163 | TAZEMETOSTAT PLUS AMDIZALISIB IN RELAPSED/REFRACTORY PERIPHERAL T-CELL LYMPHOMA PATIENTS WITH DIVERSE GENOMIC SIGNATURES: RESULTS FROM A PHASE 2 STUDY
Introduction: Peripheral T-cell lymphomas (PTCL) are aggressive, heterogeneous T cell neoplasms lacking effective treatments. Patients (pts) with distinct gene signatures respond differently to therapies. Tazemetostat (TAZ) is the first enhancer-of-zeste-homolog-2 (EZH2) inhibitor approved by FDA in 2020. Amdizalisib is a novel and highly potent selective inhibitor of phosphatidylinositol 3-kinase p110δ isoform (PI3Kδ). This open label, single-arm, phase 2 trial (NCT05713110) evaluated the efficacy and safety of TAZ plus Amdizalisib in relapsed/refractory (R/R) lymphoma. Herein, we report the results in PTCL pts with genomic data.
Methods: Pts with histologically confirmed R/R PTCL after at least one systemic therapy were eligible. TAZ 800 mg BID plus Amdizalisib 20 or 30 mg QD were administered orally until disease progression, intolerability, or withdrawal. The responses were assessed by investigators according to LUGANO 2014 criteria. Somatic gene alterations of tumor and blood samples were detected by next generation sequencing (NGS) (188-gene panel, Genetron).
Results: Up to 31 Dec 2024, 29 PTCL pts were enrolled with median age of 61 years, 69.0% were male, 44.8% were stage IV and 20.7% with bone marrow involvement at baseline. Median prior systemic therapy lines were 3 (range 1–13), and 24 (82.8%) pts were refractory to the last regimen.
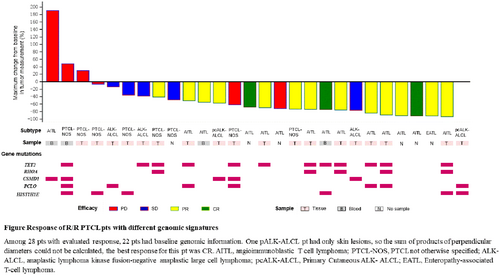
The overall response rate (ORR) was 60.7% (4 complete responses [CRs], 13 partial responses [PRs]) in 28 evaluable pts. The ORRs were 85.7% in angioimmunoblastic T cell lymphoma (AITL) (3 CRs, 9 PRs/14 evaluable pts), 25.0% in PTCL, not otherwise specified (2 PRs/8 evaluable pts), 100.0% in primary cutaneous anaplastic lymphoma kinase fusion-negative anaplastic large cell lymphoma (pcALK-ALCL) (1 CR, 1 PR/2 evaluable pts) and no response was observed in systemic ALK-ALCL pts (3 stable disease/3 evaluable pts). Median duration of response and progression-free survival were 6.47 and 8.25 months (m), respectively within a median follow-up of 10.97m. The overall survival has not been reached due to insufficient death events observed.
Genomic characteristics were assessed in 22 pts. TET2 (55%) was the most prevalent mutation, followed by PCLO (32%), HIST1H1E (27%), CSMD1 (23%), and RHOA (23%). ORR of 100% was observed in 5 pts with TET2 and RHOA co-mutations. CSMD1 and RHOA mutations were mutually exclusive. CSMD1 alteration was associated with low ORR of 20%.
Median drug administration duration was 7.33m for 29 pts. Most pts (96.6%) experienced at least one treatment-related adverse event (TRAE), including 48.3% ≥ grade 3. The most common (> 10%) grade ≥ 3 TRAEs were neutropenia (24.1%), anaemia (17.2%), lymphopenia (13.8%), and thrombocytopenia (13.8%). No treatment-related death occurred.
Conclusions: TAZ plus Amdizalisib has shown favorable responses in R/R PTCL, especially in AITL. TET2 and RHOA co-mutations are associated with better outcome. More patients are necessary for further validation.
Research funding declaration: This study is funded by HUTCHMED, Shanghai, China.
Keywords: genomics, epigenomics, and other -omics; aggressive T-cell non-Hodgkin lymphoma; molecular targeted therapies
Potential sources of conflict of interest:
X. Jia
Employment or leadership position: HUTCHMED Limited
L. Jiao
Employment or leadership position: HUTCHMED Limited
J. Wangwu
Employment or leadership position: HUTCHMED Limited
X. Luo
Employment or leadership position: HUTCHMED Limited
C. Guan
Employment or leadership position: HUTCHMED Limited
D. Chen
Employment or leadership position: HUTCHMED Limited
S. Fan
Employment or leadership position: HUTCHMED Limited
M. M. Shi
Employment or leadership position: HUTCHMED Limited
W. Su
Employment or leadership position: HUTCHMED Limited