Prevalence, molecular characterization, and clinical features of human bocavirus in children under 5 years of age with acute gastroenteritis admitted to a specialized children's hospital in Iran: A cross-sectional study
Abstract
Background and Aims
Although some reports have confirmed the role of human bocavirus (HBoV) in respiratory infections, the importance of this virus in causing acute gastroenteritis has not yet been proven. This study aimed to determine the molecular prevalence of HBoV in children under 5 years old with gastroenteritis and to compare the clinical symptoms of HBoV-positive and -negative gastroenteritis cases.
Methods
A total of 100 stool samples were collected from children with gastroenteritis hospitalized in a pediatric hospital in Tehran, Iran. Demographic and clinical data were collected from patients' medical records. Viral genomic DNA was extracted from stool samples and amplified using the PCR assay. Finally, sequencing was used to determine the genotype of HBoV.
Results
The HBoV genome was detected in 14 samples (14%). The highest prevalence of HBoV was observed in the age range of 24–60 months (n = 5; 35.7%); However, no statistically significant relationship was observed between the prevalence of HBoV and age groups (p = 0.09). Nine (64.3%) and 5 (35.7%) HBoV-positive cases were boys and girls, respectively (p = 0.45). Fever, vomiting, and heartache were seen in 5 (35.7%), 3 (21.4%), and 1 (7.1%) HBoV-positive patients, respectively. Overall, no significant difference was observed in any of the investigated clinical manifestations between patients positive or negative for HBoV. Five HBoV-positive samples were subjected to sequencing and all five sequenced samples were genotype 3.
Conclusion
HBoV infections can be considered a risk factor for causing at least a portion of acute gastroenteritis cases in children under 5 years of age.
1 INTRODUCTION
Acute gastroenteritis is a very common infectious disease that causes a combination of abdominal pain, nausea, vomiting, and diarrhea.1 It is estimated that 3–6 million people die each year from gastroenteritis worldwide, with children under the age of five accounting for approximately 20% of all child deaths. Diarrhea is the most common symptom in most gastroenteritis cases, and it is the second most common cause of death in infants and children under the age of five (about 800,000 fatalities each year).2
Among the various etiological agents of acute gastroenteritis, viruses are considered the most common. Around 75%–90% of all acute gastroenteritis cases in children are caused by viruses worldwide.3 Rotavirus and Norovirus are the most frequently detected viral pathogens, followed by Sapovirus, enteric adenovirus, and Astrovirus. Aichi virus A, Picobirnavirus, human bocavirus (HBoV), Torovirus, Parechovirus, Safford virus, and Parechovirus are some of the less prevalent viral agents involved in acute gastroenteritis etiology.4
In 2005, HBoV was found in the respiratory secretions of Swedish children suffering from acute respiratory infections.5 HBoV is a non-enveloped virus with a 5 kb linear, single-stranded DNA molecule that can be positive- or negative-sensed.6 There are three open reading frames (ORFs) in the HBoV genome. Nonstructural proteins are encoded by the first ORF, NP1 protein by the second ORF, and VP1 and VP2 proteins by the third ORF.6, 7 Four genotypes of HBoV have been identified based on the genetic diversity of the VP1 gene (i.e., HBoV-1, HBoV-2, HBoV-3, and HBoV-4). Several studies have found that HBoV-1 is associated with respiratory tract infections, while HBoV-2, 3, and 4 are linked to gastrointestinal tract infections.7-9
HBoVs have received a lot of attention since their discovery in 2005, owing to their widespread distribution in clinical samples. Depending on the country, the prevalence of HBoVs has been reported between 1.3% and 63% in stool samples, and between 1% and 56.8% in respiratory tract samples. The overall prevalence of HBoVs was calculated to be 6% worldwide.6 High prevalence does not always imply high clinical importance, and it has been a challenge to establish the virus' causal role, in part because it is frequently discovered alongside other respiratory and gastrointestinal viruses at co-detection rates as high as 75%. These facts call into question how truly significant and important HBoV is as a causal agent in human illnesses.
A significant reduction of rotavirus-related diarrhea episodes has been observed after the introduction of the rotavirus vaccine, but acute gastroenteritis cases that are related to other viruses like HBoV are more commonly found. This study aims to assess whether the presence of HBoV in stool samples can be a risk factor for severe gastroenteritis. To answer this question, we assessed the molecular prevalence of HBoV in stool samples of children under 5 years of age who were hospitalized with severe gastroenteritis.
2 MATERIALS AND METHODS
2.1 Ethical considerations
Under the ethical reference number IR.IUMS.REC.1399.467, the ethics committee of the Iran University of Medical Sciences reviewed and approved the protocol of the study. Before participating in this study, the patients' parents were fully informed and provided written consent for participation and publication of the results.
2.2 Patients and samples
For this cross-sectional study, a total of 100 stool samples were collected from children with acute gastroenteritis admitted to a public pediatric hospital in Tehran, Iran, between March 2021 and March 2022. The non-probability sampling approach was used to calculate the sample size, as the sample size varies depending on the time of the study. Samples were transported to the laboratory at the Research Center of Pediatric Infectious Diseases, and stored at −70°C until further analysis. This study included children under the age of five who were suffering from acute gastroenteritis (defined as at least three loose or watery stools or episodes of vomiting per day). The common bacterial and parasitic infections were eliminated by microbiological tests before the stool samples were included in this study. Patients with chronic illnesses and those with bacterial and parasitic gastroenteritis were excluded.
2.3 Data acquisition
Demographic data was obtained from medical records for each patient. Clinical symptoms and manifestations including acute respiratory distress syndrome (ARDS), rash, fever, vomiting, cough, and stomach pain were recorded by the primary physician. Furthermore, the presence of SARS-CoV-2 infection was also examined by real-time reverse transcription PCR assay using Viga Sars-CoV-2 Molecular Diagnostic kit (ROJE Technologies).
2.4 Stool preparation and DNA extraction
Stool suspensions were prepared as 10% (wt/vol) in phosphate-buffered saline (pH 7.2) and cleared by centrifugation at 2500g for 5 min. The supernatants were then kept at −70°C until genomic DNA isolation. Using the HiPurA® Multi-Sample DNA Purification kit (HiMedia), 200 μL of each suspension were used for DNA extraction as per the manufacturer's instructions. The extracted DNA was then kept at −20°C before polymerase chain reaction (PCR) analysis.
2.5 Detection of HBoV using PCR
For this purpose, the PCR method was used to amplify the VP1 gene. The following primer pair was used in the PCR test procedure to generate an amplicon of 579-bp:
Forward primer 5′-GGCTCCTGCTCTAGGAAATAAAGAG-3′
Reverse primer 5′-CCTGCTGTTAGGTCGTTGTTGTATGT-3′
The PCR reaction mix was prepared in a total volume of 25 μL consisting of 5 μL of the template DNA, 12.5 μL of 2x red PCR Master Mix (Ampliqon), 0.5 μL of the forward and 0.5 μL of the reverse primers (10 μM solutions), and 6.5 μL of DNase and RNase-free water. The PCR reaction program consisted of 1 cycle at 95°C for 5 min, followed by 40 cycles at 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s, and a final extension at 72°C for 5 min. PCR products were electrophoresed on 1% agarose gel, then visualized under UV light after staining with DNA safe stain.
2.6 Nucleotide sequencing and sequence analyses
The VP1 gene PCR products were purified by High Pure PCR Product Purification Kit (Roche Diagnostic GmbH) according to the manufacturer's protocol. VP1 gene-specific primers were used for direct sequencing via an ABI 3730 XL sequencer. Multiple sequence alignment, trimming, and analysis of raw sequences were performed by CLC Workbench 5 and MEGA6 software against the HBoV reference sequence (GenBank accession number NC_012564). All sequences of VP1 from our 5 strains were submitted to the GeneBank via BankIt online submission system.
2.7 Statistical analysis
Statistical analysis of the current study was conducted in accordance with the guidelines suggested by Assel et al.10 Normal distribution of age data was assessed using the Kolmogorov–Smirnov test, and based on the results, the Mann–Whitney U test (two-sided) was used for study group comparison. All other variables including gender, age group, and clinical manifestations accompanying the HBoV infection were categorical and thus were assessed using the χ2 or Fisher exact tests. A p value of less than 0.05 was considered as statistical significance threshold. All statistical tests were performed using the SPSS software version 22.0 (SPSS Inc.).
3 RESULTS
3.1 Study population characteristics
Among the 100 children with acute gastroenteritis enrolled in the study, 55 (55%) were male and 45 (45%) were female. The median age of the children with acute gastroenteritis was 12 (interquartile range: 8–24) months. Eight (8.1%) children were younger than 6 months, 25 (25%) were aged between 7 and 12 months, 38 (38%) were aged between 13 and 24 months, 28 (28%) were aged between 25 and 60 months, and for one patient the age data was missing. In terms of clinical symptoms, all patients had diarrhea and symptoms of gastroenteritis. Coughing and ARDS were observed in 7 (7%) and 4 (4%) patients, respectively. Also, rash, fever, vomiting, and stomach pain were reported in 6 (6%), 31 (31%), 34 (34%), and 7 (7%) patients, respectively. Of all 100 patients, 7 (7%) were laboratory-confirmed COVID-19 patients with oropharyngeal/nasopharyngeal swab samples that tested positive for SARS-CoV-2 (Table 1).
| Total (n = 100) | HBoV negative (n = 86) | HBoV positive (n = 14) | p value | |
|---|---|---|---|---|
| Gender (male) | 55 (55%) | 46 (53%) | 9 (64%) | 0.45 |
| Agea | 12 (8–24) | 12 (8.5–24) | 11 (5.75–36.75) | 0.44 |
| Age group (months) | ||||
| 0–6 | 8 (8.1%) | 5 (5.9%) | 3 (21%) | 0.09 |
| 7–12 | 25 (25%) | 21 (25%) | 4 (29%) | |
| 13–24 | 38 (38%) | 36 (42%) | 2 (14%) | |
| 25–60 | 28 (28%) | 23 (27%) | 5 (36%) | |
| Clinical symptoms | ||||
| Cough | 7 (7%) | 6 (7%) | 1 (7.1%) | >0.9 |
| ARDS | 4 (4%) | 3 (3.5%) | 1 (7.1%) | 0.46 |
| Rash | 6 (6%) | 6 (7%) | 0 | 0.59 |
| Fever | 31 (31%) | 26 (30%) | 5 (36%) | 0.76 |
| Vomiting | 34 (34%) | 31 (36%) | 3 (21%) | 0.37 |
| Stomach pain | 7 (7%) | 6 (7%) | 1 (7.1%) | >0.9 |
| Covid-19 | 7 (7%) | 7 (8.1%) | 0 (0%) | 0.59 |
- Abbreviations: ARDS, acute respiratory distress syndrome; HBoV, human bocavirus; IQR, interquartile range.
- a Data presented as median (IQR).
3.2 Molecular detection of HBoV
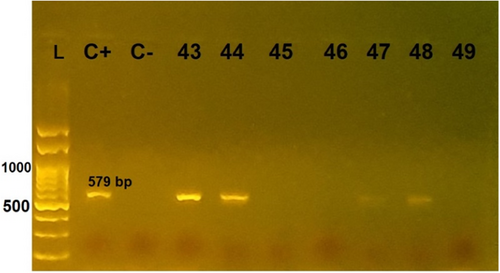
In total, HBoV DNA was detected in 14 out of 100 patients (14%) (Figure 1). HBoV was detected at a higher rate in males (n = 9/14; 64%) than in females (n = 5/14; 36%) but the difference was not statistically significant (p = 0.45). Among the 14 positive samples for HBoV, 3 (21%) belonged to the younger than 6-month-age group, 4 (29%) were in the 7–12-month-age group, 2 (14%) were in the 13–24-month-age group, and 5 (36%) were in the 25–60-month-age group. The difference in the prevalence of HBoV in different age groups was not statistically significant (p = 0.09). None of the HBoV-positive patients showed skin manifestations in the form of a rash, but five HBoV-positive patients (36%) had a fever. Vomiting and stomach pain were seen in 3 (21%) and 1 (7.1%) of all the HBoV-positive patients, respectively. Interestingly, none of the 14 HBoV-positive cases were infected with SARS-CoV-2. Overall, no significant relationship was observed between any of the investigated clinical manifestations and HBoV test results (Table 1).
3.3 Nucleotide sequencing and phylogenetic analyses
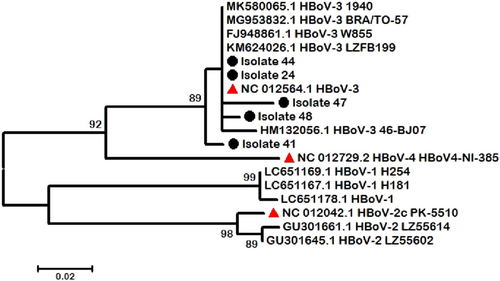
From the 14 detected positive HBoV–DNA specimens, we selected 5 sharp and high-quality PCR products based on agarose gel visualization and purified them. These were sequenced using the Sanger sequencing method, and the raw data were analysed using CLC Main Workbench 5 software. A comparison of the reference sequence with sequences of differentiated regions among our isolates revealed that all of our cases were HBoV genotype 3, which makes it the predominant genotype in our study (Figure 2). Phylogenetic analysis was performed using the maximum likelihood method to identify the ancestor sequence by their similarity, which showed that isolate 41 was rather different from other isolates categorized in the HBoV-3 node.
4 DISCUSSION
HBoV was first identified in 2005 in nasopharyngeal aspirates from children with respiratory tract infections.5 Later, other studies also reported the presence of HBoV in stool samples from children with acute gastroenteritis.11-22 In Iran, the prevalence of HBoV infection in children with acute gastroenteritis ranged from 7.5% to 12.8% from 2010 to 2014.23, 24 However, there is a lack of up-to-date information about HBoV infection in this age group of patients from 2014 until now. In the current study, the prevalence of HBoV infection in children with acute gastroenteritis was investigated during a 1-year period from 2021 to 2022, which coincided with the Covid-19 pandemic in Iran and other parts of the world. According to our findings, the molecular prevalence of HBoV infection in children with acute gastroenteritis hospitalized in a public pediatric hospital was 14%, which shows a slight increase compared to the prevalence in previous years.
One of the reasons for the difference in the prevalence of HBoV in this study with those reported in other studies conducted in Iran, and even other parts of the world, can be the techniques used to detect HBoV in the stool samples of children with gastroenteritis. Nadji et al.23 and Romani et al.25 used nested the PCR technique, while Monavari et al.26 and Shokrollahi et al.24 used the real-time PCR technique. It should be noted that the highest infection rates in Iran were previously reported by Nadji et al.23 (with a prevalence of 12.8%) and Romani et al.25 (with a prevalence of 9.18%), respectively, and in both studies the nested PCR technique was used. However, to detect HBoV infection in the present study, the conventional PCR assay was used, which showed a prevalence of 14%. It could be argued that if we had used the nested PCR technique, which is a more sensitive technique, the prevalence rate would have possibly been higher than 14%. Thus, it is possible that the difference in the prevalence of HBoV infection in different regions is related to the method used to detect the presence of HBoV. This conclusion, which is in line with the report of Campos et al.,27 points to the importance of performing active surveillance of HBoV in all different parts of the world using the same method.
Another interesting finding of the present study is the difference in the prevalence of HBoV infection in different age groups of children with acute gastroenteritis. Based on our results, the highest prevalence of HBoV infection was observed in children with gastroenteritis in the age group 25–60 months. HBoV seroepidemiological studies have shown high seroprevalence rates in infants and children less than 5 months old. However, this serum prevalence gradually decreases with age. In this way, the probability of acquiring a HBoV infection at a young age is low due to the presence of more anti-HBoV antibodies, and high at older ages due to the presence of fewer anti-HBoV antibodies. This hypothesis is consistent with the findings of the present study. It is believed that the high seroprevalence of HBoV in young children is related to maternal antibodies, which are over time removed from children's blood circulation and the child becomes susceptible to infection. Breastfeeding is also another effective factor in lowering the prevalence of HBoV infection in young children compared to older children. The reason is that mother's breast milk contains various antiviral compounds, and as a result, the child is protected from HBoV infections.28, 29
The clinical findings of this study have also shown that none of the HBoV-positive cases manifested skin rashes, and all the cases with skin rashes were patients who were negative for HBoV. This observation can indicate that, unlike some other viral diseases such as human herpes virus 6, human herpes virus 7, and human parvovirus B19, HBoV infection is not associated with skin manifestations of rash in children. Also, none of the HBoV-positive cases were infected with SARS-CoV-2. From one point of view, the interpretation could be that the presence of some acute viral infections in children, such as HBoV infection, can prevent the replication of the SARS-CoV-2 in their bodies. However, such a conclusion requires further studies with a larger sample size.
Also, the results of our study showed that boys have a greater tendency to suffer from HBoV-related gastroenteritis than girls, which can partly indicate the role of sex hormones. In general, the immune reactions in women are high, which can lead to their more effective resistance to infection. Therefore, women are less prone to viral infections. Another explanation for the higher rate of HBoV infection among boys could be that they are more exposed to infection due to their activities and hobbies than girls.30
One of the limitations of the present study was the lack of investigations about co-infections with other gastrointestinal viruses that cause acute gastrointestinal diseases. Considering the fact that there is a possibility of the presence of other viral pathogens causing gastroenteritis in the stool samples examined in the present study, it is wrong to completely attribute the gastroenteritis disease in this situation only to the HBoV infection. Also, another limitation of the present study was the small sample size and collection of samples from only one hospital, which reduces the strength of the study and does not show the true picture of the prevalence of HBoV infection in children with gastroenteritis. Also, not including a healthy control group can be mentioned as another limitation of the present work.
5 CONCLUSION
The present study was conducted to determine the molecular prevalence of HBoV infection in children less than 5 years old with gastroenteritis, and it is considered the first study after 8 years since the last study conducted in Iran. Based on the findings of the present study, the molecular prevalence of HBoV in this group of people was reported as 14%. Also, the prevalence of HBoV infection was highest in the oldest age group (25–60 months) and the male gender; However, these findings were not statistically significant, and there is a need to conduct more studies with larger sample sizes. The findings of this study show that HBoV infection can be considered a risk factor for at least a portion of acute gastroenteritis cases in children under 5 years of age.
AUTHOR CONTRIBUTIONS
Atefeh Kachooei: data curation; investigation; writing—original draft. Mohammad Hadi Karbalaie Niya: investigation; methodology. Pegah Khales: conceptualization; writing—original draft; writing—review & editing. Milad Sabaei: writing—review & editing. Soheil Rahmani Fard: formal analysis. Malihe Hamidzade: investigation; writing—original draft. Ahmad Tavakoli: conceptualization; funding acquisition; project administration; supervision; writing—original draft; writing—review & editing.
ACKNOWLEDGMENTS
The authors would like to acknowledge the members of the Institute of Immunology and Infectious Diseases. This study was financially supported by the Iran University of Medical Sciences, Grants Number: 99-1-78-18430.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
The lead author Ahmad Tavakoli affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
The original data presented in this study are included in this article, further data can be available upon reasonable request to the corresponding author. The corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.