Safety and efficacy of cerebral embolic protection devices for patients undergoing transcatheter aortic valve replacement: An updated meta-analysis
Abstract
Background and Aims
Cerebral embolic protection (CEP) devices are employed to capture embolic debris and reduce the risk of stroke during transcatheter aortic valve replacement (TAVR). Evidence is mixed regarding the safety and efficacy of CEP. We aimed to summarize the safety and effectiveness of CEP use during TAVR.
Methods
Electronic databases, including PubMed, PubMed Central, Scopus, Cochrane Library, and Embase, were searched using relevant search terms for articles relating to CEP. All relevant data from 20 studies were extracted into a standardized form. Statistical analyses were performed using Revman 5.4. Odds ratio (OR) or mean differences (MDs) were used to estimate the desired outcome with a 95% confidence interval (CI).
Results
Twenty studies (eight randomized controlled trials [RCTs]) involving 210,871 patients (19,261 in the CEP group and 191,610 in TAVR without the CEP group) were included. The use of CEP was associated with a lower odds of 30-day mortality by 39% (OR: 0.61, 95% CI: 0.53–0.70) and stroke by 31% (OR: 0.69, 95% CI: 0.52–0.92). Comparing devices, benefit in terms of mortality and stroke was observed with the use of the Sentinel device (Boston Scientific), but not among other devices. No differences were observed in the outcomes of acute kidney injury, major or life-threatening bleeding events, or major vascular complications between groups. When only RCTs were included, there were no observed differences in the primary or secondary outcomes for CEP versus no CEP use during TAVR.
Conclusions
The totality of evidence suggests a net benefit for the use of CEP, weighted by studies in which the Sentinal device was used. However, given the RCT subanalysis, additional evidence is needed to identify patients at the highest risk of stroke for optimal decision-making.
1 INTRODUCTION
Transcatheter aortic valve replacement (TAVR) is an established treatment method for severe symptomatic aortic stenosis. TAVR has shown to be associated with improved clinical outcomes compared with medical therapy and surgical aortic valve replacement (SAVR) in patients with an elevated risk for mortality with surgery.1-3 Despite a very high procedural success rate, cerebral ischemic events remain unpredictable and substantially impact long-term morbidity and mortality after TAVR.4, 5 During the TAVR, there is a possibility of debris embolizing from the aorta and aortic valve, which may result in cerebrovascular events (CVEs).6, 7 Several studies have demonstrated a high incidence of new cerebral ischemic lesions (~70%) following TAVR, identified by diffusion-weighted magnetic resonance imaging (DW-MRI).8-10
Transcranial Doppler studies during TAVR have revealed that embolic phenomena occur most commonly during the positioning of the prosthetic valve and valve insertion.11-13 Approximately half of the periprocedural CVEs become clinically apparent at least 24 h after TAVR.14-16
TAVR is shown to be associated with improved post-procedural outcomes compared with standard medical therapy, including lower mortality and better quality of life in surgically high-risk population,2, 17 and the rate of overt stroke following TAVR is also relatively low (~2%) in current practice.18 However, the burden of microembolization and small ischemic cerebral injuries may still contribute to cognitive decline.19-21
Cerebral embolic protection (CEP) devices have been employed to capture embolic debris and mitigate adverse neurological events. Several observational and randomized controlled trials (RCTs) have been conducted, but the safety and efficacy of using CEPs during TAVR remain inconclusive. Thus, we have conducted this systematic review and meta-analysis to evaluate the safety and efficacy of CEPs during TAVR.
2 METHODS
2.1 Literature search strategy
This systematic review and meta-analysis followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.22 Our protocol for this meta-analysis is available in the publicly available international prospective register of systematic reviews (PROSPERO) registry (CRD42022325385). We searched for relevant articles from web-based medical libraries, including PubMed, PubMed Central, Scopus, Cochrane Library, and Embase. We used the terms “cerebral protection system,” “transcatheter aortic valve replacement,” and “TAVR,” as the keywords for search. The reference list of the retrieved article was then imported into Covidence software.23
2.2 Selection criteria
Title and abstract screening and full-text screening were the two initial steps applied to filter the desired papers and exclude irrelevant articles for our study. Three independent researchers (SL, AB, and MS) were involved in screening and conflict management. Discrepancies were further resolved by mutual discussion and consensus.
We included the published articles such as RCTs, prospective and retrospective cohort studies, and cross-sectional studies that had compared TAVR with or without CEP through 1/9/2023.
This study did not include case reports, case series, review articles, editorials, expert opinions, studies with poorly defined outcomes, or meta-analyses. In addition, abstracts with no available full text, unpublished studies, and single-arm studies whose results had evaluated the feasibility of TAVR with only CEP were also excluded during the full-text review.
2.3 Data extraction
We extracted variables under sub-headings including baseline characteristics (participant number, mean age, male population, and other comorbid medical and surgical conditions), procedural characteristics (TAVR site, valve type, CEP type, procedural time, imaging assessment time frame, and neurocognitive assessment). Primary endpoints were 30-day all-cause mortality and 30-day stroke. Secondary endpoints were related to imaging evidence of emboli after TAVR, as measured by DW-MRI, acute kidney injury (AKI), significant or life-threatening bleeding, and major vascular complications. Imaging endpoints included the number of patients with new ischemic lesions, the total volume of lesions (TVL), and the number of new lesions.
2.4 Data analysis
Data were analyzed using RevMan 5.4.24 Random/fixed-effects models were used to determine the pooled odds ratio (OR) to estimate the outcome with a 95% confidence interval (CI) based on heterogeneity. We used the mean difference (MD) for DW-MRI findings for the volume of lesions and the number of new lesions. The median and standard deviations were calculated using the median and interquartile range, if those values were not provided in the studies.25 A forest plot was used to represent the degree of variation between studies.
2.5 Assessment of risk of bias in included studies
Risk of bias assessment was performed using the Cochrane Risk of Bias (ROB) 2.0 tool for RCTs, shown in the (Supporting Information: Figure S1). We used the Joanna Briggs Institute critical appraisal checklist for non-RCT studies (Supporting Information: Table 1).26, 27 RevMan 5.4 was used to summarize biases for RCTs using the Cochrane ROB 2.0 tool.
2.6 Assessment of heterogeneity
The I-squared (I2) test was employed to assess heterogeneity, and interpretation was done based on the Cochrane Handbook for Systematic Reviews of Interventions.
2.7 Subgroup analyses and sensitivity analysis
Subgroup analyses were performed between RCTs and observational studies and between types of CEP devices to evaluate their impact on the overall result. A sensitivity analysis was performed by excluding studies with fewer than 50 patients in a particular group to omit the skewed result based on the shared weight in the result.
3 RESULTS
3.1 Study selection and study population
A total of 1057 studies were identified after the databases were searched. After removing 125 duplicates, the title and abstract of 932 studies were screened, and 106 studies were eligible for full-text review. A total of 86 records were excluded for reasons described in Figure 1. We included 20 studies for our quantitative synthesis, as represented in the PRISMA flow diagram (Figure 1).
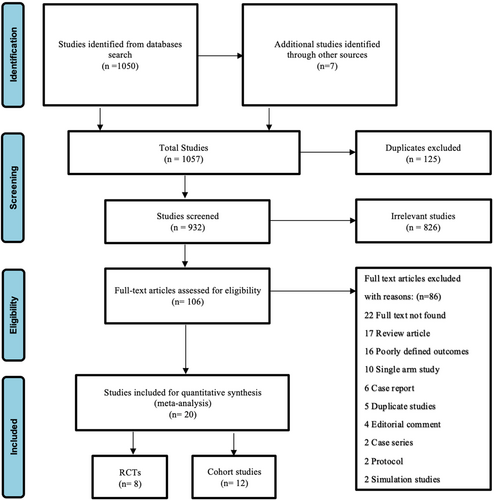
Among 20 studies, 8 RCTs and 12 cohort studies were included comparing the efficacy of CEP with no CEP in patients undergoing TAVR. These studies included a total of 210,871 patients, with 19,261 undergoing TAVR with CEP and 191,610 undergoing TAVR without CEP. 53.3% were male.
Baseline characteristics of the participants in the included studies are shown in Table 1. Procedural and outcome details are shown in Table 2.
| Study | Year | Type of study | Intervention | N | Mean age (years) | Sex (male) | DM | HTN | CKD | CAD | PVD | H/O CVD (stroke, TIA) | TAVR access site |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wendt et al. (EMBOL-X)28 | 2015 | RCT | With CEP | 14/30 | (81.0 ± 5.0) | 4/14 | – | – | 6/14 | – | 5/14 | 1/14 | Transaortic 100% |
| Without CEP | 16/30 | (82.1 ± 4.1) | 8/16 | 6/16 | 6/16 | 3/16 | |||||||
| Lansky et al. (DEFLECT III)29 | 2015 | RCT | With CEP | 46/85 | (82.5 ± 6.5) | 20/46 | 10/46 | 37/46 | 11/46 | 6/46 | 6/46 | 6/46 | Transfemoral 96.47%, Transapical 3.53% |
| Without CEP | 39/85 | (82.3 ± 6.0) | 19/39 | 9/39 | 28/39 | 10/39 | 8/39 | 5/39 | 7/39 | ||||
| Kapadia et al. (SENTINEL)30 | 2017 | RCT | With CEP | 244/363 | 82.8 | 113/244 | 82/244 | 127/244 | 37/244 | 17/244 | Transfemoral 94.7% | ||
| Without CEP | 119/363 | 85 | 61/119 | 45/119 | 66/119 | 18/119 | 7/119 | ||||||
| Lind et al.31 | 2022 | Retrospective study | With CEP | 18/51 | (91.7 ± 2.1) | 8/18 | 3/18 | – | 10/18 | 10/18 | 3/18 | 8/18 | Transfemoral |
| Without CEP | 33/51 | (92 ± 1.85) | 14/33 | 8/33 | – | 22/33 | 27/33 | 11/33 | 5/33 | ||||
| Butala et al.32 | 2021 | Retrospective study | With CEP | 12,409/123,186 | 79.0 ± 8.9 | 7341/12,409 | – | – | – | – | 3059/12,398 | 1253/12,396 | Transfemoral |
| Without CEP | 110,777/123,186 | 79.4 ± 8.8 | 60,571/110,777 | 25,793/110,657 | 10,180/11,0 628 | ||||||||
| Megaly et al.33 | 2020 | Retrospective study | With CEP | 525/36,220 | 81 (76–87) | 280/525 | 210/525 | 105/525 | 165/525 | 65/525 | 85/525 | 60/525 | |
| Without CEP | 35,695/36,220 | 81 (75–86) | 19,215/35,695 | 13,590/35,695 | 9505/35,695 | 11,685/35,695 | 4695/35,695 | 8310/35,695 | 4195/35,695 | ||||
| Rodés-Cabau et al. (PROTAVI-C)34 | 2014 | Pilot N.R. study | With CEP | 41/52 | 83 (79–86) | 19/41 | 14/41 | 36/41 | 18/41 | 24/41 | 6/41 | 0 | Transfemoral |
| Without CEP | 11/52 | 84 (78–89) | 8/11 | 5/11 | 10/11 | 6/11 | 5/11 | 1/11 | 0 | ||||
| Samim et al.35 | 2015 | Retrospective study | With CEP | 15/52 | 84 (73–87) | 8/15 | 5/15 | 8/15 | - | 8/15 | 1/15 | 3/15 | Transfemoral 100% |
| Without CEP | 37/52 | 81 (78–84) | 21/37 | 9/37 | 21/37 | 25/37 | 3/37 | 5/37 | |||||
| Stachon et al.36 | 2021 | Retrospective study | With CEP | 1564/41,654 | 80.62 ± 6.36 | 722/1564 | 539/1564 | 935/1564 | 45/1564 | 822/1564 | 174/1564 | Transfemoral | |
| Without CEP | 40,090/41,654 | 81.14 ± 6.02 | 18,778/40,090 | 13,009/40,090 | 25,437/40,090 | 1800/40,090 | 19,528/40,090 | 3311/40,090 | |||||
| Van Mieghem et al. (MISTRAL-C)37 | 2016 | RCT | With CEP | 32/65 | 82 (79–84) | 17/32 | 4/32 | 21/32 | – | 2/32 | 9/32 | 6/32 | Transfemoral |
| Without CEP | 33/65 | 82 (77–86) | 17/33 | 9/33 | 23/33 | 2/33 | 11/33 | 6/33 | |||||
| Seeger et al.38 | 2017 | Prospective study | With CEP | 280/802 | 80.6 ± 6.0 | 128/280 | 84/280 | 81/280 | 166/280 | 18/280 | 26/280 | Transfemoral 100% | |
| Without CEP | 522/802 | 80.5 ± 6.2 | 256/522 | 155/522 | 180/522 | 330/522 | 53/522 | 63/522 | |||||
| Seeger et al. (RESPOND)39 | 2020 | Prospective study | With CEP | 92/996 | 80.0 ± 6.4 | 37/92 | 21/92 | 80/92 | 53/92 | 11/92 | Transfemoral | ||
| Without CEP | 904/996 | 80.0 ± 6.5 | 452/904 | 202/904 | 709/904 | 505/904 | 83/904 | ||||||
| Voss et al.40 | 2019 | Retrospective study | With CEP | 39/391 | 79.1 ± 7.3 | 18/39 | – | – | – | 23/39 | – | 2/39 | Radial (35), brachial (4) |
| Without CEP | 352/391 | 79.5 ± 7.2 | 174/352 | 192/352 | 28/352 | ||||||||
| Haussig et al. (CLEAN TAVI)41 | 2016 | RCT | With CEP | 50/100 | 80 ± 5.1 | 21/50 | 20/50 | 44/50 | 43/50 | 26/50 | 2/50 | 1/50 | Transfemoral |
| Without CEP | 50/100 | 79.3 ± 4.1 | 22/50 | 25/50 | 47/50 | 39/50 | 25/50 | 4/50 | 3/50 | ||||
| Dona et al.42 | 2022 | Prospective study | With CEP | 213/411 | 80.4 ± 6.7 | 118/213 | 71/213 | 189/213 | – | 133/213 | 27/213 | 15/213 | Transfemoral |
| Without CEP | 198/411 | 80.4 ± 6.8 | 98/198 | 65/198 | 175/198 | 127/198 | 19/198 | 15/198 | |||||
| Nazif et al. (REFLECT II)43 | 2021 | RCT | With CEP | 157/214 | 80.31 ± 7.73 | 86/157 | 61/156 | – | 36/157 | – | 20/155 | 27/157 | Transfemoral 100% |
| Without CEP | 57/214 | 78.05 ± 8.19 | 35/57 | 23/57 | 17/57 | 11/57 | 3/57 | ||||||
| Lansky et al. (REFLECT I)44 | 2021 | RCT | With CEP | 141/204 | 79.8 ± 7.3 | 80/141 | 60/140 | – | 27/136 | – | 15/134 | 18/137 | Transfemoral |
| Without CEP | 63/204 | 81.5 ± 7.1 | 42/204 | 20/63 | 11/62 | 8/59 | 7/62 | ||||||
| Kemp et al.45 | 2022 | Prospective study | With CPS | 78/157 | 84 ± 4 | 43/78 | 25/78 | 60/78 | 28/78 | 13/78 | Badial. Brachial Transfemoral | ||
| Without CPS | 79/157 | 84 ± 4 | 36/79 | 26/79 | 59/79 | 26/79 | 9/79 | ||||||
| Isogai et al.46 | 2022 | Retrospective cohort study | With CPS | 1802/2839 | 78.6 ± 9.4 | 1081/1802 | 678/1802 | 1611/1802 | 45/1802 | 344/1802 | 385/1802 | Transfemoral | |
| Without CPS | 1037/2839 | 80.2 ± 9.5 | 581/1037 | 379/1037 | 948/1037 | 43/1037 | 274/1037 | 238/1037 | |||||
| Kapadia et al. (PROTECTED-TAVR)47 | 2022 | RCT | With CPS | 1501/3000 | 78.9 ± 8.0 | 870/1501 | 501/1501 | 1306/1500 | 850/1493 | 165/1484 | 114/1496 | Transfemoral | |
| Without CPS | 1499/3000 | 78.9 ± 7.8 | 933/1499 | 522/1499 | 1312/1497 | 880/1493 | 162/1481 | 122/1491 |
| Study | Intervention | Type of valve | Type of CEP | All-cause mortality | All stroke | Major bleeding complications | AKI (Stage II/III) | Major vascular complications | Procedural success | 30-day events | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | Overt stroke | Life-threatening bleeding | AKI | Major vascular complications | ||||||||||
| Wendt et al. (EMBOL-X)28 | With CEP | SAPIEN-XT | EMBOL-X | 0 | 0 | – | – | – | 100% | NA | – | – | – | – |
| Without CEP | 0 | 0 | NA | |||||||||||
| Lansky et al. (DEFLECT III)29 | With CEP | SAPIEN-XT | TriGuard | 1/46 | 1/46 | 1/46 | 1/46 | 7/46 | 88.90% | 1/46 | 2/46 | 2/46 | 1/46 | 8/46 |
| Without CEP | SAPIEN-XT, CoreValve | 2/39 | 2/39 | 2/39 | 0/39 | 6/39 | 2/39 | 2/39 | 3/39 | 0 | 8/46 | |||
| Kapadia et al. (SENTINEL)30 | With CEP | SAPIEN-XT, SAPIEN-3, CoreValve, Evolut-R | Sentinel | 94.40% | 3/234 | 13/231 | 1/231 | 21/244 | ||||||
| Without CEP | 2/111 | 10/110 | 0/111 | 7/119 | ||||||||||
| Lind et al.31 | With CEP | CoreValve Evolut, Sapien S3 | TriGuard 3 | – | – | 7/18 | 2/18 | – | 100% | 0/18 | 2/18 | – | – | – |
| Without CEP | 17/33 | 1/33 | – | 100% | 4/33 | 10/33 | ||||||||
| Butala et al.32 | With CEP | CoreValve, Sapien | Sentinel | 99/12,409 | 158/12,409 | 491/12,266 | – | – | 96.90% | 162/11,658 | 216/11,682 | – | – | – |
| Without CEP | 1317/110,777 | 1716/110,777 | 4808/108,858 | 97.30% | 2297/102,877 | 2224/102,919 | ||||||||
| Megaly et al.33 | With CEP | Sentinel | 0/525 (in hospital) | 5/525 | – | – | 0/525 | – | – | – | – | – | – | |
| Without CEP | 510/35,695 | 920/35,695 | 30/35,695 | |||||||||||
| Rodés-Cabau et al. (PROTAVI-C)34 | With CEP | SAPIEN-XT | Embrella Embolic Deflector | – | – | – | – | – | 100% | 3/41 | 3/41 | 3/41 | 3/41 | 5/41 |
| Without CEP | 0/11 | 0/11 | 0 | 0 | 1/11 | |||||||||
| Samim et al.35 | With CEP | Medtronic core valve, Edwards SAPIEN XT | Embrella Embolic Deflector System | - | 0/15 | – | – | – | 100% | 0/15 | 0/15 | – | – | – |
| Without CEP | 0/37 | 100% | 0/37 | 0/37 | ||||||||||
| Stachon et al.36 | With CEP | 30/1564 (in hospital) | 44/1564 | – | 123/1564 | – | – | – | – | – | – | |||
| Without CEP | 1033/40,090 | 849/40,090 | 2897/40,090 | |||||||||||
| Van Mieghem et al. (MISTRAL-C)37 | With CEP | SAPIEN-XT, SAPIEN-3, Medtronic CoreValve, Balloon dilatation, Portico | Sentinel | 93.75% | 1/32 | 0/32 | 1/32 | 0/32 | 0/32 | |||||
| Without CEP | 3/33 | 2/33 | 5/33 | 1/33 | 6/33 | |||||||||
| Seeger et al.38 | With CEP | Claret dual valve sentinel type | 2/280 (7-day follow-up) | 4/280 | 4/280 | 3/280 | 5/280 | 91.8% | – | – | – | – | – | |
| Without CEP | 11/522 | 22/522 | 21/522 | 7/522 | 19/522 | |||||||||
| Seeger et al. (RESPOND)39 | With CEP | lotus valve | 1/92 | 1/92 | NA | NA | ||||||||
| Without CEP | 30/904 | 31/904 | ||||||||||||
| Voss et al.40 | With CEP | Medtronic CoreValve, Edwards Sapien 3 valve | Claret Sentinel | – | – | 1/39 | 3/39 | 1/39 | 94.90% | – | – | – | – | – |
| Without CEP | 11/352 | 39/352 | 9/352 | |||||||||||
| Haussig et al. (CLEAN TAVI)41 | With CEP | Medtronic Core Valve | Claret Montage Dual Filter System | – | 5/50 | – | – | – | 90% | 0 | 4/50 | 1/50 | 1/50 | 5/50 |
| Without CEP | 5/50 | 1/50 | 4/50 | 1/50 | 5/50 | 6/50 | ||||||||
| Dona et al.42 | With CEP | Sentinel | 1/213 (in hospital) | 5/213 | – | – | – | 88.7% | 19/213 | – | – | – | – | |
| Without CEP | 1/198 | 13/198 | 32/198 | |||||||||||
| Nazif et al. (REFLECT II)43 | With CEP | Medtronic Core Valve, Edwards SAPIEN | TriGuard 3 | 4/157 | 13/157 | 9/157 | 4/157 | 11/157 | 67.60% | 4/157 | 13/157 | 9/157 | 4/157 | 11/157 |
| Without CEP | 1/57 | 3/57 | 0/57 | 0/57 | 0/57 | 1/57 | 3/57 | 0/57 | 0/57 | 0/57 | ||||
| Lansky et al. (REFLECT I)44 | With CEP | Medtronic Corevalve | TriGuard HDH | 0/141 | 13/141 | 4/141 | 0/141 | 16/141 | 93.40% | 2/131 | 14/131 | 4/130 | 0/129 | 16/130 |
| Without CEP | 0/63 | 4/63 | 0/63 | 0/63 | 1/63 | 0/59 | 4/59 | 0/59 | 0/59 | 1/59 | ||||
| Kemp et al.45 | With CPS | Boston Accurate Neo, Boston Lotus Edge and Medtronic evolut R | Sentinel | 0/78 | 0/78 | 3/78 | 0/78 | |||||||
| Without CPS | 2/79 | 5/79 | 6/79 | 99.00% | 2/79 | |||||||||
| Isogai et al.46 | With CPS | Sentinel | 2/1802 | 8/1802 | 2/1802 | 8/1802 | ||||||||
| Without CPS | 8/1037 | 15/1037 | 8/1037 | 15/1037 | ||||||||||
| Kapadia et al. (PROTECTED-TAVR)47 | With CPS | Sentinel | 8/1501 | 34/1501 | 8/1501 | 94.4% | ||||||||
| Without CPS | 4/1499 | 43/1499 | 7/1499 | |||||||||||
4 QUANTITATIVE SYNTHESIS
ORs were used to measure outcome estimation for 30-day all-cause mortality and stroke as co-primary outcomes. DW-MRI findings (number of patients with new lesions, volume of lesions, and number of new lesions) and other complications (AKI, significant/life-threatening bleeding, and major vascular complications) were secondary outcome variables.
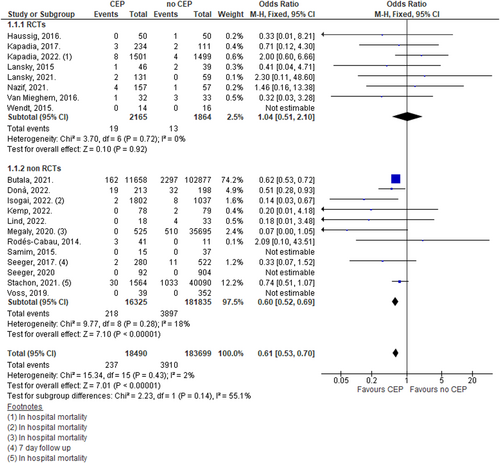
4.1 Thirty-day all-cause mortality
Pooling data using a fixed effect model from 20 studies demonstrated a 39% lower odds of 30-day mortality amongst patients undergoing TAVR with CEP (OR: 0.61, 95% CI: 0.53–0.70; n = 202,189; I2 = 2%). Analysis including only RCTs did not show a significant difference (OR: 1.04, 95% CI: 0.51–2.10; n = 4029; I2 = 0%). Thirty-day mortality was significantly lower among patients undergoing TAVR with CEP group compared with patients undergoing TAVR without CEP when only observational real-world studies were included (OR: 0.60, 95% CI: 0.52–0.69; n = 198,160; I2 = 18%; Figure 2).
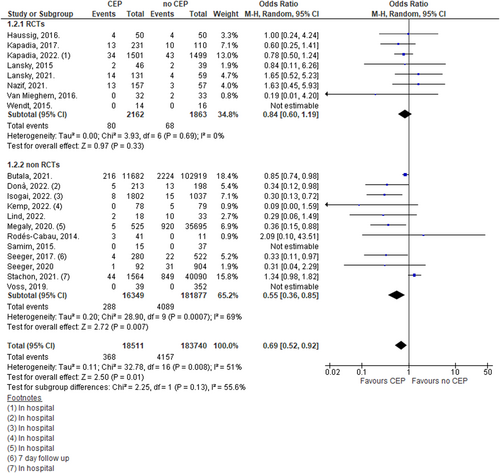
4.2 Thirty-day stroke events
Overall analysis using random effect models demonstrated a significant reduction in the occurrence of stroke at 30 days in TAVR with the CEP group in comparison to TAVR only (OR: 0.69, 95% CI: 0.52–0.92; n = 202,251; I2 = 51%). However, when only RCTs were included, there was no significant benefit of CEP over no CEP (OR: 0.84, 95% CI: 0.60–1.19; n = 4025; I2 = 0%; Figure 3).
4.3 Sensitivity analysis
A sensitivity analysis excluding studies with fewer than 50 patients demonstrated a lower odds of mortality in TAVR with CEP group compared to those without CEP, however when only RCTs were included, this became nonsignificant (Supporting Information: Figure 2).
Excluding studies with fewer than 50 patients, there was a reduction in stroke when CEP was used with TAVR versus no CEP (OR: 0.72, 95% CI: 0.53–0.98; n = 201,368; I2 = 60%). When only RCTs were included, this finding did not reach significance (OR: 0.86, 95% CI: 0.60–1.22; n = 3845; I2 = 0%; Supporting Information: Figure 3).
4.4 Subgroup analysis
Thirty-day mortality based on CEP device: We performed a subgroup analysis to compare individual CEP devices. Thirteen studies used the Sentinel (Boston Scientific) device, 4 studies used Triguard (Keystone Heart) device and the rest used the Embrella (Edwards Lifesciences) and Embol-x systems (Edwards Lifesciences). In subgroup analysis performed based on the device used, using fixed effect model from 13 studies, 4 RCTs and 9 non-RCTs, we noted a 39% lower odds of 30-day mortality in the TAVR with CEP group when the Sentinel device was used as the CEP while comparing TAVR without CEP (OR: 0.61, 95% CI: 0.53–0.70; n = 201,515; I2 = 21%). However, analysis performed only on the four RCTs showed no significant difference. The analysis on other devices showed no significant difference. (Supporting Information: Figure 4).
A subgroup analysis using a random effect model from the 13 studies using the Sentinel device showed a 37% lower odds of 30-day stroke in TAVR with the CEP group where the Sentinel device was used as the CEP while comparing TAVR without CEP (OR: 0.63, 95% CI: 0.46–0.87; n = 201,577; I2 = 61%; Supporting Information: Figure 5).
4.5 DW-MRI assessment
4.5.1 Patients with new lesions
DW-MRI was performed in seven studies, five RCTs and two non-RCTs that included a total of 586 patients. The overall incidence of a patient with a new lesion was 85.1% (274/322) with the use of CEP and 86.7% (229/264) in patients without the use of CEP. The statistical analysis showed no difference in the number of new lesions (MD: 0.79, 95% CI: 0.49–1.28; n = 586; studies = 7; I2 = 0%; Supporting Information: Figure 6).
4.5.2 Total volume of lesions
The TVL was 88–511 mm³ in patients with CEP and 168–942 mm³ in patients without CEP. The use of CEP during TAVR was not associated with lower total volume of lesions (MD: −76.03, 95% CI: −169.44 to 17.38; n = 765; studies = 8; I2 = 69%; Supporting Information: Figure 7).
4.5.3 Number of new lesions per patient
Meta-analysis of the number of new lesions per patient among those treated with CEP with TAVR versus TAVR only showed no statistical difference between the two (MD: −0.26, 95% CI: −2.08 to 1.56; n = 728; studies = 7; I2 = 80%;Supporting Information: Figure 8).
4.6 Major complications
4.6.1 Acute kidney injury
There was no statistical difference in the odds of AKI among those treated with CEP with TAVR versus TAVR only (OR: 1.06, 95% CI: 0.89–1.27; n = 47,101; studies = 13; I2 = 0%; Supporting Information: Figure 9).
4.6.2 Major or life-threatening bleeding
The odds of major or life-threatening bleeding on Day 30 did not differ between the two groups (OR: 0.86, 95% CI: 0.70–1.06; n = 123,073; I2 = 3%; Supporting Information: Figure 10).
4.6.3 Major vascular complications
There was no difference in the odds for developing major vascular complications among those treated with CEP with TAVR versus TAVR only (OR: 1.08, 95% CI: 0.59–1.95; n = 38,488; studies = 10; I2 = 30%; Supporting Information: Figure 11).
4.7 Publication bias
Publication bias of included studies was assessed using Egger's funnel plots (Supporting Information: Figures 12 and 13).
5 DISCUSSION
The transcatheter approach to aortic valve replacement reduces cardiac symptoms, hastens recovery time, and has been shown to reduce the 1-year mortality rate by 20% in patients at high surgical risk.2 However, the risk of stroke remains a substantial concern, with rates of stroke around 5% in RCTs comparing TAVR with SAVR in high-risk patients.1, 2 Thus, the CEP was developed to diminish the risk of CVEs, shorten the length of stay, and improve the overall survival rate.32, 33, 38, 42 This meta-analysis investigated the safety and efficacy of CEP use during TAVR. Our analysis showed overall 39% lower odds in 30-day mortality (OR: 0.61, 95% CI: 0.53–0.70), and 31% lower odds of stroke at 30 days (OR: 0.69, 95% CI: 0.52–0.92). In our subgroup analysis, these findings were weighted by the findings from the Sentinel CEP; however, four RCTs including Sentinel devices did not reach significance. These findings are congurent with previous results of Butala et al. which study described lower odds of mortality and lower trend in stroke in national inpatient registry-based data.32 Giustino et al. previously did not observe any significant reduction in clinically overt stroke or all-cause mortality.48 We believe that the differences between our findings and Giustino et al. can be explained by inclusion of only four RCTs with total population of 252.
Despite similar stroke rates with and without the use of CEP, disabling stroke is thought to occur less commonly among the CEP group.47 CEP devices are designed to primarily protect the carotid arterial system and current studies are inconclusive about the stroke distribution and stroke size. Further RCTs that compare stroke distribution and stroke severity should be conducted to assess the potential benefit of CEP devices.
In this study, the secondary outcomes of imaging-based embolic phenomena did not show significant differences between patients treated with or without CEP during TAVR. Among prior studies, only Haussaig et al.41 described a statistically significant decrease in the volume of lesions, while all other studies failed to show differences.28, 30, 34, 35, 37, 43, 44 Most RCT studies have used 3 T DW-MRI brain imaging, aside from a study by Wendt et al.28 that used 1.5 T DW-MRI, which is maybe less sensitive. Studies consistently obtained postprocedure MRIs at 7- and 30-day post-TAVR; some studies were limited by not having access to preprocedural MRIs.29
We did not observe any differences in the odds of major or life-threatening bleeding, acute kidney injury, or major vascular complications. Our findings can be compared with the results of Ndunda et al. for AKI and major vascular complications.49
5.1 Limitations
Inclusion of only randomized studies failed to show a reduction in mortality or stroke when CEP was used in conjunction with TAVR versus TAVR alone. It is possible that the findings are due to insufficient total sample size and are not powered adequately enough to show significant differences. The relative infrequency of clinically evident strokes and also heterogeneity among the studies may have contributed to this lack of differences. Included studies used real-world data and included different CEP devices, which may differ in their design and efficacy. Also, included studies did not analyze the neurocognitive outcome of patients due to the limited availability of data.
6 CONCLUSIONS
This meta-analysis suggests that the use of CEP is associated with lower odds of 30-day mortality only when non-randomized studies were pooled with data from RCTs. There was no overall reduction in stroke when CEP was used with TAVR compared with patients treated only with TAVR. Our subanalysis suggests that outcomes were more compelling when the Sentinel CEP device was used. Operators will need to determine CEP use based on clinical judgment until new iterations of the device are available, or additional studies compel more or less utilization.
AUTHOR CONTRIBUTIONS
Dhan Bahadur Shrestha: Conceptualization; data curation; formal analysis; methodology; project administration; resources; software; validation; visualization; writing—original draft; writing—review and editing. Jurgen Shtembari: Conceptualization; methodology; visualization; writing—original draft; writing—review and editing. Sandesh Lamichhane: Data curation; investigation; methodology; project administration; resources; software; writing—original draft; writing—review and editing. Abinash Baniya: Data curation; investigation; resources; software; writing—original draft; writing—review and editing. Manoj Shahi: Data curation; investigation; methodology; project administration; resources; software; writing—original draft; writing—review and editing. Swati Dhungel: Project administration; supervision; writing—review and editing. Kailash Pant: Project administration; supervision; validation; writing—review and editing. Nadia R. Sutton: Conceptualization; investigation; methodology; project administration; supervision; validation; visualization; writing—review and editing. Pedro Villablanca: Investigation; methodology; project administration; supervision; validation; writing—review and editing. Sudhir Mungee: Project administration; supervision; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Abinash Baniya affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Open Research
DATA AVAILABILITY STATEMENT
All data are available in the manuscript and Supporting Information files.