Blood C-peptide concentration as a proxy marker of cardiovascular disease: An observational cross-sectional study
Abstract
Background and Aims
Cardiovascular diseases (CVDs) are among the leading causes of disability and early death in sub-Saharan Africa. Most of the current blood tests for CVD diagnosis involve performing about three test profiles; often at additional cost to patients. C-peptide, a cleavage product of proinsulin, is a promising marker that has the potential to serve as a proxy marker for diagnosing CVDs in resource-poor settings.
Methodology
The study was an observational cross-sectional one and involved 127 consenting persons diagnosed with CVD and 127 individuals without CVD. The socio-demographic and clinical characteristics of participants were obtained. Blood levels of C-peptide, fasting plasma glucose (FPG), total creatinine kinase (CK), creatine kinase myocardial bound (CKMB), lactate dehydrogenase (LDH), propeptide of brain natriuretic peptide (PBNP), Troponin T, lipids, and biomarkers of kidney and liver function were analyzed using ELISA and an automated analyzer. Insulin resistance was computed using the modified homeostatic model assessment (HOMA-IR).
Results
The CVD Group had significantly higher levels of C-peptide, CK, CKMB, troponin T, PBNP, FPG, HOMA-IR, and several selected kidney, liver, and lipid parameters compared to the non-CVD Group (p < 0.05 for all). Troponin T recorded a positive correlation (r = 0.34, p < 0.001) with C-peptide among the CVD Group. The sensitivity and specificity of C-peptide in identifying CVD were 96.1% and 91.3% respectively (area under the curve = 0.938, p < 0.001).
Conclusion
C-peptide levels were higher in the CVD Group and appeared to be a valuable (high sensitivity and specificity) biomarker in detecting CVD.
Key points
- •
Cardiovascular diseases (CVDs) are primary contributors to both disability and early death in sub-Saharan Africa. Most of the current blood tests for CVD diagnosis involve performing about three test profiles often at additional cost to patients.
- •
C-peptide, a product of proinsulin, could potentially serve as a proxy marker for diagnosing CVDs in resource-poor settings.
- •
The sensitivity and specificity of C-peptide in identifying CVD in this study were 96.1% and 91.3%, respectively (area under the curve = 0.938, p < 0.001).
1 INTRODUCTION
Cardiovascular diseases (CVDs) are a group of disorders of the heart and blood vessels and comprise both atherosclerotic (such as coronary artery disease [CAD], cerebrovascular disease, and peripheral artery disease) and non-atherosclerotic disorders (e.g., venous thromboembolism, valvular, rheumatic, and congenital heart diseases [CHD]).1, 2 CVDs are a major cause of death in both industrialized and developing countries.3, 4 Mortality attributed to CVDs, which was estimated to be 17.9 million in 2015, is projected to rise to more than 23.6 million by the year 2030.5 Aside from contributing to early mortality, CVDs are responsible for incapacitation to work, decreased family income, and diminished productivity.6 Over the years, the prevalence, morbidity, and mortality associated with CVDs have risen in Sub-Saharan African.7 More than 60% of CVD-related deaths in Africa occur in adults aged 30–65 years old, which is about 10 years younger than the average bracket age of mortality for people in the industrialized world.7, 8
The incidence of CVD tends to increase with age, and several modifiable risk factors, including alcohol misuse, sedentariness, excessive weight gain, and tobacco use continue to be associated with CVD development and progression.9 The growing incidence of CVDs in sub-Saharan Africa highlights the importance of investigating potential markers that could assist in early diagnosis. The majority of the currently available blood diagnostic tests for CVD diagnosis include at least three test profiles. These tests are not readily available, costly, and out of reach for most people in resource-poor settings.
Connecting peptide (C-peptide) is a 31-amino-acid cleavage precursor of the production of insulin. C-peptide was previously thought to be a physiologically inactive molecule.10 It is released into the bloodstream at the same concentration as insulin, but in contrast to insulin, it undergoes only a tiny amount of first-pass metabolism in the liver.11, 12 Importantly, C-peptide has been associated with inflammation and is touted to have a protective role in blood glucose metabolic disorders in both humans and rats.13-15 This peptide and related inflammatory mechanisms have also been implicated in conditions such as obesity,16 metabolic syndrome,17 hypertension,18 and hepatosteatosis.15, 19 This has led to an increase in the clinical usefulness of C-peptide, as it may readily exhibit hormone-like properties and offer promise as a diagnostic marker in pathologies such as CVDs. Although data shows that there is some relationship between C-peptide and CVDs in the Caucasian population, there is sparse data among sub-Saharan Africans. Furthermore, the majority of the existing data supporting C-peptide as a possible CVD marker have been derived from diabetic patients. These data are insufficient to validate the use of C-peptide as a diagnostic marker for CVD. This study, therefore, aimed to evaluate the role of blood C-peptide in the detection of CVDs among Ghanaians and to elucidate factors that contribute to the incidence of CVDs.
2 MATERIALS AND METHODS
2.1 Study design, site, and participants
This was an observational cross-sectional study conducted at the 37 Military Hospital, Accra, Ghana. A total of 354 consenting volunteers, comprising 127 with CVD and 127 without CVD, were recruited. The Ethical and Protocol Review Committee (EPRC) of the College of Health Sciences at the University of Ghana approved this study (ID: CHS-Et/M.4-5.6/2020-2021). Additionally, permission was obtained from the Management at the 37 Military Hospital. Clinicians at the hospital referred potential participants to the research team, after which the objectives, rationale, and outcomes of the study were explained. Those willing to participate in the study were offered appointment dates and times. Those who refused to participate in the experiment or expressed reservations were allowed to continue receiving their customary care at the hospital. Patients who had a severe condition that required immediate medical attention, such as severe chest pain, altered mental status, breathing difficulties, and persistent bleeding, were excluded from the study. The volunteers were to fast for 8–12 h overnight. Participants in the Control Group were seemingly healthy, between the ages of 18 and 65, and free of any CVD, diabetes, or any severe condition that required immediate medical attention. The participants were instructed to complete a questionnaire that had information on socio-demographic and clinical information; age, gender, weight and height, educational level, lifestyle (degree of cigarette usage, alcohol intake), and medical history (personal or family history of CVD). A minimum sample size of 100 participants was adequate for this study.
2.2 Clinical assessment and laboratory procedures
Blood pressure was measured with an OMRON digital sphygmomanometer (OMRON HEALTHCARE Company Limited). Height and weight were assessed using a digital stadiometer and an electronic digitized scale (both from SECA). Venous blood samples (5 mL) were taken from each study participant, after which 3 mL was placed in a serum separator tube and 2 mL in a sodium fluoride tube. Samples in the serum separator tubes were allowed to clot for 10–20 min before centrifugation at 3000 rpm for 10 min (at room temperature) together with the samples in the sodium fluoride tubes. The sera and plasma were aliquoted into 0.5 mL Eppendorf tubes and stored at −20°C until analysis. The plasma was used to determine fasting blood glucose (FPG) whereas the serum was used to assess liver function [aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin (TBIL), direct bilirubin (DBIL), total proteins, and albumin], kidney function (sodium, potassium, chloride, urea, creatinine), lipids [total cholesterol, triglycerides and high-density lipoproteins cholesterol (HDL)], total creatinine kinase (CK), creatine kinase myocardial bound (CKMB) and lactate dehydrogenase (LDH) concentrations using dry reagent slides from the VITROS 5.1FS Chemistry Autoanalyser (Ortho Clinical Diagnostics, Neckargemünd, Germany). The Sandwich enzyme-linked immunosorbent assay (ELISA) method was used to analyze serum concentrations of C-peptide, troponin T, and propeptide of brain natriuretic peptide (PBNP) on a micro-ELISA strip-plate analyzer (SUNLONG BIOTECH Company Limited) pre-coated with an antibody specific to these tests. The modified homeostasis model assessment for insulin resistance (HOMA-IR) formula was used to measure insulin resistance (IR) as previously described.20 Low-density lipoprotein cholesterol (LDL) was derived using Frieldewald's equation.21 The coronary risk was computed as a ratio of total cholesterol and HDL cholesterol.
2.3 Statistical analysis
STATA, version 14, was used to analyze the data. The biodata of the participants was summarized using descriptive statistics and the normality of the data determined by the Shapiro–Wilk test. The biochemical parameters of the participants with CVD were compared to those without CVD using independent-sample t tests. Associations between these biochemical parameters and C-peptide levels were determined separately for the CVD and control participants using Pearson's product-moment correlation. Furthermore, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of C-peptide in distinguishing between CVD and non-CVD participants were determined, as previously described.22 These analyses were bolstered by plotting a receiver operating characteristic (ROC) curve and computing Kappa's measure of agreement between the C-peptide-based diagnosis and the reference CVD diagnosis of the participants. All inferential statistics were performed at a 0.05 alpha level.
3 RESULTS
3.1 Sociodemographic and clinical (categorical) features of the study participants
The socio-demographic and clinical (categorical) characteristics of the participants are shown in Table 1. In this study, 127 individuals with CVD (Case Group) and 127 individuals without CVD (Control Group) were recruited. Each of these Groups had identical distributions for males (54.3%, n = 69) and females (45.7%, n = 58) respectively. In both Groups, the majority of the participants had tertiary education [CVD Group (52.0%); Control Group (62.2%)]. In the CVD Group, retired individuals and traders, as a composite, made up the highest proportion (54.3%) with regard to participant occupation, whereas in the Control Group, it was military personnel who dominated (72.4%). Again, 67.7% of the participants in the CVD Group reported having a family history of CVD, compared to 26% in the Control Group. Majority of the participants were non-smokers [CVD Group = 94.5%; Control Group = 98.4%)] and did not consume alcohol [CVD Group = 82.7%; Control Group = 89.8%)] (Table 1).
| Features | CVD group | Control group | p value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Gender | |||||
| Female | 58 | 45.7 | 58 | 45.7 | 0.71 |
| Male | 69 | 54.3 | 69 | 54.3 | |
| Marital status | |||||
| Single | 35 | 27.6 | 105 | 82.7 | <0.001 |
| Married | 72 | 56.7 | 22 | 17.3 | |
| Widowed | 10 | 7.9 | 0 | 0.0 | |
| Divorced | 10 | 7.9 | 0 | 0.0 | |
| Educational level | |||||
| None | 5 | 3.9 | 0 | 0.0 | 0.01 |
| Basic | 19 | 15.0 | 3 | 2.4 | |
| Secondary | 37 | 29.1 | 45 | 35.4 | |
| Tertiary | 66 | 52.0 | 79 | 62.2 | |
| Occupation | |||||
| Unemployed | 7 | 5.5 | 2 | 1.6 | 0.79 |
| Student | 1 | 0.8 | 3 | 2.4 | |
| National service personnel | 0 | 0.0 | 5 | 3.9 | |
| Retired | 28 | 22.0 | 5 | 3.9 | |
| Military personnel | 13 | 10.2 | 92 | 72.4 | |
| Trader | 41 | 32.3 | 8 | 6.3 | |
| Finance officer | 4 | 3.1 | 5 | 3.9 | |
| Healthcare worker | 4 | 3.1 | 0 | 0.0 | |
| Artisan | 9 | 7.1 | 0 | 0.0 | |
| Civil servant | 5 | 3.9 | 2 | 1.6 | |
| Teacher | 15 | 1.8 | 5 | 3.9 | |
| Monthly family income (in Ghana cedis) | |||||
| ≤500 | 45 | 35.4 | 51 | 40.2 | 0.18 |
| 501–1000 | 35 | 27.6 | 30 | 23.6 | |
| 1001–2000 | 4 | 3.1 | 7 | 5.5 | |
| >2000 | 43 | 33.9 | 39 | 30.7 | |
| Family history of CVD | |||||
| No | 31 | 24.4 | 55 | 43.3 | <0.001 |
| Yes | 86 | 67.7 | 33 | 26.0 | |
| Unsure | 10 | 7.9 | 39 | 30.7 | |
| Smoking status | |||||
| No | 120 | 94.5 | 125 | 98.4 | 0.09 |
| Yes | 7 | 5.5 | 2 | 1.6 | |
| Alcohol consumption status | |||||
| No | 105 | 82.7 | 114 | 89.8 | 0.10 |
| Yes | 22 | 17.6 | 13 | 10.2 | |
- Note: The sociodemographic and clinical (categorical) characteristics of the study participants. % = percentages.
- Abbreviation: CVD, cardiovascular diseases.
3.2 Clinical (continuous) and biochemical parameters of study participants
The independent-sample t test conducted to compare the clinical (continuous) and biochemical parameters of the study participants is shown in Table 2. C-peptide, FPG, HOMA-IR, urea, creatinine, total CK, CKMB, troponin T, PBNP, ALT, ALP, total cholesterol, triglycerides, LDL, very low-density lipoprotein cholesterol (VLDL), coronary risk, systolic, and diastolic blood pressure levels were higher (p < 0.001, respectively) whereas potassium, chloride, total protein and albumin were lower (p < 0.001 for all) in the CVD Group when compared with the Control group.
| Features | CVD group | Control group | 95% CI | p value |
|---|---|---|---|---|
| x̄ ± SD | x̄ ± SD | |||
| Clinical/metabolic | ||||
| Age (years) | 57.18 ± 14.74 | 56.20 ± 13.60 | −2.52 to 4.49 | 0.58 |
| BMI (Kg/m2) | 37.59 ± 8.58 | 24.83 ± 3.28 | 11.15 to 14.37 | <0.001 |
| Systolic blood pressure (mmHg) | 131.71 ± 23.87 | 98.54 ± 11.00 | 28.57 to 37.76 | <0.001 |
| Diastolic blood pressure (mmHg) | 89.27 ± 14.04 | 74.96 ± 10.79 | 11.21 to 17.40 | <0.001 |
| Insulin resistance | 1.74 ± 0.20 | 1.55 ± 0.02 | 0.16 to 0.23 | <0.001 |
| C peptide (ng/mL) | 6.75 ± 4.57 | 1.56 ± 0.69 | 4.39 to 6.00 | <0.001 |
| FPG (mmol/L) | 5.44 ± 2.58 | 4.41 ± 0.49 | 0.57 to 1.49 | <0.001 |
| Cardiac parameters | ||||
| Total Creatine kinase (U/L) | 141.06 ± 86.56 | 51.21 ± 26.40 | 74.04 to 105.72 | <0.001 |
| CKMB (U/L) | 66.95 ± 30.60 | 14.09 ± 5.60 | 47.40 to 58.30 | <0.001 |
| LDH (U/L) | 56.30 ± 11.85 | 51.32 ± 29.98 | −0.67 to 10.62 | 0.08 |
| Troponin T (ng/L) | 1.06a ± 0.73 | 0.31 ± 0.08 | 0.62 to 0.88 | <0.001 |
| PBNP (pg/mL) | 176.42 ± 136.84 | 103.22 ± 23.84 | 18.66 to 54.17 | <0.001 |
| Coronary risk | 4.25 ± 1.17 | 3.28 ± 0.59 | 0.75 to 1.21 | <0.001 |
| Liver parameters | ||||
| AST (U/L) | 27.23 ± 14.93 | 23.47 ± 11.06 | 0.51 to 7.00 | 0.02 |
| ALT (U/L) | 27.58 ± 15.89 | 20.20 ± 10.63 | 4.04 to10.72 | <0.001 |
| GGT (U/L) | 55.26 ± 36.69 | 28.96 ± 11.27 | 19.59 to 33.07 | <0.001 |
| TBIL (mg/dL) | 12.92 ± 8.13 | 13.15 ± 7.15 | −2.12 to 1.66 | 0.81 |
| DBIL (µmol/L) | 5.47 ± 3.48 | 4.85 ± 2.12 | −0.09 to 1.33 | 0.09 |
| Total protein (g/L) | 71.16 ± 15.33 | 77.21 ± 6.08 | −8.94 to −3.17 | <0.001 |
| ALP (U/L) | 81.39 ± 34.88 | 63.29 ± 18.07 | 11.23 to 24.96 | <0.001 |
| Albumin (g/L) | 40.73 ± 5.08 | 46.08 ± 2.57 | −6.34 to −4.35 | <0.001 |
| Kidney parameters | ||||
| Sodium (mmol/L) | 140.44 ± 5.34 | 140.26 ± 4.54 | −1.04 to 1.41 | 0.77 |
| Potassium (mmol/L) | 3.97 ± 0.59 | 4.33 ± 0.64 | −0.51 to −0.20 | <0.001 |
| Chloride (mmol/L) | 102.57 ± 6.09 | 106.46 ± 2.00 | −5.01 to −2.77 | <0.001 |
| Urea (mmol/L) | 7.03 ± 5.29 | 3.81 ± 0.09 | 2.28 to 4.15 | <0.001 |
| Creatinine (µmol/L) | 123.02 ± 69.88 | 94.87 ± 16.43 | 15.55 to 40.74 | <0.001 |
| Lipid parameters | ||||
| Total cholesterol (mmol/L) | 5.41 ± 1.11 | 4.47 ± 0.52 | 0.73 to 1.15 | <0.001 |
| Triglycerides (mmol/L) | 1.56 ± 0.71 | 0.75 ± 0.27 | 0.68 to 0.94 | <0.001 |
| HDL (mmol/L) | 1.43 ± 0.36 | 1.40 ± 0.21 | −0.04 to 0.10 | 0.42 |
| LDL (mmol/L) | 3.28 ± 1.19 | 2.74 ± 0.52 | 0.32 to 0.77 | <0.001 |
| VLDL (mmol/L) | 0.71 ± 0.32 | 0.34 ± 0.13 | 0.31 to 0.43 | <0.001 |
- Note: A comparison of levels of C-peptide and other biochemical and clinical (continuous) parameters of the study participants. Data presented as mean (x̄), SD = standard deviation.
- Abbreviations: ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; CKMB, creatinine kinase myocardial bound; DBIL, direct bilirubin; FPG, fasting plasma glucose; GGT, gamma glutamyl transferase; HDL, high-density lipoprotein cholesterol; LDH, lactate dehydrogenase; LDL, low-density lipoprotein cholesterol; PNBP, propeptide of brain natriuretic peptide; TBIL, total bilirubin; VLDL, very low-density lipoprotein.
- a Significant at 0.05 alpha level.
3.3 Associations between levels of C-peptide and other biochemical markers
The Pearson product-moment correlation was conducted to determine associations between the levels of C-peptide and several cardiac, liver, kidney, and lipid biomarkers among individuals with or without CVDs. The levels of troponin T in the CVD Group were found to be associated with C-peptide levels (r = 0.34, p < 0.001). In the Control Group, GGT showed a weak relationship with C-peptide levels (r = 0.19, p = 0.04). The correlation analysis is shown in Table 3.
| Features | CVD group | Control group | ||
|---|---|---|---|---|
| r | p | r | p | |
| Cardiac parameters | ||||
| Total creatine kinase | 0.02 | 0.80 | −0.09 | 0.33 |
| Creatine kinase myocardial bound | −0.16 | 0.86 | −0.12 | 0.20 |
| Lactate dehydrogenase | −0.03 | 0.73 | 0.04 | 0.67 |
| Troponin T | 0.34 | 0.001 | 0.07 | 0.41 |
| Propeptide of brain natriuretic peptide | −0.10 | 0.25 | 0.10 | 0.26 |
| Coronary risk | −0.14 | 0.12 | 0.13 | 0.14 |
| Liver parameters | ||||
| Aspartate aminotransferase | 0.05 | 0.57 | 0.01 | 0.87 |
| Alanine aminotransferase | −0.07 | 0.43 | 0.06 | 0.49 |
| Gamma-glutamyl transferase | −0.10 | 0.25 | 0.19 | 0.04 |
| Total bilirubin | −0.16 | 0.08 | −0.09 | 0.31 |
| Direct bilirubin | −0.17 | 0.06 | −0.12 | 0.18 |
| Total protein | −0.02 | 0.82 | −0.03 | 0.72 |
| Alkaline phosphatase | 0.01 | 0.94 | 0.01 | 0.95 |
| Albumin | −0.06 | 0.51 | 0.07 | 0.43 |
| Kidney parameters | ||||
| Sodium | 0.03 | 0.75 | 0.13 | 0.13 |
| Potassium | 0.07 | 0.45 | −0.10 | 0.27 |
| Chloride | 0.07 | 0.42 | 0.08 | 0.38 |
| Urea | −0.07 | 0.45 | −0.13 | 0.16 |
| Creatinine | −0.01 | 0.91 | −0.04 | 0.63 |
| Lipid parameters | ||||
| Total cholesterol | −0.11 | 0.23 | 0.11 | 0.23 |
| Triglycerides | −0.02 | 0.83 | 0.04 | 0.66 |
| High-density lipoprotein cholesterol | 0.13 | 0.14 | −0.08 | 0.39 |
| Low-density lipoprotein cholesterol | −0.13 | 0.13 | 0.12 | 0.19 |
| Very low-density lipoprotein cholesterol | −0.02 | 0.85 | 0.03 | 0.70 |
- Note: Associations between levels of C-peptide and other biochemical parameters of the study participants; significant at 0.05 alpha level, r = Pearson's correlation coefficient.
3.4 Performance of the C-peptide as a CVD diagnostic marker
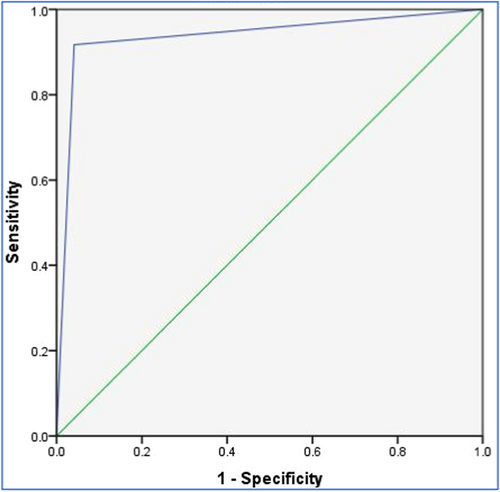
As observed in Table 4, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of C-peptide in distinguishing between CVD and non-CVD participants ranged between 91.3% and 96.1%. The ROC curve (Figure 1) showed high sensitivity and specificity. The area under the curve (AUC) for the model was large (AUC = 0.938, p < 0.001; SE = 0.017; 95% confidence interval = 0.90–0.97). Furthermore, the Kappa measure (k = 0.874, p < 0.001) indicated a high degree of agreement between the C-peptide assay and the reference CVD diagnosis.
| Metric | Score (%) |
|---|---|
| Sensitivity | 96.1 |
| Specificity | 91.3 |
| Accuracy | 93.7 |
| Positive predictive value | 91.7 |
| Negative predictive value | 95.9 |
- Note: The performance of C-peptide as a diagnostic marker of CVD. The Kappa (k) measure was 0.874 (p < 0.001).
- Abbreviation: CVD, cardiovascular diseases.
4 DISCUSSION
The present investigation sought, among others, to compare the levels of C-peptide in individuals with and without CVD. Participants in the CVD Group had considerably higher C-peptide levels (Table 2), corroborating the findings of Li et al.,23 where C-peptide was strongly associated with cardiovascular risk factors. Further, Harnishsingh and colleagues found a relationship between C-peptide levels, disease severity, and the existence of CAD.24 Other studies have found significant relationships between C-peptides, cardiovascular events, and mortality.25, 26
Basal C-peptide levels have also been linked with carotid artery intima-media thickness in T2DM patients27, 28 and myocardial infarction (MI) in the general population.29 As such, the current study contributes to the expanding body of knowledge supporting the use of C-peptide as a stand-in biomarker for CVD. In this study, C-peptide had high specificity (96.1%) and sensitivity (91.3%), indicating its potential as a CVD diagnostic marker. Any good biomarker for any disease should be simple to assess and must have high precision and accuracy. A prior study used C-peptide with a sensitivity of 83% and specificity of 89% to discriminate T1DM from T2DM in pediatric diabetes typology.30
This study also evaluated the levels of various cardiac, lipids, liver and kidney biomarkers between the two study groups, as well as their relationship with C-peptide. Participants with CVD exhibited significantly higher levels of cardiac biomarkers in this study and corroborated previous studies,31, 32 lending credence to the fact that our Case Group was indeed persons with CVD. Importantly, troponin T showed a significant association with C-peptide levels in the CVD Group. Cardiac troponins are key diagnostic markers for CVD.33 The observation of a positive association between troponin T (considered a key marker of MI and by extension, CVD) and C-peptide levels are further evidence of the potential appropriateness of C-peptide in CVD. However, C-peptide levels did not correlate with the other established CVD diagnostic markers like CK, CKMB, and PBNP.
In the current study, the findings that patients with CVD had higher levels of total cholesterol, triglycerides, and LDL confirms earlier observations.34-36 The combined action of these lipid biomarkers has been linked to plaque development and CVD progression.37, 38 Indeed, whiles raised HDL levels are connected to lower CVD risks, high levels of LDL have long been linked to increased CVD risk. HDL is known to create an enabling environment for endothelial repair and function improvement, as well as decrease inflammation, vascular thrombosis, and oxidation.39, 40
Among the liver function biomarkers ALT, AST, ALP, and GGT were higher in the CVD Group than in the Control Group, and these results were consistent with other studies.41, 42 Wannamethee et al.43 for instance, investigated the link between ALP and CVD as well as total mortality in older adults. In that study, a retrospective assessment was performed on a total of 3381 patients who had stable angina pectoris. During their hospitalization, their ALP levels were measured by an automated analyzer. A link was found by the researchers between ALP levels versus systolic blood pressure and DM.43 The CVD group had higher urea and creatinine levels compared with the control Group. Indeed, CVD and kidney disease are inexorably intertwined. Instability in one organ encourages dysfunction in the other, eventually leading to the loss of both organs.
This study does have some limitations. The fact that this research was conducted using a cross-sectional methodology places restrictions on the degree to which C-peptide could be considered a potential contributor to the observed relationships seen. To determine whether or not our findings are the product of causal connections, follow-up data will be extremely helpful. Because of time constraints as well as the specific characteristics of the CVD Group that were being targeted, it was not possible to sample a larger number of study participants. In this particular study, dietary habits and overall levels of physical activity were not taken into consideration and could be incorporated in the design of future studies. The procedure called a hyperinsulinemic-euglycaemic clamp, which is used to determine glucose disposal rates, is the ideal measure of insulin sensitivity rather than the calculated HOMA-IR used.
These limitations notwithstanding, it is noteworthy that this study is among the few to have evaluated the diagnostic potential of C-peptide among sub-Saharan Africans, and its findings fill important epidemiological gaps.
In conclusion, the Group that had CVD had higher circulating levels of C-peptide, and this peptide was demonstrated to be a good biomarker in cardiovascular illnesses, with sensitivity and specificity scores of 96.1% and 91.3%, respectively. C-peptide could, thus, potentially be a cost-effective CVD diagnostic-cum-prognostic tool, particularly, in resource-limited settings. Nonetheless, further research is needed to understand the role C-peptide plays in health and disease.
AUTHOR CONTRIBUTIONS
Laurinda Adusu-Donkor: Conceptualization; Data curation; Funding acquisition; Investigation; Writing—original draft. Emmanuel Kwaku Ofori: Conceptualization; Supervision; Validation; Writing—original draft; Writing—review & editing. Fleischer C N Kotey: Formal analysis; Software; Writing—review & editing. Francis Kwaku Dogodzi: Data curation; Methodology. Wormenor Dziedzorm: Data curation; Investigation; Methodology. Alfred Buabeng: Data curation; Investigation; Methodology. Segla Kwame Bernard: Data curation; Investigation; Methodology. Seth K Amponsah: Validation; Writing—review & editing. Henry Asare-Anane: Conceptualization; Supervision; Writing—original draft.
ACKNOWLEDGMENTS
We are grateful to all who volunteered to participate in the research. The authors would like to also appreciate the staff of the Chemical Pathology Unit, 37 Military Hospital, Accra Ghana. In addition, we express our gratitude to the Department of Chemical Pathology, University of Ghana for the institutional assistance provided.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
All participants provided written informed consent.
TRANSPARENCY STATEMENT
The lead author Emmanuel Kwaku Ofori affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
Emmanuel Kwaku Ofori and Fleischer C. N. Kotey have full access to the data and will make the datasets that were used throughout this study available to the interested party upon reasonable request.