221 | DETERMINING THE PROGNOSTIC SIGNIFICANCE OF THE Ki67 PROLIFERATION INDEX IN TREATMENT-NAÏVE FOLLICULAR LYMPHOMA: AN AUSTRALASIAN LYMPHOMA REGISTRY (LaRDR) Study
Introduction: The prognostic value of the Ki67 proliferation index in follicular lymphoma (FL) is uncertain. While inferior progression-free survival (PFS) and shorter time to first treatment (TTFT) have been associated with high Ki67, prior studies were limited by small sample size. Here we report associations between characteristics and outcomes of Australasian FL patients (pts) from the LaRDR according to Ki67. We aim to determine (1) the prognostic value of Ki67 expression in pts with treatment-naive FL, and (2) the concordance of Ki67 expression levels between data reported in the LaRDR database and extracted from source diagnostic pathology reports.
Methods: Pts ≥ 18y with newly diagnosed FL were included. Grade 3B or pts with any previous lymphoma diagnoses were excluded. Pt characteristics, demographics, laboratory results, staging, histological grade, proportion of Ki67 expression and treatment were extracted from LaRDR and analysed using descriptive statistics. Intrafollicular Ki67 expression and/or range from source pathology reports were extracted. FL International Prognostic Index (FLIPI) was derived using available variables. PFS and overall survival (OS) were analysed by Cox proportional regression and Kaplan-Meier method. Survival outcomes were analysed according to Ki67 percentage, or low (< 30%) versus high (≥ 30%) expression, as continuous or categorical variables, respectively.
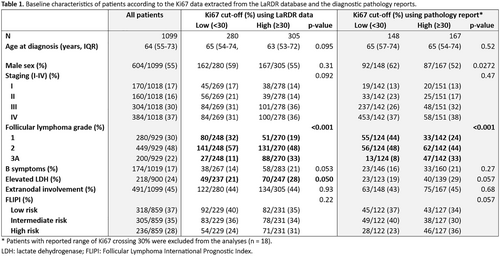
Results: 1099 FL pts were identified, with median follow-up of 23 m (Table 1). Median pt age was 64y, 55% were male, with stage III-IV disease in 67%, extranodal disease in 45%, Grade 3A in 22%, B symptoms in 17%, elevated lactate dehydrogenase in 24%. FLIPI was available in 78% pts (37% low, 35% intermediate, 28% high risk). 49% had commenced systemic therapy after a median of 2 m from diagnosis. 58% (639/1099) had Ki67 data in LaRDR. Pathology reports were accessible in 460/1099 with 333 (72%) reporting Ki67. The Ki67 expression was concordant in 91% (199/220) of pts with data in both LaRDR and pathology reports. There were no baseline characteristic differences between pts with versus without available Ki67 data. High Ki67 expression correlated with grade 3A disease (p < 0.001), in both Ki67 data sources. Based on pathology reports, high Ki67 conferred inferior PFS (HR = 1.646, 95% CI: = 1.036–2.613, p = 0.035). Ki67 as a continuous variable was also associated with inferior PFS (HR = 1.011, 95% CI: = 1.001–1.022, p = 0.036). No difference in OS nor TTFT was found according to Ki67 categories for either data source, in the context of short median duration of follow-up.
Conclusions: In this, the first registry study, and the largest FL cohort, we found inferior PFS in pts with high Ki67. The high concordance between entered data for Ki67 in LaRDR and source pathology reports supports the validity of registry data. Large-scale studies with central assessment of Ki67 expression levels by international consortia may confirm the prognostic role of Ki67 in pts with FL.
Encore Abstract: EHA 2025
Keywords: other; diagnostic and prognostic biomarkers; indolent non-Hodgkin lymphoma
Potential sources of conflict of interest:
E. A. Hawkes
Consultant or advisory role: (*Paid to institution) for Roche*, Merck Sharpe & Dohme*, AstraZeneca*, Gilead, Antengene*, Novartis*, Regeneron, Janssen*, Specialised Therapeutics*, Sobi*
Educational grants: AstraZeneca
Other remuneration: Research funding (paid to institution) from Roche, Bristol Myers Squibb, Merck KgA, AstraZeneca, TG therapeutics and Merck