Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma (R/R CLL/SLL): Impact on health-related quality of life
Introduction: Zanubrutinib (zanu) is a potent and highly selective next-generation Bruton tyrosine kinase (BTK) inhibitor designed to maximize BTK occupancy and minimize off-target effects. In the ALPINE study (NCT03734016), zanu demonstrated superiority to ibrutinib (ibr) in both progression-free survival (PFS) and overall response rate and a more favorable safety profile in R/R CLL/SLL. The purpose of this analysis was to assess health-related quality of life (HRQOL) in patients (pts) treated with zanu and ibr. Results from the data cutoff related to recent PFS analysis (8 Aug 2022) are reported here.
Methods: HRQOL was measured by EORTC QLQ-C30 and EQ-5D-5L at baseline, cycle 1, and every 3rd 28-day cycle until end of treatment. Key pt-reported outcome (PRO) endpoints included global health status (GHS), physical and role functions, fatigue, pain, diarrhea, and nausea/vomiting. Descriptive analysis was conducted on all the scales; a mixed-model repeated-measure analysis using key PRO endpoints at the key clinical cycles of cycles 7 (6 months) and 13 (12 months) was performed. Adjusted completion rates were defined as the number of pts who completed the questionnaires at each cycle divided by number still on treatment. Clinically meaningful was defined as a ≥5% mean change difference from baseline.
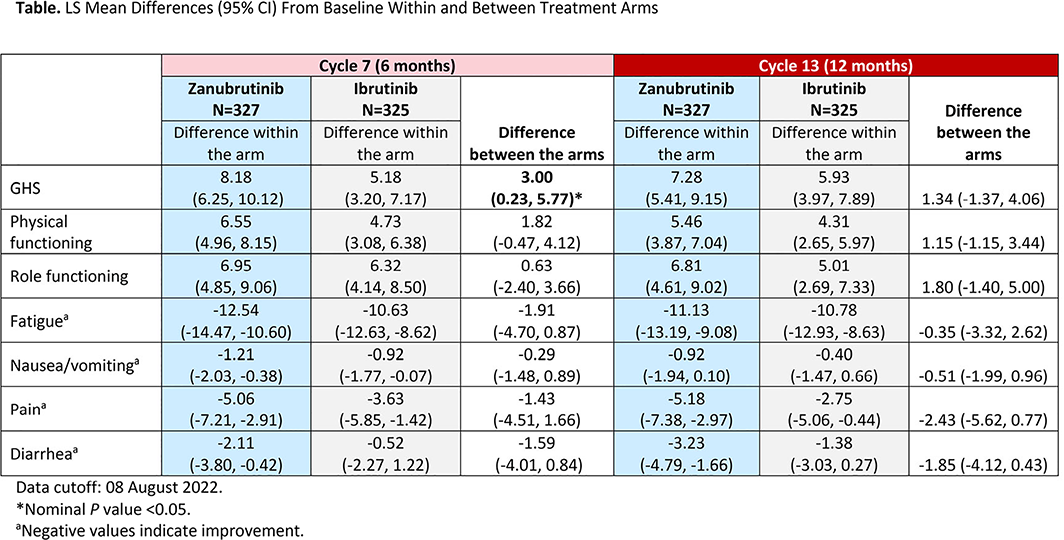
Results: A total of 652 pts were randomized to receive zanu (n = 327) or ibr (n = 325); baseline characteristics were generally similar between arms, although the zanu arm had fewer males versus ibr arm (65.1% vs. 71.4%). At baseline, GHS, functional, and symptom scales scores were similar between arms. Although more ibr-treated pts discontinued treatment due to adverse events versus zanu (22.2% vs. 15.4%), adjusted PRO completion rates were high at cycles 7 and 13 in both the zanu (89.6% and 94.3%) and ibr arm (87.7% and 92.3%), respectively. By cycle 7, GHS scores were improved with zanu versus ibr and by cycle 13, the difference in GHS scores from baseline was no longer significant (Table). Pts in the zanu arm experienced clinically meaningful improvements in physical and role functioning as well as pain and fatigue at cycles 7 and 13, but the difference between arms was not significant. Although pts in the zanu arm reported lower diarrhea scores, the difference between treatments was not significant. Nausea/vomiting scores were maintained in both arms with no measurable difference. VAS scores showed greater improvement from baseline at both cycle 7 (7.92 vs. 3.44) and cycle 13 (7.75 vs. 3.92) of treatment with zanu versus ibr, respectively.
Conclusions: In ALPINE, pts with R/R CLL/SLL treated with zanu demonstrated improvement versus ibr in the QLQ-C30 GHS/QoL scale at cycle 7. Other endpoints continued to improve, suggesting treatment with zanu positively affected and improved HRQOL over time. As expected, given the generally good HRQOL at baseline in both arms, the differences between the arms were small and not significant.
 |
Encore Abstract - previously submitted to EHA 2023
The research was funded by: BeiGene
Keyword: Chronic Lymphocytic Leukemia (CLL)
Conflicts of interests pertinent to the abstract.
B. Eichhorst
Consultant or advisory role: Janssen, AbbVie, Gilead, AstraZeneca, BeiGene, MSD, Lilly
Honoraria: Roche, AbbVie, BeiGene, AstraZeneca, MSD
Research funding: Janssen, Gilead, Roche, AbbVie, BeiGene, AstraZeneca
Educational grants: BeiGene
N. Lamanna
Consultant or advisory role: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Janssen, Pharmacyclics
Research funding: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Octapharma, Oncternal, MingSight, TG Therapeutics
S. M. O'Brien
Consultant or advisory role: AbbVie, Alexion, Amgen, Aptose Biosciences Inc., Astellas, AstraZeneca, Autolus, BMS, Celgene, DynaMed, Eli Lilly and Company, Gilead, GSK, Janssen Oncology, Johnson and Johnson, Juno Therapeutics, MEI Pharma Inc., Merck, NOVA Research Company, Pfizer, Pharmacyclics, TG Therapeutics, Vaniam Group Inc., Verastem, Vida Ventures
Research funding: TG Therapeutics, Pharmacyclics, Pfizer, Nurix Therapeutics, Inc., Mustang, Loxo Oncology Inc., Kite, Gilead, Caribou Biosciences, Inc. BeiGene, Ltd., Alliance, Acerta
C. S. Tam
Honoraria: BeiGene, AbbVie, Janssen
Research funding: Janssen, AbbVie
Other remuneration: BeiGene
L. Qiu
Consultant or advisory role: Janssen, Astra Zeneca, Takeda, Roche, Abbvie, Beigene
K. Yang
Employment or leadership position: BeiGene
Stock ownership: BeiGene
Research funding: BeiGene
K. Wu
Employment or leadership position: BeiGene (Self), Roche/Genentech (Spouse)
Stock ownership: BeiGene (Self), Roche/Genentech (Spouse)
T. Salmi
Employment or leadership position: BeiGene
G. Barnes
Employment or leadership position: BeiGene
Stock ownership: BeiGene
J. R. Brown
Consultant or advisory role: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Octapharma, Oncternal, MingSight, TG Therapeutics
Research funding: BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics