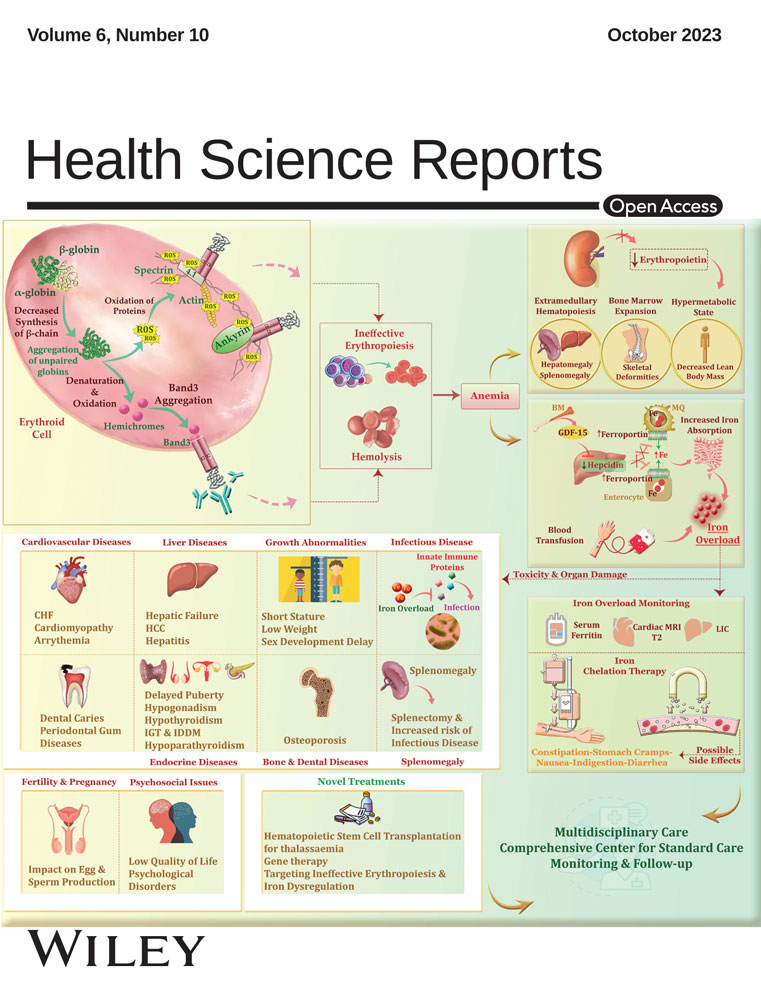
The association between periodontal disease and risk of adverse maternal or neonatal outcomes: A systematic review and meta-analysis of analytical observational studies
Abstract
Background and Aim
The aim of this meta-analysis was to find the association between periodontal disease (PD) and the risk of adverse pregnancy outcomes, including Pre-eclampsia (PE), premature rupture of the amniotic sac, gestational diabetes (GDM), or low birth weight (LBW) in pregnant women, which should be investigated in a systematic meta-analysis.
Methods
Studies that reported the association between PD and pregnancy or neonatal outcomes and were published from January 1990 to December 2022, were identified by an extensive search in PubMed (Medline), Scopus, Web of Sciences, and Medline (Elsevier). After retrieving the studies, the screening stage was performed based on their titles, abstracts, and full texts, and after selecting the final articles, their information was extracted and their quality was assessed using the Newcastle Ottawa Scale checklist.
Results
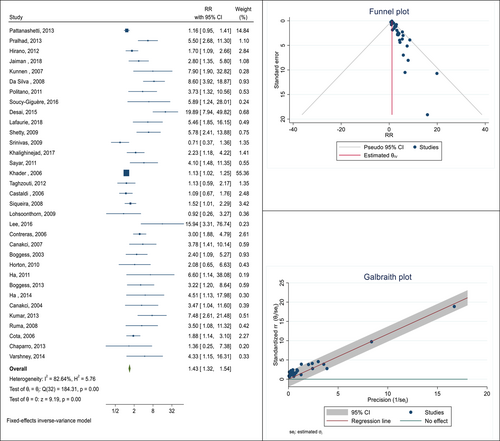
Pregnant women with PD had a 1.39 higher chance of developing GDM than those who did not have the infection (risk ratio [RR]: 1.39; 95% confidence interval [CI]: 1.21−1.61; I square: 49.67%; p: 0.03). Additionally, the pooled RR of LBW was 2.19, which indicates that pregnant women with PD had a 2.19-fold higher risk of LBW than pregnant women who do not have the infection (RR: 2.19; 95% CI: 1.82−2.64; I square: 0.00%; p: 0.65). The relationship between the risk of PE and the existence of PD was examined in 33 cohort and case-control studies for this meta-analysis. These results were combined, and the pooled RR was 1.43. This indicates that pregnant women with PD are 1.43 times more likely to experience PE than pregnant women without PD (RR: 1.43; 95% CI: 1.32−1.54; I square: 82.64%; p: 0.00).
Conclusion
According to the findings of the current meta-analysis, PD may contribute to a higher risk of poor maternal and newborn outcomes in pregnant women.
1 INTRODUCTION
Periodontal disease (PD) is the most common chronic infectious disease among humans.1 50−70% of the world's adult population has PD, depending on the definition of this disease and the geographical location.2 PD is a chronic and destructive inflammatory disease that affects the supporting structures of the tooth and is one of the chronic infectious diseases in humans, and over the past several years, the percentage of people getting this infection has increased significantly.3 In previous research related to PD, no uniform criteria have been determined to define this disease clearly. Epidemiological studies have considered a wide range of symptoms, such as gingivitis, probing depth, clinical attachment level, and alveolar bone loss, that are evaluated through radiography in a specific and nonuniform way to diagnose this disease. Between the numbers of thresholds used to define periodontal pockets as deep or pathological or for the number determined for the distance of the attachment surface and the state of the alveolar bone and to check whether the periodontal supporting tissue is destroyed or not, there are considerable differences.4 Based on the results of previous studies,5-7 the diagnostic criteria for determining the severity of periodontitis include severe periodontitis (two or more than two nonadjacent teeth with interproximal areas with clinical attachment loss (CAL) ≥ 6 mm, periodontal probing depth (PPD) ≥ 4 mm); moderate periodontitis (two or more than two nonadjacent teeth with interproximal areas with CAL ≥ 5 mm, PPD ≥ 4 mm); mild periodontitis (one tooth or more than two nonadjacent teeth with interproximal areas with CAL ≥ 4 mm, PPD ≥ 4 mm); and finally, people who are not from any of these groups are considered healthy.5 Measuring and defining this infection in key groups of society, such as pregnant women, is very important. The periodontal condition of pregnant mothers has been investigated in several studies to determine the relationship between periodontitis and pregnancy outcomes.8-11 Infection of pregnant mothers with periodontal bacteria and activation of immune-inflammatory mediators' cascades such as prostaglandin E2 (PGE2), IL-6, IL-1, and TNF-alpha may be related to adverse pregnancy outcomes. PD can act as a source of bacteria, and then inflammatory mediators are transferred through the oral cavity to the fetus-placental unit through blood circulation and ultimately cause adverse pregnancy outcomes in pregnant women.8-10 In the last two decades, many epidemiological studies have been conducted to investigate the relationship between PD during pregnancy and the occurrence of pregnancy outcomes such as Pre-eclampsia (PE), premature rupture of the amniotic sac, premature birth, or low birth weight (LBW), and different results have been reported in this field. According to past studies, PD in pregnant mothers may have a positive relationship with the risk of adverse pregnancy outcomes,12, 13 but this relationship needs to be investigated through more detailed studies. Considering that periodontitis is a relatively common disease among pregnant mothers, on the other hand, the occurrence of adverse pregnancy outcomes can impose a significant financial- and emotional burden on the family, health system, and society. It is necessary to evaluate the previous articles related to this issue more accurately and coherently and to report the results in a more up-to-date and complete manner. A meta-analysis study was conducted by Xiong et al.14 and published in 2006. Due to the passage of a long period of time after the publication of this study and the failure to consider the structure and principles of the methodology, such as the failure to perform subgroup analyses based on the type of studies and different definitions of PD, updating this study is of great importance. These results can help improve prevention and care programs before, after, and during pregnancy. Also, these results can help update clinical guidelines. Considering that several clinical studies with different work methods and conflicting results investigated the relationship between PD and pregnancy outcomes, in this study the researchers decided that the relationship between PD and the risk of adverse pregnancy outcomes, including PE, premature rupture of the amniotic sac, gestational diabetes (GDM), or LBW in pregnant women, should be investigated in a systematic meta-analysis.
2 METHODS AND MATERIALS
This systematic review and meta-analysis study was written and reported based on the structure of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).15 The desired structure for this meta-analysis included the steps of search strategy, screening of articles, final selection of articles, extraction of information, qualitative assessment, and data analysis.
2.1 Search strategy and screening
- 1.
The cohort studies, whose main goal was to determine the relationship between PD and the occurrence of maternal outcomes in pregnant women. A group of pregnant women with PD and a group without PD are selected, then tracked until the end of pregnancy. And finally, the desired maternal and neonatal outcomes (pre-eclampsia, premature rupture of the amniotic sac, GDM, or low weight at birth) were measured and reported in two groups.
- 2.
Case-control studies that selected two groups of women with adverse pregnancy outcomes (including pre-eclampsia, premature rupture of the amniotic sac, GDM, or LBW) and healthy women and determined the frequency of periodontal infection in these two groups had paid.
- 3.
There was no specific limit for the studied population, and every pregnant woman, whether healthy or suffering from any other underlying disease that was investigated and reported in the studies, was considered to perform subgroup analyses.
Other studies such as review studies or systematic reviews, cross-sectional, case or case reports, clinical, laboratory, animal trials, letters to the editor, or short communication were excluded from the study. Also, non-English and inaccessible articles were excluded from the study. All stages of screening articles were done by two authors independently. If there was any dispute, the dispute was resolved by a third person. To carry out the search strategy correctly and accurately, the researchers in the present meta-analysis performed a manual search based on the reviews of all the references in the final selected studies and a Google Scholar search based on the relevant keywords.
2.2 Data extraction
To extract information, first, the opinions of all the authors about the items and variables were collected from the selected articles. Then a checklist was designed, which included the name of the author of the article, the year of publication, the country of the study, the type of study, the age of the people, the population under investigation, the sample size, the desired effect size (risk ratio in cohort studies and chance ratio in case-control studies), and finally, the definition of PD. All data extraction steps were done by two authors independently. If there was any dispute, the dispute was resolved with a third party.
2.3 Quality evaluation of articles
Two of the authors conducted a qualitative evaluation of the studies based on the Newcastle-Ottawa Quality Assessment Scale (NOS). A checklist was designed to evaluate the quality of observational studies.16 This tool examines each research question with eight items in three groups. Including how to select study samples, how to compare and analyze study groups, and how to measure and analyze the desired outcome. Each of these items is given a score of one if it is observed in the studies, and the maximum score for each study is 9-points. In cases of discrepancies in the score assigned to the published articles, the discussion method and the third researcher were applied to reach an agreement.
2.4 Data analysis
To calculate the association of cumulative relative risk (RR) with the 95% confidence interval (CI), and the meta set command was used, considering the logarithm and logarithm standard deviation of the RR. Heterogeneity was assessed between studies using the I2 and Q Cochrane tests. According to Cochrane's reported criteria, 0−25% indicate no heterogeneity, 25−50% indicate low heterogeneity, 50−75% indicate high but acceptable heterogeneity, and 75−100% indicate high and unacceptable heterogeneity.17, 18 The Egger test was used to evaluate the publication bias. Statistical analysis was performed using STATA 16.0, and a p < 0.05 was considered.
3 RESULTS
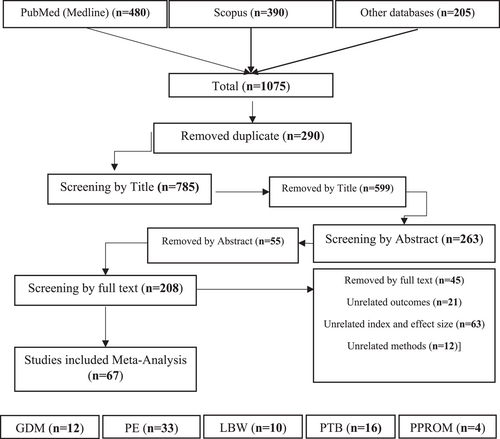
In this meta-analysis, a total of 1075 related articles were retrieved from the target databases. Of these, 290 articles were duplicates, and after removing them, 785 articles entered the screening stage based on the title. After screening based on the title, 263 articles remained and entered the stage of screening based on the abstract, and finally, after this stage and the removal of 55 articles, 208 articles were screened based on the full text, and 67 articles were finally selected for meta-analysis. 12 articles were related to investigating the relationship between PD and the occurrence of GDM; 33 articles were related to PE; 10 articles were related to LBW; 16 articles were related to preterm birth; and 4 articles were related to PROM (Figure 1 and Table 1).
| Authors | Country | Years | TOS | Exposer detect | TO | SS | Age | BMI | Effect size | Lower | Upper |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kumar et al. | India | 2018 | CO | ≥1 site with PD ≥ 4 mm, CAL ≥ 3 mm, BOP | GDM | 584 | 20 | 2.85 | 1.47 | 5.53 | |
| Novak et al. | United States | 2006 | CC | ≥1 site with PD ≥ 4 mm, CAL ≥ 2 mm, BOP | GDM | 4070 | 1.73 | 0.87 | 3.11 | ||
| Dasanayake et al. | United States | 2008 | CC | ≥1 site with PD > 3 mm | GDM | 200 | 27.65 | 28 | 1.70 | 0.97 | 2.99 |
| Xiong et al. | United States | 2009 | CC | ≥1 site with PD ≥ 4 mm or CAL ≥ 4 mm | GDM | 159 | 30.75 | 26.4 | 2.52 | 1.19 | 5.33 |
| Chokwiriyachit et al. | Thailand | 2013 | CC | ≥1 site with both PD ≥ 5 mm and CAL ≥ 2 mm | GDM | 100 | 33.2 | 23.95 | 2.85 | 1.23 | 6.60 |
| Esteves Lima et al. | Brazil | 2013 | CC | of ≥4 teeth having ≥1 sites with PD ≥ 4 mm and CAL ≥ 3 mm, BOP | GDM | 360 | 27.2 | 0.74 | 0.4 | 1.38 | |
| Bullon et al. | Spain | 2014 | CC | of ≥2 interproximal sites with CAL ≥ 6 mm (not on the same tooth) and ≥1 interproximal site with PD ≥ 5 mm | GDM | 188 | 3.09 | 0.88 | 10.89 | ||
| Mishra et al. | India | 2014 | CC | Any site with PD ≥ 4 mm and clinical AL ≥ 3 mm | GDM | 90 | 26 | 0.48 | 0.06 | 3.51 | |
| Mishra et al. | India | 2014 | CC | Any site with PD ≥ 4 mm and clinical AL ≥ 3 mm | GDM | 90 | 26 | 0.54 | 0.08 | 3.79 | |
| Zhang et al. | china | 2021 | CO | as ≥2 interproximal sites with AL ≥ 3 mm and ≥2 interproximal sites with PD ≥ 4 mm (not on same tooth) or one site with PD ≥ 5 mm | GDM | 69 | 7 | 28 | 7 | ||
| Tansriratanawong et al. | Thailand | 2021 | CC | interdental CAL ≥ 2 mm, or buccal or oral CAL ≥ 3 mm. is detectable at ≥2 30 teeth | GDM | 128 | 32.18 | 24.39 | 2.28 | 1.12 | 4.64 |
| Habib et al. | Saudi Arabia | 2009 | CC | CPITN | GDM | 200 | 31.75 | 30.25 | 1.44 | 0.79 | 2.60 |
| Chaparro et al. | Chile | 2018 | CO | interdental CAL at ≥2 nonadjacent teeth, or buccal or oral CAL ≥ 3 mm with pocketing >3 mm is detectable at ≥2 teeth, gingivitis | GDM | 212 | 29 | 28.3 | 1.20 | 0.99 | 1.45 |
| Louro et al. | Brazil | 2001 | CC | the areas in a tooth in which AL exceeded 1 mm | LBW | 26 | 21 | 7.2 | 0.4 | 125.4 | |
| Kumar et al. | India | 2013 | CO | CAL and PD 4 ≥ mm in one or more sites | LBW | 340 | 22 | 3.03 | 1.53 | 5.97 | |
| Jacob and Nath et al. | India | 2014 | CC | pocket PD of ≥4 mm in at least one site | LBW | 340 | 23.74 | 2.85 | 1.62 | 5.5 | |
| Lafaurie, et al. | Colombia | 2018 | CC | classified according to the presence of periodontal pockets | LBW | 535 | 2.52 | 1.36 | 4.70 | ||
| Novák et al. | Hungary | 2020 | CC | probing depth (PD) ≥ 4 mm and bleeding on probing (BOP) ≥ 50% | LBW | 242 | 29.3 | 2.28 | 1.06 | 4.89 | |
| Castaldi et al. | Argentina | 2006 | CO | Severe periodontal disease: ≥4 teeth with ≥1 sites with CAL ≥ 3 mm | LBW | 1562 | 1.05 | 0.74 | 1.47 | ||
| Figueiredo MGOP et al. | Brazil | 2019 | CO | NR | LBW | 138 | 2.93 | 0.46 | 2.36 | ||
| Figueiredo MGOP et al. | Brazil | 2019 | CO | NR | LBW | 138 | 4.81 | 0.68 | 33.92 | ||
| Boggess et al. | United States | 2003 | CO | PD ≥ 4 and, CAL ≥ 3 mm | PE | 802 | 2.1 | 1.0 | 4.4 | ||
| Boggess et al. | United States | 2003 | CO | PD ≥ 4 and, CAL ≥ 3 mm | PE | 802 | 2.4 | 1.1 | 5.3 | ||
| Canakci et al. | Turkey | 2004 | CC | ≥4 teeth with ≥1 sites with PD ≥ 4 mm and BOP + and CAL ≥ 3 mm | PE | 82 | 25 | 3.47 | 1.07 | 11.95 | |
| Contreras et al. | Colombia | 2006 | CC | ≥4 sites showed ≥4 mm), CAL ≥ 4 mm, and BOP | PE | 373 | 24.7 | 3.0 | 1.91 | 4.87 | |
| Cota et al. | Brazil | 2006 | CC | ≥4 teeth with ≥1 sites with a PD ≥ 4 mm and CAL ≥ 3 mm at the same site | PE | 588 | 1.88 | 1.1 | 3.0 | ||
| Kunnen et al. | Netherlands | 2007 | CC | PD ≥ 4 mm and BOP + , Sev periodontal disease: PD ≥ 4 mm, ≥15 tooth sites | PE | 52 | 30.6 | 26.05 | 7.9 | 1.9 | 32.8 |
| Siqueira et al. | Brazil | 2008 | CC | PD ≥ 4 mm and CAL ≥ 3 mm at the same site in ≥4 teeth | PE | 1206 | 1.52 | 1.01 | 2.29 | ||
| Shetty et al. | India | 2009 | CC | CAL of ≥3 mm and a PD of ≥4 mm | PE | 130 | 26.8 | 5.78 | 2.41 | 13.89 | |
| Politano et al. | Brazil | 2011 | CC | two or more sites PD ≥ 4 mm, CAL ≥ 4 mm | PE | 116 | 26.64 | 3.73 | 1.32 | 10.58 | |
| Sayar et al. | Iran | 2011 | CC | Mild: CAL ≤ 2 mm Moderate to Severe: CAL ≥ 3 mm | PE | 210 | 4.1 | 1.5 | 11.5 | ||
| Taghzouti et al. | Canada | 2012 | CC | as ≥ 4 site PD ≥ 5 mm and CAL ≥ 3 mm at the same sites | PE | 337 | 1.13 | 0.59 | 2.17 | ||
| Chaparro et al. | Chile | 2013 | CC | PD ≥ 4 mm and CAL ≥ 3 mm at the same site of ≥4 teeth, BOP | PE | 54 | 26.5 | 26.42 | 1.36 | 0.25 | 7.37 |
| Hirano, et al | Japan | 2012 | CC | having over 60% of sites with CAL ≥ 3 mm | PE | 127 | 1.7 | 1.1 | 2.7 | ||
| Kumar et al. | India | 2013 | CO | CAL and PD 4 ≥ mm in one or more sites | PE | 340 | 22 | 7.48 | 2.72 | 22.42 | |
| Da Silva et al. | Brazil | 2008 | CC | ≥4 teeth with ≥ 1 sites with a PD ≥ 4 mm and AL ≥ 3 mm in the same site | PE | 574 | 8.60 | 3.92 | 18.88 | ||
| Pralhad et al. | India | 2013 | CC | PD > 4 mm; and CAL > 3 mm | PE | 200 | 5.5 | 2.7 | 11.4 | ||
| Ha et al. | Korea | 2014 | CO | CAL ≥ 4.0 mm on two or more sites on different teeth | PE | 283 | 32.93 | 4.51 | 1.13 | 17.96 | |
| Varshney and Gautam et al. | India | 2014 | CC | PD ≥ 4 mm and CAL ≥ 3 mm at the same site on at least 4 different non-neighboring teeth | PE | 40 | 4.33 | 1.15 | 16.32 | ||
| Desai et al. | India | 2015 | CO | PD ≥ 4 mm and CAL ≥ 3 mm at the same site in at least four teeth | PE | 1240 | 19.89 | 7.80 | 48.94 | ||
| Soucy-Giguère et al. | Canada | 2016 | CO | probing depths ≥4 mm and ≥10% bleeding on probing | PE | 248 | 35 | 23 | 5.89 | 1.24 | 28.05 |
| Lee et al. | Korea | 2016 | CO | two or more interproximal sites with CAL ≥ 4 mm that were not on the same tooth | PE | 328 | 33 | 15.94 | 3.31 | 76.71 | |
| Khalighi-nejad et al. | United States | 2017 | CC | ≥4 teeth with 1 or more sites with PD ≥ 4 mm and with CAL ≥ 3 mm at the same site | PE | 100 | 25 | 2.23 | 1.92 | 6.88 | |
| Lafaurie et al. | Colombia | 2018 | CC | (code 3: periodontal pockets of 4-5 mm or code 4: periodontal pockets > 5 mm) | PE | 380 | 5.46 | 1.84 | 16.1 | ||
| Jaiman et al. | India | 2018 | CC | According to the criteria of Löe and Silness | PE | 30 | 14 | 1 | 5 | ||
| Ruma et al. | United States | 2008 | CO | 1 or more tooth sites PD ≥ 4 mm or >3 mm that bled on probing | PE | 775 | 27.5 | 3.5 | 1.1 | 11.5 | |
| Srinivas et al. | United States | 2009 | CO | CAL ≥ to 3 mm on 3 or more teeth | PE | 786 | 23.9 | 0.71 | 0.37 | 1.36 | |
| Boggess et al. | United States | 2013 | CO | Had a history of treatment ever for gum disease In the 6 months before pregnancy | PE | 599 | 29 | 3.22 | 1.20 | 8.64 | |
| Lohsoonthorn et al. | Thailand | 2009 | CC | Severe: ≥2 nonadjacent teeth with interproximal ≥6 mm CAL and ≥4 mm PD | PE | 300 | 0.92 | 0.26 | 3.28 | ||
| Horton et al. | United States | 2010 | CO | severe: ≥15 sites demonstrated a probing depth ≥4 mm | PE | 791 | 27.25 | 2.08 | 0.65 | 6.60 | |
| Pattanashetti et al. | India | 2013 | CC | Moderate/Severe: 15 or more sites with periodontal probing ≥4 mm | PE | 200 | 72 | 28 | 62 | ||
| Castaldi et al. | Argentina | 2006 | CO | Severe periodontal disease: ≥4 teeth with ≥1 sites with CAL ≥ 3 mm | PE | 1562 | 0.99 | 0.70 | 1.40 | ||
| Ha et al. | Korea | 2011 | CC | Generalized P:CAL ≥ 3.5 mm on ≥4 sites not on the same tooth localized on 2 or 3 sites | PE | 64 | 32.8 | 6.60 | 1.25 | 41.61 | |
| Khader et al. | Jordan | 2006 | CC | PD ≥ 3 or≥4 mm, percentages of sites with CAL ≥ 3 mm | PE | 345 | 29.49 | 1.13 | 1.02 | 1.25 | |
| Offenbacher et al. | United States | 1996 | CC | CAL ≥ 2, 3, or 4 mm | PLB | 124 | 23.5 | 7.9 | 1.52 | 41.4 | |
| Savitha et al. | India | 2022 | CO | pocket depths of ≥5 mm | PLB | 130 | 1.90 | 1.48 | 2.45 | ||
| Vergnes et al. | France | 2011 | CC | PD ≥ 4 mm and CAL ≥ 3 mm on the same site | PROM | 2201 | 1.14 | 0.91 | 1.42 | ||
| Lafaurie et al | Colombia | 2018 | CC | classified according to the presence of periodontal pockets | PROM | 535 | 2.04 | 1.17 | 3.56 | ||
| Castaldi, et al. | Argentina | 2006 | CO | Severe periodontal disease: ≥4 teeth with ≥1 sites with CAL ≥ 3 mm | PROM | 1562 | 1.06 | 0.74 | 1.50 | ||
| Figueiredo MGOP et al. | Brazil | 2019 | CO | NR | PROM | 138 | 5.59 | 1.36 | 22.92 | ||
| Figueiredo MGOP et al. | Brazil | 2019 | CO | NR | PROM | 138 | 2.62 | 0.96 | 7.11 | ||
| Radnai et al. | Hungary | 2004 | CC | PD ≥ 4, BOP ≥ 50% | PTB | 85 | 28.3 | 5.46 | 1.72 | 17.32 | |
| Nabet et al. | France | 2010 | CC | PD ≥ 4 mm and CAL ≥ 3 mm (Armitage 2004) | PTB | 2202 | 2.46 | 1.58 | 3.83 | ||
| Srinivas et al. | United States | 2009 | CO | CAL ≥ to 3 mm on 3 or more teeth | PTB | 786 | 23.9 | 0.77 | 0.49 | 1.21 | |
| Vergnes et al. | France | 2011 | CC | PD ≥ 4 mm and CAL ≥ 3 mm on the same site | PTB | 2201 | 1.10 | 0.91 | 1.32 | ||
| Agueda et al. | Spain | 2008 | CO | ≥4 teeth with ≥1 site with PPD ≥ 4 mm and CAL ≥ 3 mm | PTB | 1296 | 29.6 | 1.77 | 1.08 | 2.88 | |
| Kumar, et al | India | 2013 | CO | CAL and PD 4 ≥ mm in one or more sites | PTB | 340 | 22 | 2.72 | 1.30 | 5.68 | |
| da Mota Krüger et al. | Brazil | 2018 | CC | PD ≥ 4 mm and CAL ≥ 3 mm in the same site | PTB | 444 | 0.94 | 0.61 | 1.46 | ||
| de Oliveira et al. | Brazil | 2020 | CO | mild to severe periodontitis according to CDC | PTB | 585 | 28 | 1.20 | 0.88 | 1.64 | |
| Uwambaye et al. | Rwanda | 2021 | CC | PD > 3 mm, CAL ≥ 3 mm | PTB | 555 | 27.35 | 6.36 | 3.9 | 10.4 | |
| Micu et al. | Romania | 2020 | CC | PD ≥ 4 mm and with clinical CAL ≥ 3 mm at the same site. | PTB | 194 | 29.1 | 22.9 | 2.26 | 1.06 | 4.82 |
| Lafaurie et al. | Colombia | 2018 | CC | classified according to the presence of periodontal pockets | PTB | 535 | 2.04 | 1.10 | 3.64 | ||
| Novák et al. | Hungary | 2020 | CC | probing depth (PD) ≥ 4 mm and bleeding on probing (BOP) ≥ 50% | PTB | 242 | 29.3 | 2.02 | 1.23 | 4.22 | |
| Marquez-Corona et al. | Mexico | 2019 | CC | The severity of PD according to CDC | PTB | 111 | 24 | 26 | 10 | 6 | |
| Erchick et al. | Nepal | 2020 | CO | Gingival inflammation was defined as BOP ≥ 10% | PTB | 1394 | 23 | 1.37 | 0.81 | 2.32 | |
| Chan et al. | Taiwan | 2010 | CO | periodontal pathogens measured with the benzoyl-DL-arginine-naphthylamide (BANA) test. | PTB | 268 | 5.89 | 1.5 | 31.6 | ||
| Lee et al. | Taiwan | 2022 | CO | PD subjects diagnosed with gingivitis, acute or chronic periodontitis were coded as 523.0–523.5 in the ICD-9-CM | PTB | 1762 | 1.09 | 1.07 | 1.11 | ||
| Gallagher-Cobos et al. | Spain | 2022 | CO | CAL ≥ 3 mm with PD > 3 mm detectable in at least 2 teeth | PTB/LBW | 98 | 23.43 | 23.11 | 1.43 | 0.53 | 3.88 |
| Canakci et al. | Turkey | 2007 | CC | BOP and ≥4 mm PD, Sev periodontal disease: PD ≥ 4 mm, ≥15 tooth sites | PE | 59 | 24 | 3.78 | 1.77 | 12.74 | |
| Boggess et al. | United States | 2005 | CO | Moderate/severe PD:15 or more tooth sites with pocket depths >4 mm | SGA | 1017 | 28 | 2.3 | 1.1 | 4.5 |
- Abbreviations: CC, case-control; CO, cohort; GDM, gestational diabetes mellitus; LBW, low birth weight; PE, pre-eclampsia; PTB, Preterm Birth; PROM, premature rupture of membranes.
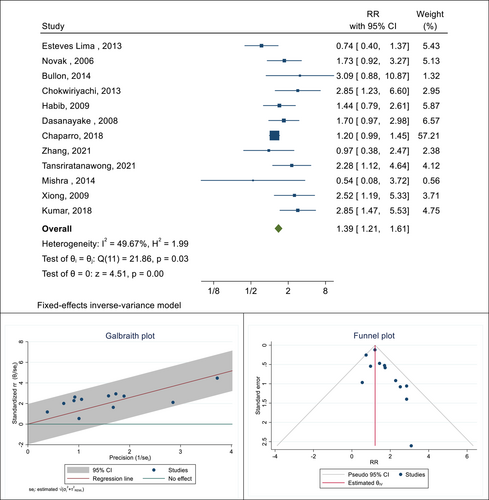
In this meta-analysis, there were 12 cohort and case-control studies aimed at determining the association between the presence of PD and the occurrence of GDM in pregnant women. The total sample size in these studies was 6636, of which 1518 had PD and 5118 had no PD. The highest and lowest reported correlations were 3.09 (RR: 3.09; 95% CI: 0.87−10.87) and 0.54 (RR: 0.54; 95% CI: 0.08−3.72), respectively, which belonged to the study of Bullon et al. and Mishra et al. After combining these results, the pooled risk ratio was 1.39. This means that the risk of GDM in pregnant women with PD was 1.39 times that of pregnant women without this infection (RR: 1.39; 95% CI: 1.21−1.61; I square: 49.67%; p: 0.03) (Figure 2). The results of publication bias using a funnel plot and Galbraith diagram to check heterogeneity are reported in Figure 2. The Eggers test was also performed to evaluate publication bias, and the results showed that there was no distortion in these findings (B: 1.02; SE: 0.55; p: 0.229).
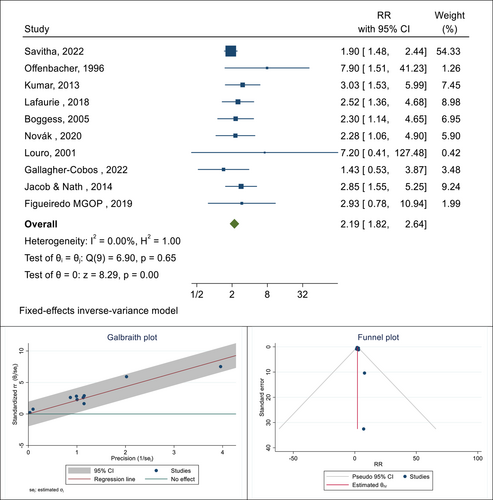
Also, there were 16 cohort and case-control studies related to determining the association between the presence of PD and the occurrence of LBW in pregnant women. The total sample size in these studies was 3575 people, and respectively, the lowest and highest reported associations were 1.43 (RR: 1.43; % 95 CI: 0.53−3.87) and 7.90 (RR: 7.90; % 95 CI: 1.51−41.23), which belonged to the study of Gallagher-Cobos, 2022 and Offenbacher, 1996, respectively. After combining these results, the pooled RR was equal to 2.19, which means that the risk of LBW in pregnant women with PD is 2.19 times that of pregnant women without this infection (RR: 2.19; 95% CI: 1.82−2.64; I square: 0.00%; p: 0.65) (Figure 3). The results of publication bias using a funnel plot and Galbraith diagram to check heterogeneity are reported in Figure 3. The Eggers test was also performed to evaluate publication bias, and the results showed that there was no bias in these findings (B: 1.49; SE: 0.29; p: 0.887).
In the next step of this meta-analysis, the association between the presence of PD and the occurrence of PE was studied. In this relationship, 33 cohort and case-control studies were selected. The total sample size was equal to 12586 pregnant women, of which 2153 had PE. The lowest and highest reported associations were 0.71 (RR: 0.71; 95% CI: 0.37−1.36) and 19.89 (RR: 19.89; 95% CI: 7.94−49.82), respectively, which belong to the studies of Srinivas19 and Desai.20 After combining these results, the pooled RR was 1.43. This means that the risk of PE in pregnant women with PD is 1.43 times that of pregnant women without this infection (RR: 1.43; 95% CI: 1.32−1.54; I square: 82.64%; p: 0.00) (Figure 4). The Eggers test was also conducted to evaluate publication bias, and the results showed that there was no bias in these findings (B: 1.99; SE: 0.46; p: 0.340).
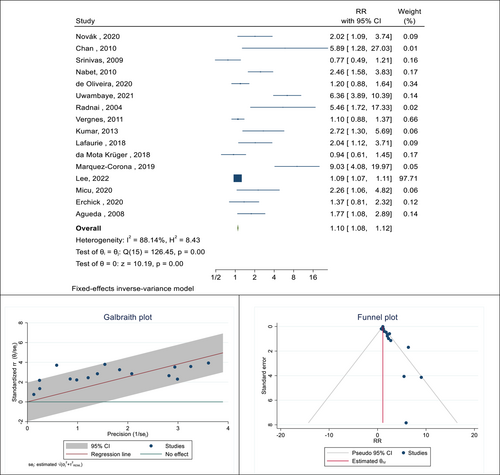
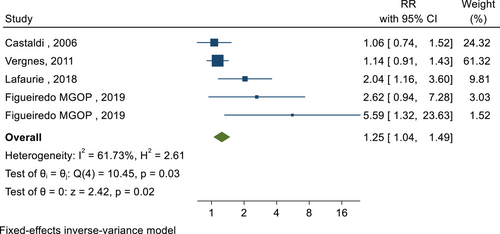
In the next step of this meta-analysis, the association between the presence of PD and the occurrence of premature birth was investigated. In this relationship, 33 cohort and case-control studies were selected. The total sample size was 13098 pregnant women. The lowest and highest reported associations were 0.77 (RR: 0.77; 95% CI: 1.23−0.49) and 9.03 (RR: 9.03; 95% CI: 19.97−4.08), respectively. After combining these results, the pooled RR was 1.10, which means that the risk of preterm birth in pregnant women with PD was 1.10 times that of pregnant women without PD (RR: 1.10; 95% CI: 1.08−1.12; I square: 88.14%; p: 0.00) (Figure 5). The Eggers test was also conducted to evaluate publication bias, and the results showed that publication bias occurred in these findings (B: 0.99; SE: 0.09; p: 0.294). The relationship between the presence of PD and premature rupture of the water sac in pregnant women was reported in four studies with a sample size of 4436 people. After combining these results, the pooled RR was 1.25, which means that the risk of premature rupture of the water sac in pregnant women with PD is 1.25 times that of pregnant women without this infection (RR: 1.25; 95% CI: 1.04−1.49; I square: 61.73%; p: 0.03) (Figure 6). The Eggers test was also conducted to evaluate publication bias, and the results showed there was no distortion in these findings (B: 1.03; SE: 0.44; p: 0.931). Considering that PD was not defined in the reviewed studies, in each of these studies, based on the indicators that were considered to define this disease and its subgroup analysis, the pooled risk RR was calculated (Table 2).
| Outcomes | Categories | RR (% 95 CI) | Heterogeneity assessment | ||
|---|---|---|---|---|---|
| I square | Q | p Value | |||
| GDM | Definition of PD | ||||
| CP | 1.44 (0.78–2.64) | - | - | - | |
| AL | 1.32 (1.10–1.57) | 69.88% | 6.64 | 0.04 | |
| TIS | 1.57 (1.10–2.10) | 44.00% | 14.29 | 0.07 | |
| Low Birth Weight | Definition of PD | ||||
| AL | 2.61 (1.73–3.96) | 0.00% | 0.56 | 0.91 | |
| TIS | 1.54 (1.17–2.04) | 76.99% | 13.04 | 0.00 | |
| Pre-eclampsia | Definition of PD | ||||
| AL | 1.71 (1.44–2.02) | 80.23% | 55.63 | 0.00 | |
| TIS | 1.34 (1.23–1.46) | 82.65% | 67.75 | 0.00 | |
| Preterm Birth | Definition of PD | ||||
| AL | 1.09 (1.07–1.11) | 88.00% | 41.6 | 0.00 | |
| TIS | 1.37 (1.21–1.55) | 87.28% | 62.92 | 0.00 | |
| PROM | Definition of PD | ||||
| AL | 2.39 (1.50–3.83) | 0.00% | 1.66 | 0.44 | |
| TIS | 1.12 (0.92–1.35) | 0.00% | 0.11 | 0.74 | |
- Abbreviations: CI, confidence interval; GDM, gestational diabetes mellitus; PD, periodontal diseases; PROM, premature rupture of membranes; Q, Q Cochrane test; RR, relative risk.
In studies that examined the association between PD and GDM by using the CPITN (Community Periodontal Index of Treatment Needs) or the Index of Periodontal Treatment Requirements to evaluate PD, the pooled RR was 1.44 (RR: 1.44; 95% CI: 0.78–2.64). After pooling the results of studies that considered PD based on a specified amount of probing depth and probing bleeding, the pooled RR value was 1.32 (RR: 1.32; 95% CI: 1.10–1.57; I square: 69.88%; p: 0.04).
In the studies that examined the relationship between PD and LBW of babies in pregnant mothers, two definitions of AL and TIS, were used. Subgroup analysis showed that according to the definition of AL, the pooled RR of this association is equal to 2.61 (RR: 2.61; % 95 CI: 1.73−3.96; I square: 0.00%; p: 0.91) and according to the definition of TIS is equal to 1.54 (RR: 1.54; % 95 CI: 1.17−2.04; I square: 76.99%; p: 0.00). Also, selected studies related to determining the association between PD and PE in pregnant mothers also used two definitions of AL and TIS. The pooled RR of this association based on the definition of AL is equal to 1.71 (RR: 1.71; % 95 CI: 1.44−2.02; I square: 80.23%; p: 0.00), and based on the definition of TIS is equal to 1.34 (RR: 1.34; % 95 CI: 1.23−1.46; I square: 82.65%; p: 0.00). The pooled RR between PD with preterm birth and PROM in pregnant mothers based on TIS definition is 1.37 (RR: 1.37; % 95 CI: 1.21–1.55; I square: 87.28%; p: 0.00) and 1.12 (RR: 1.12; % 95 CI: 0.92–1.35; I square: 0.00%; p: 0.74) respectively (Table 2).
4 DISCUSSION
The results of this meta-analysis indicated that PD can be a risk factor in causing adverse pregnancy outcomes such as PE, GDM, premature rupture of the amniotic sac in pregnant mothers, LBW, and premature birth in infants. Concerning GDM, we can say that pregnancy is not the primary cause of PD, but it may prepare and provide conditions for the development of this disease in pregnant mothers. The increase in inflammation of the gums and blood vessels as a result of the increase in estrogen and progesterone levels during pregnancy leads to changes in the oral flora. In reaction to this infection, the host mediates a complex cascade of tissue-destructive pathways. The PD acts as a reservoir for Gram-negative anaerobic flora, lipopolysaccharides, and inflammatory mediators, and it triggers a systemic inflammatory response in pregnant women, which can increase insulin resistance. Therefore, it may increase insulin resistance caused by pregnancy and cause mild GDM.21-24
Hyperglycemia from GDM is transient and short-lived and may not be long enough to initiate or exacerbate PD. As a result, periodontitis patients are likely to be the cause of GDM and not a result of it.25-28 The results of the present meta-analysis also confirm this hypothesis and confirm the development of GDM in pregnant women with periodontitis. The meta-analysis results of Lima et al. in 2015 showed that there is no positive and significant relationship between periodontitis and GDM, which contradicts the results of the present meta-analysis.29, 30 The reason for this difference can be attributed to the increase in the number of studies since 2015 and the use of more accurate analyses and tools to evaluate the selected studies in this meta-analysis. The results of a 2020 meta-analysis study by Mauricio Baeza et al. showed that PD in pregnant mothers increases the mean HbA1C by an average of 0.56.31 Data from the Chaparro Padilla study show that MMP-8 and MMP-9 GCF concentrations measured between 11 and 14 weeks of gestation are increased in pregnancies that develop GDM.32 In addition, the first 3 months of GCF MMP-8 concentration can be subsequently associated with the subsequent development of GDM. Moreover, the increase of both MMPs has a direct relationship with the severity of periodontitis and is also associated with several clinical periodontal inflammatory parameters. Early pregnancy levels of gingival crevicular fluid matrix metalloproteinases are associated with periodontitis severity and GDM.33, 34
In association with PE, there is an important interaction between chronic PD and the presence of Tannerella forsythensis, Eikenella corrodens, and Porphyromonas gingivalis in the development of PE.35 Evidence suggests that increased numbers of S. haemolyticus in women with PE are mild. Kunnen et al. investigated the possibility of a relationship between PD and PE in a systematic review, and the results showed that due to differences in the definition of diseases, unclear timing, and failure to consider confounding factors, decision-making related to the impact of PD It is difficult with the occurrence of PE.36, 37 In the previous study, the presence of several statistically significant correlations between biochemical and clinical periodontal parameters indicated that both serum and GCF levels of IL-1b, TNF-a, and PGE2 were significantly higher in PE groups than in women with normal blood pressure, which can indicate that there is a relationship between PD and PE.38, 39 Of course, the current meta-analysis showed a significant relationship between PD and PE, which is due to the existence of a sufficient number of studies and the precise combination of the results of these studies. In a study, the periodontal status and the presence of 15 oral pathogens were investigated in pairs of women who had a full-term delivery compared to those who had a preterm delivery. The results showed that periodontal pathogens were more common in the pairs of women with PD. These pathogens included the gram-negative anaerobic Fusobacterium nucleatum, which is also associated with preterm birth or LBW.40 F. nucleatum has been suggested to be involved in many adverse pregnancy outcomes, including hypertensive disorders, preterm delivery, LBW, chorioamnionitis, miscarriage, stillbirth, and early-onset neonatal sepsis.40, 41 Fusobacterium and Streptococcus thermophiles species were also associated with chorioamnionitis in preterm labor.42 The detection of periodontal pathogens P. gingivalis and F. nucleatum in the vagina, as well as the placenta in those with adverse birth outcomes, also suggests that known oral pathogens may play a role.43 Another important oral pathogen is Porphyromonas gingivalis, which has been found in amniotic fluid. In another study, this pathogen was isolated from several pregnant women, some of whom had experienced the risk of preterm delivery, which is considered to be the main cause of fetal growth restriction.44-46
The results of previous studies and reviews showed that the clinical criteria for evaluating PD are not the same in research, and different classifications have been considered for periodontitis. For this reason, according to the considered definitions, the desired effect size in determining the relationship between PD and the occurrence of maternal and neonatal outcomes may also be affected. In the current meta-analysis, subgroup analyses were performed based on different definitions of PD, and the results showed differences in the estimated effect size. Therefore, a specific and accurate definition related to PD needs to be considered for future research.47-49 Two main pathways were identified in the consensus report of the Joint European Federation of Periodontology/American Academy of Periodontology Workshop on periodontitis and systemic diseases.50, 51 In the first path, or direct mechanisms, with oral microorganisms or their particles that attack the placental fetal unit through blood diffusion, or in the ascending path through the genitourinary system. In the second pathway or indirect mechanisms, mediated by inflammatory mediators that are produced locally in periodontal tissues, directly affect the embryo-pair unit, or circulate to the liver and induce a systemic inflammatory state through acute phase protein responses, such as they increasing C-reactivity, which later affects the placental fetal unit.
The superiority of the current meta-analysis compared to previous meta-analyses is the placement of a wider range of studies, the updating of the collected data in some way, as well as the examination of more variables and outcomes compared to the previous meta-analyses, which can lead to the achievement of more reliable and consistent results than the previous meta-analyses. Given that PDs do not have a clear and uniform definition in the reviewed studies, and this problem can place a variable range of pregnant women in this group, this issue itself affects the calculated cumulative ratio and is considered one of the limits of this study. Some PD studies were used to evaluate PD by evaluating probing depth (PD), some PD, CAL (clinical attachment loss), and some other indicators such as CPITN and DMF, which are the advantages of the present meta-analysis. Previously, the grouping of cumulative effect was based on the definition of PD in different studies based on the three subgroups of PD, PD + CAL, and CPTIN, and there was a clear difference in the size of the reported effects.
A large number of studies have investigated the potential association between maternal periodontitis and adverse pregnancy outcomes, but there is a high degree of variability in study populations as well as in methods of diagnosis and assessment. In addition, exposure to other risk factors influencing the outcomes mentioned in this study may not have been adequately considered in all studies. Therefore, the presence or absence of multivariate analysis was included in the quality scores assigned to the studies in the tables. The range of variation in the quality of the selected articles was limited, possibly due to compliance with the predetermined inclusion criteria. Some confounding variables, such as adverse pregnancy history, infections (such as bacterial vaginosis and chorioamnionitis), usage of antibiotics during pregnancy, body mass index, and maternal disorders (hypertension, diabetes), were not fully considered in some studies. Therefore, in this study, researchers could not perform subgroup analyses based on these variables.
5 CONCLUSION
Based on the results of the present meta-analysis, the presence of PD can play a role in increasing the risk of adverse maternal and neonatal outcomes in pregnant mothers. Therefore, we recommend improving healthcare programs related to dentistry for pregnant mothers before, during, and after pregnancy. Considering that these consequences can have huge effects and costs, both material and spiritual, for people in society, especially pregnant women, and centers related to health and hygiene, prevention and planning to improve oral and dental health, and follow-up along with effective treatment of PDs in pregnant women will be of great importance. In addition, more accurate methodology studies, such as cohort studies with a large sample size, should be conducted to produce more accurate evidence by considering confounding variables to determine the relationship between PD and the occurrence of other pregnancy outcomes in the world.
AUTHOR CONTRIBUTIONS
Newsha Karimi: Investigation; supervision; validation; writing—original draft; writing—review & editing. Negin Samiee: Data curation; investigation; project administration; writing—original draft; writing—review and editing. Yousef Moradi: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; supervision; visualization; writing—original draft; writing—review and editing.
ACKNOWLEDGMENTS
This article has resulted from a thesis approved and supported by the Kurdistan University of Medical Sciences with the code of ethics IR.MUK.REC.1401.449.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Negin Samiee, Yousef Moradi affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Data and materials are available within the complementary materials, and further information can be available by request to the corresponding author.