibitor”. For an advanced literature search, MeSH (medical subject headings) and Emtree terms were also utilized to find the preceding search terms in PubMed and Embase databases. The literature search was expanded to cover additional references from thesis or dissertation repositories, preprint servers, and manual searching of reference lists from identified articles.
4 STUDY SELECTION CRITERIA
We included randomized and observational prospective and retrospective cohort studies of CVT patients in all age groups that compared the long-term anticoagulation effects of DOACs and warfarin.6-30 We excluded case reports, case series, review papers, and studies based on phenprocoumon anticoagulant.32, 33 The intervention was defined as direct oral anticoagulants (apixaban, rivaroxaban, edoxaban, or dabigatran), while the control group was warfarin treatment. We excluded phenprocoumon, another vitamin K antagonist, as its pharmacodynamics is distinct from warfarin and could be a potential source of outcome bias.
5 DATA VARIABLES
Detailed demographic information on studies was documented, including study type and population, treatment duration of DOACs and warfarin, age, gender, and prior medical history related to CVT. The safety outcomes were measured by all-cause mortality and new onset of intracranial hemorrhage (ICH) events between the DOACs and warfarin arms. In contrast, the efficacy of the DOACs and warfarin was observed using a good modified Rankin scale (mRS) score defined as 0–2, nonrecanalisation, and recurrence of thrombosis events.34-40 Nonrecanalisation events were defined as any case that failed to completely or partially resolve its thrombus following further imaging. Recurrence of venous thrombosis was defined as the occurrence of second events of CVT or any DVT despite long-term anticoagulation therapy. Although included studies evaluated anticoagulation exposure for 3 to ≥12 months, for our meta-analysis, we defined ≥6 months of anticoagulation with either warfarin or DOACs as a long-term treatment. Results reporting outcomes at fewer months were extrapolated to 6 months. Studies with no events in either DOACs or warfarin treatment arms were excluded from the individual analysis.
6 RISK OF BIAS ANALYSIS
Two independent investigators evaluated each study for risk of bias using the Cochrane Risk of Bias tool 2.0 (ROB 2) for randomized trials41 and the Risk of Bias in Nonrandomised Studies (ROBINS-I) tool for observational cohorts.42 Publication bias across individual studies was graphically assessed using the ROBINS-I tool for the appropriate outcomes. The ROB 2 and ROBINS-I tools assess bias in seven domains covering the randomization process, selection of participants and intervention, deviation from the intended intervention, lack of outcome data, outcome measures and selection of reported outcomes.
7 STATISTICAL ANALYSIS
This meta-analysis was performed using RevMan v5.4 software and the Cochran-Mantel-Haenszel statistical method with a random effects model. Pooled risk ratios (RRs) with 95% confidence intervals (CI) and heterogeneity, were calculated for each study. We explored all forms of clinical, methodological, and statistical heterogeneity when considering pooled results. We calculated the inter-study heterogeneity I2 index, and the statistical significance threshold was set at p ≤ 0.05.
8 RESULTS
8.1 Summary of search and screening
We identified a total of 2274 published articles, of which 25 studies (5 RCTs and 20 observational studies) involving 2301 CVT patients met our study inclusion criteria, illustrated in the PRISMA flow diagram (Figure 1). RCTs evaluated 370 patients (DOACs n = 200: warfarin n = 170)6-10 while observational cohort studies comprised 1931 patients (DOACs; n = 699: warfarin n = 1232).11-30 Most common reasons for the exclusion of articles were duplication (n = 115), studies not compatible with inclusion criteria (n = 1967), and review articles (n = 141). The clinical characteristics and outcomes of the patients from each study are shown in Supplementary Table 1. However, RCTs and observational cohorts with no events in either the DOACs or warfarin treatment arms were excluded from the forest plots.
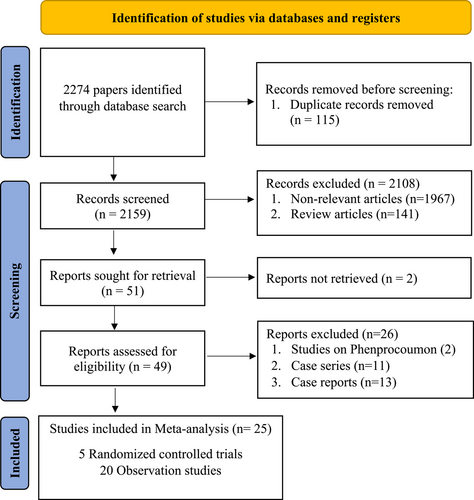
8.2 Disability and functional recovery
Approximately 90.6% of the 797 CVT patients from RCT and observational studies had a good clinical outcome.6-8, 11, 15-17, 19, 21-24, 26, 28 Analysis based on the eventful RCTs and observational cohorts found comparable functional recovery events between the DOACs and warfarin arms in observational and randomized control studies (93.8% vs 89.6%, I2 = 0%, p = 0.61; RR = 1.01, 95% CI = 0.98–1.03) (Figure 2).6-8, 11, 15-17, 19, 21-24, 26, 28 There was no evidence of interstudy heterogeneity (I2 = 0%, p = 0.76) or publication bias.
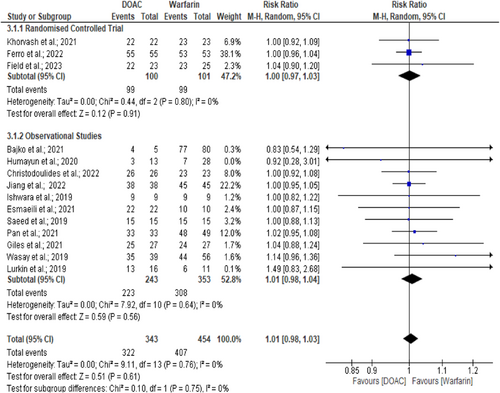
8.3 New onset intracranial hemorrhage
A total of 920 CVT patients (RCT and observational) were assessed for new ICH events. Only 2.8% of patients experienced new ICH events.6, 8-11, 13-27, 29, 30 However, new ICH events were similar across the DOACs and warfarin arms (2.6% vs. 2.9%, p = 0.99; RR = 1.00, 95% CI = 0.48–2.08, I2 = 0%) (Figure 3).6, 8, 10, 13, 14, 16, 18, 22, 24, 25, 29, 30 Further analysis excluding pediatric CVT study10 also found similar outcomes between DOACs and warfarin arms (RR = 1.10, 95% CI = 0.52–2.33, p = 0.81, I² = 0%).6, 8, 13, 14, 16, 18, 22, 24, 25, 29, 30 The symmetrical funnel plot suggests no publication bias. There was no evidence of heterogeneity (I2 = 0%, p = 0.80).
8.4 All-cause mortality
A total of 2142 CVT patients were evaluated for all-cause mortality. Overall mortality was 2.6%, with observational cohorts all-cause mortality being comparable (2.4% vs 2.7%, I2 = 0%, p = 0.99; RR = 1.00, 95% CI = 0.50–1.98) among the DOACs and warfarin group patients (Figure S1).12, 13, 16-18, 20, 22, 24 The funnel plot showed no evidence of publication bias. There was no evidence of heterogeneity (I2 = 0%, p = 0.60).
8.5 Nonrecanalisation events
The overall occurrence of nonrecanalisation events was 19.6% of 1371 CVT patients. The analysis yielded similar events between the DOACs and warfarin groups (19.4% vs. 19.7%, I2 = 0%, p = 0.65; RR = 0.95, 95% CI = 0.77–1.18) (Figure S2).7, 10-12, 14-16, 18, 19, 21, 23-25, 28, 29 Additionally, analysing only studies of adult CVT also observed identical findings between DOACs and warfarin groups (RR = 0.97, 95% CI = 0.77–1.21, p = 0.78, I² =0%).7, 11, 12, 14-16, 18, 19, 21, 23-25, 28, 29 The funnel plot was symmetrical suggesting no evidence of publication bias. There was no evidence of interstudy heterogeneity (I2 = 0%, p = 0.88).
8.6 Recurrence of venous thrombosis
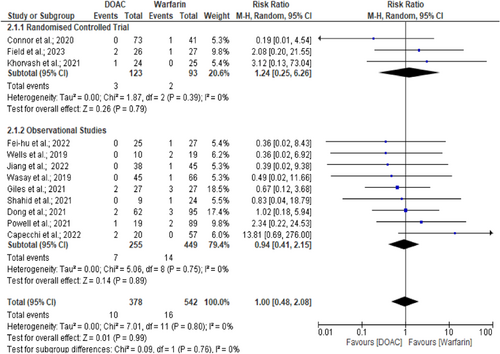
There was no consistent time frame for defining recurrence, with each study describing the event during its own follow-up period (Supplementary Table 1). However, insofar as can be analysed, the overall mean recurrence of venous thrombosis, either CVT or DVT events, was 2.9% among all 1436 patients (1.8% vs. 3.7% between the DOACs and warfarin arms, respectively) from both RCTs and observational cohorts.6, 7, 9-11, 13-17, 19-24, 26-30 Analysis of eventful RCTs and cohort studies found similar outcomes between the two treatment arms (3.5% vs. 7.5%, I2 = 0%, p = 0.17; RR = 0.63, 95% CI = 0.33–1.22) (Figure S3).6, 10, 11, 14-16, 23, 24, 27-30 Further analysis excluding Connor et al.10 also found similar events between DOACs and warfarin arms (RR = 0.69, 95% CI = 0.35–1.35, p = 0.28, I² = 0%). There was no interstudy heterogeneity (I2 = 0%, p = 0.83) or publication bias.
8.7 Subgroup analysis: Safety and efficacy of rivaroxaban versus warfarin
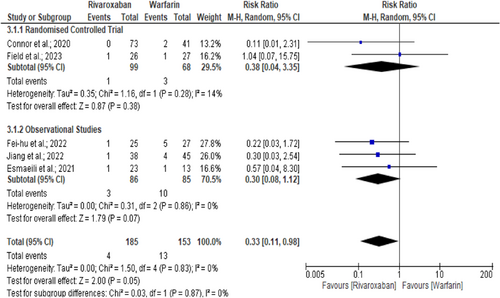
A subgroup analysis observes that recurrence of venous thrombosis, either CVT or DVT, was higher in the warfarin group than in rivaroxaban (8.5% vs. 2.2%, RR = 0.33, 95% CI = 0.11–0.98; I2 = 0%, p = 0.05) (Figure 4).6, 10, 15, 24, 30 However, disability and functional recovery (RR = 1.00, 95% CI = 0.97–1.04; I2 = 0%, p = 0.77), (Figure S4)6, 8, 15, 17, 19, 21, 24 new intracranial hemorrhage (RR = 0.82, 95% CI = 0.22–3.06; I2 = 0%, p = 0.77), (Figure S5)6, 8, 10, 24, 30 all-cause mortality (RR = 0.74, 95% CI = 0.15–3.59; I2 = 0%, p = 0.71), (Figure S6)6, 17, 24 and nonrecanalisation rate (RR = 0.91, 95% CI = 0.61–1.34; I2 = 0%, p = 0.63), (Figure S7)10, 15, 19, 21, 24 were comparable among rivaroxaban and warfarin arms. Further analysis excluding pediatric CVT study10 also observed similar recurrence (RR = 0.38, 95% CI = 0.12–1.24, p = 0.11, I² = 0%),6, 15, 24, 30 new intracranial hemorrhage (RR = 1.11, 95% CI = 0.26–4.70, p = 0.88, I² = 0%),6, 8, 24, 30 and nonrecanalisation (RR = 0.96, 95% CI = 0.59–1.56, p = 0.87, I² = 0%)15, 19, 21, 24 events between rivaroxaban and warfarin arms. There was no evidence of interstudy heterogeneity or publication bias for the recurrence of venous thrombosis (I2 = 0%, p = 0.83),6, 10, 15, 24, 30 good functional recovery (I2 = 0%, p = 0.98),6, 8, 15, 17, 19, 21, 24 new intracranial hemorrhage (I2 = 0%, p = 0.63),6, 8, 10, 24, 30 all-cause mortality (I2 = 0%, p = 0.70),6, 17, 24 and nonrecanalisation rate (I2 = 0%, p = 0.89)10, 15, 19, 21, 24 events between rivaroxaban and warfarin treatment arms.
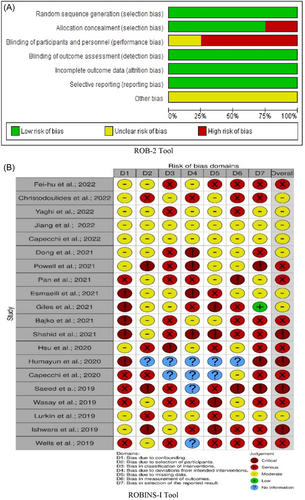
8.8 Quality and bias assessment
The risk of bias analysis for RCT6-10 and observational cohort studies11-30 is illustrated in Figure 5. One of the 5 RCTs had methodological concerns relating to randomization and allocation concealment,9 and four other studies6-8, 10 were judged to be at high risk of bias mostly due to randomization processes, deviations from intended interventions, and bias in the measurement of the outcome (Figure 5A). We judged 20 observational cohorts to have at least moderate risk bias; 8 papers14, 17, 19, 20, 22, 27, 29, 30 had serious, and four studies18, 21, 26, 28 had a critical risk of bias (Figure 5B). To ensure the robustness of our results in light of these identified biases, all analyses were repeated excluding studies14, 17-22, 26-30 with serious and critical risk of bias, but the overall results remained unchanged. Furthermore, to ensure the reliability of our findings, we also repeated all analyses, excluding the pediatric CVT study,10 but the outcome remained the same. Despite no significant heterogeneity documented, we utilized the random effects model in our study.
9 DISCUSSION
We have shown that the use of either DOACs or warfarin in treating CVT achieves comparable clinical outcomes in terms of efficacy, safety, all-cause mortality and recurrence of venous thrombosis. A subgroup analysis between rivaroxaban and warfarin found a lower recurrence of venous thrombosis in the rivaroxaban group than in warfarin, with similar other safety and efficacy profiles.
To ensure a comprehensive analysis, we exclude phenprocoumon-based observational studies32, 33 as their pharmacodynamics is distinct from warfarin, which would otherwise have lead to a potential risk of bias compared to previous meta-analyses.35-40 In addition, we present a detailed risk of bias assessment for included studies, which needed to be demonstrated in the previous papers35, 37, 39 and focus on warfarin anticoagulant specifically as that is the world's most commonly used anticoagulant.
Long-term oral anticoagulation in CVT aims to prevent thrombus propagation, reduce recurrence, and enhance endogenous fibrinolysis to expedite thrombus resolution and recanalisation.30, 44-46, 43 Current European and American guidelines recommend using warfarin following bridging with heparin as a long-standing anticoagulant in CVT treatment.3, 4 However, concerns about the use of warfarin relate to the need for regular follow-up with international normalized ratio (INR), dose adjustment, interactions with food and drugs and risk of bleeding.47 Conversely, the advantage of DOACs includes the rapid onset of action and not requiring regular blood testing or dose adjustments, added to few food and drug interactions.48-51 A recent RCT, RE-SPECT CVT7 and a multicentre observational cohort study12 also observed similar results to our findings between DOACs and warfarin regarding all-cause mortality, thrombus recanalisation, and recurrence events.
10 STRENGTHS AND LIMITATIONS
This is the first comprehensive meta-analysis involving a large CVT cohort of pediatric and adult subjects, yielding comparable safety and efficiency between DOACs and warfarin in long-term CVT management. Additionally, we find a lower recurrence of venous thromboembolism in rivaroxaban-treated arms than in warfarin with similar safety and efficacy profiles. However, as with any study, several limitations need to be noted. Our meta-analysis was based on English-language papers, and not all clinical outcomes were well-defined in every study, potentially limiting our overall findings.52 Nevertheless, considering the rarity of CVT occurrence and the absence of large and powered RCTs, our current comprehensive meta-analysis is, to the best of our knowledge, the largest conducted to-date. Although most studies6-8, 11, 12, 14, 15, 17-23, 25, 27-29 use anticoagulation therapy for at least 6 months, the diversity in the duration of anticoagulation use in some studies9, 10, 13, 16, 24, 26, 30 could lead to heterogeneity and bias. However, the absence of significant heterogeneity in our study results demonstrates that the risk of outcome bias is minimal. The length of treatment for anticoagulation varies from 3 to 12 months, but the most common treatment time is 6 months, hence our choice in deciding the timescale for analysis,53 consistent with ESO guidelines.3 For studies9, 10, 13, 16, 24, 26, 30 with shorter outcome timescales, we have extrapolated to 6 months, but we accept that we make this an assumption. The small sample size in RCTs, the lack of events in some studies, and the use of different DOACs may limit interpretation of our results. Although the etiology of childhood and adult CVT may be different, when we did the analysis for the two groups separately no significant difference to our overall conclusion was found.
11 CONCLUSION
Compared to warfarin, long-term (≥6 months) use of DOACs in managing CVT provides similar clinical outcomes regarding functional recovery, new ICH, all-cause mortality, recurrence and recanalisation events. However, DOACs may be preferred over warfarin in long-term CVT management because of ease of clinical management.
AUTHOR CONTRIBUTIONS
Redoy Ranjan: Conceptualization; data curation; formal analysis; methodology; resources; validation; visualization; writing—original draft; writing—review and editing. Gie Ken-Dror: Conceptualization; methodology; supervision; validation; writing—review and editing. Pankaj Sharma: Conceptualization; data curation; formal analysis; investigation; methodology; resources; supervision; validation; writing—review and editing.
ACKNOWLEDGMENTS
The authors received no specific grant or financial support for this article's research work, authorship, or publication.
CONFLICT OF INTEREST STATEMENT
Redoy Ranjan is an Editorial Board member of Health Science Reports but was excluded from all editorial decision-making related to the acceptance of this article for publication. The other authors declare no conflict of interest.
ETHICS STATEMENT
The authors have nothing to report.
TRANSPARENCY STATEMENT
The lead author Pankaj Sharma affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article and its supplementary materials.