Zanubrutinib safety/tolerability profile and comparison with ibrutinib profile in B-cell malignancies: Post hoc analysis of a large clinical trial safety database
Introduction: Bruton tyrosine kinase inhibitors (BTKi) block B-cell receptor pathway signaling, leading to growth inhibition and cell death in malignant B-cells. First-generation BTKi, ibrutinib (ibr) revolutionized treatment; however, inhibition of off-target kinases such as EGFR, HER2, TEC, and CSK may be associated with toxicities, including diarrhea, rash, bleeding, and atrial fibrillation (Afib), that limit its use. Zanubrutinib (zanu), a potent and selective next-generation BTKi, maximizes BTK occupancy and minimizes off-target effects. Here, we characterized the overall safety/tolerability of zanu and compared it with ibr in patients (pts) with B-cell malignancies using the zanu clinical safety database.
Methods: Safety data were pooled from 10 clinical trials of zanu monotherapy; 2 of the included studies (ASPEN; ALPINE) compared zanu head to head with ibr. Pts with CLL/SLL, MCL, MZL, WM, FL and other B-cell malignancies were included. Treatment-emergent adverse events (TEAEs) were summarized using MedDRA preferred terms; adverse events of special interest (AESI) were defined using pooled terms. Rates of TEAEs, exposure-adjusted incidence rates (EAIRs), and prevalence over time of AESI were assessed.
Results: Pooled analyses included 1550 pts treated with zanu. Median zanu exposure was 28.6 months with 31.2% of pts having treatment exposure of ≥36 mo. Most common nonhematologic AEs were upper respiratory tract infection (29.0%), diarrhea (19.9%), contusion (19.4%), cough (17.2%), and rash (16.2%); grade ≥3 nonhematologic AEs occurring in ≥5% of pts included pneumonia (7.9%) and hypertension (7.4%). The most common serious AE was pneumonia (7.5%). Zanu discontinuation due to AE occurred in 12.3% of pts; AEs leading to dose reduction occurred in 9.6%. Disease progression was the most common cause of death (7.2%); deaths attributed to AEs occurred in 5.6% of pts, and most (3.2%) were due to infections including COVID-19-related AEs.
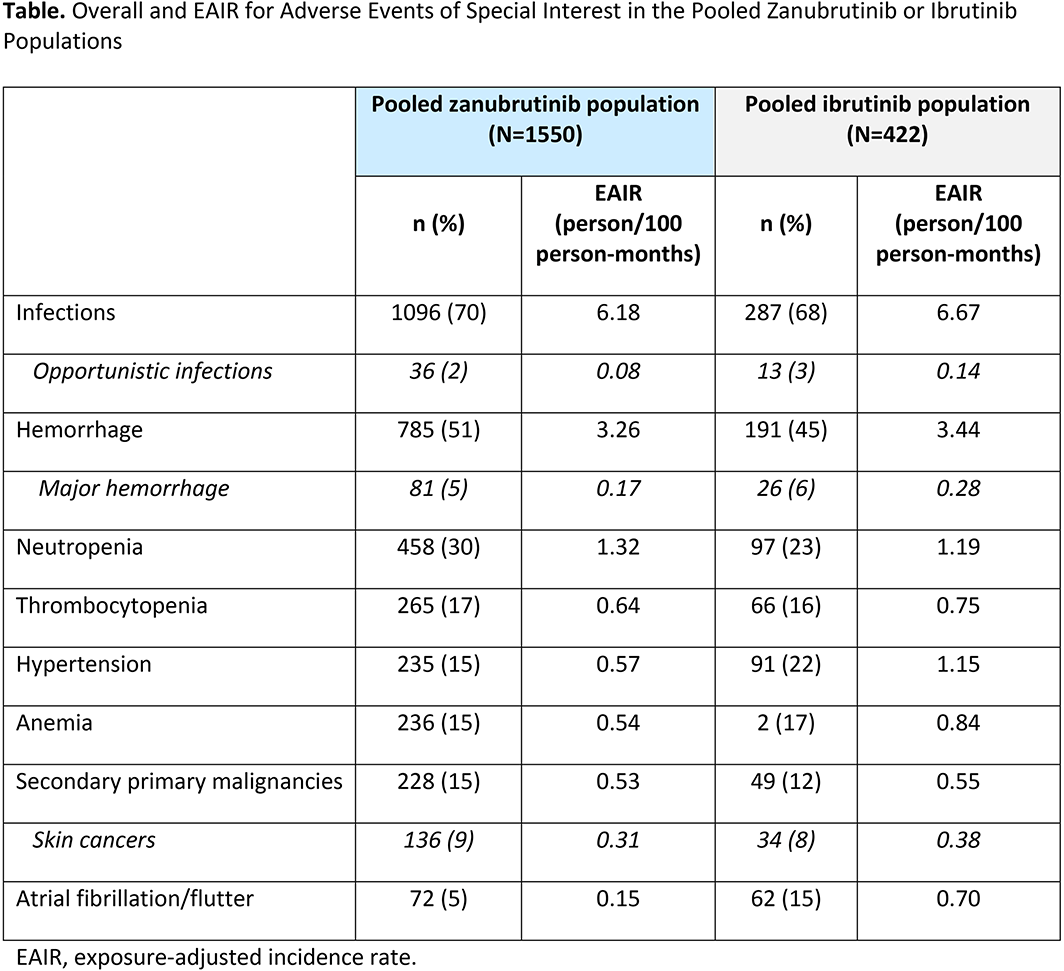
The most common AESI in the pooled zanu population and in ibr-treated pts from ASPEN and ALPINE (N = 422) were infections and hemorrhage (Table). With the exception of neutropenia, EAIRs were numerically lower for zanu versus ibr, most notably hypertension (0.57 vs. 1.15 person/100 person-months), anemia (0.54 vs. 0.84 person/100 person-months), and atrial fibrillation or flutter (0.15 vs. 0.70 person/100 person-months). Prevalence of zanu AESI tended to remain constant or decrease with longer follow-up.
Conclusions: As BTKi therapy requires continuous treatment, long-term tolerability and low treatment discontinuation rates are needed for successful outcomes. zanu was well tolerated, with generally mild-to-moderate AEs that tended not to lead to treatment discontinuation. Prevalence of AESI generally trended down over time without emergence of new safety signals, supporting zanu as a good option for long-term treatment.
 |
Encore Abstract - previously submitted to EHA 2023
The research was funded by: BeiGene
Keywords: Aggressive B-cell non-Hodgkin lymphoma, Chronic Lymphocytic Leukemia (CLL)
Conflicts of interests pertinent to the abstract.
J. R. Brown
Consultant or advisory role: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Octapharma, Oncternal, MingSight, TG Therapeutics
Research funding: BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, SecuraBio, Sun, TG Therapeutics
B. Eichorst
Consultant or advisory role: Janssen, AbbVie, Gilead, AstraZeneca, BeiGene, MSD, Lilly
Honoraria: Roche, AbbVie, BeiGene, AstraZeneca, MSD
Research funding: Janssen, Gilead, Roche, AbbVie, BeiGene, AstraZeneca
Educational grants: BeiGene
P. Ghia
Consultant or advisory role: AbbVie, AstraZeneca, BeiGene, BMS, Janssen, Lilly/Loxo Oncology, MSD, Roche
Honoraria: AbbVie, AstraZeneca, BeiGene, BMS, Janssen, Lilly/Loxo Oncology, MSD, Roche
Research funding: AbbVie, AstraZeneca, BMS, Janssen
W. Jurczak
Consultant or advisory role: Abbvie, Astra Zeneca, BeiGene, Lilly, Roche, Takeda
Research funding: Abbvie, Astra Zeneca, BeiGene, Janssen, Lilly, Roche, Takeda
B. S. Kahl
Consultant or advisory role: BeiGene, Pharmacyclics, AstraZeneca, Abbvie, Janssen
Other remuneration: BeiGene
N. Lamanna
Consultant or advisory role: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Janssen, Pharmacyclics
Research funding: Abbvie, AstraZeneca, BeiGene, Eli Lily/Loxo, Genentech, Octapharma, Oncternal, MingSight, TG Therapeutics
T. Robak
Honoraria: AstraZeneca, BeiGene, Janssen, Abbvie, Octapharma, Regeneron, GSK
Research funding: BeiGene,OctaPharma, AstraZeneca, Janssen, Regeneron, GSK,
Educational grants: AstraZeneca
M. Shadman
Consultant or advisory role: Abbvie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, Beigene, Bristol Myers Squibb, Morphosys/Incyte, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, Eli Lilly, Adaptimmune, Mustang Bio, Regeneron, Merck, Fate therapeutics, MEI pharma and Atara Biotherapeutic
Research funding: Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, Beigene, AstraZeneca, Sunesis,Atara Biotherapeutics, Genmab, Morphosys/Incyte
C. S. Tam
Honoraria: BeiGene, AbbVie, Janssen
Research funding: Janssen, AbbVie
Other remuneration: BeiGene
L. Qiu
Consultant or advisory role: Janssen, Astra Zeneca, Takeda, Roche, Abbvie, Beigene
A. Cohen
Employment or leadership position: BeiGene
Stock ownership: BeiGene
M. Zhang
Employment or leadership position: BeiGene
T. Salmi
Employment or leadership position: BeiGene International GmbH
Stock ownership: BeiGene Ltd.
J. Paik
Employment or leadership position: BeiGene
L. Wang
Employment or leadership position: BeiGene
Stock ownership: BeiGene
J. Zhang
Employment or leadership position: BeiGene
Stock ownership: BeiGene
H. Ma
Employment or leadership position: BeiGene
Stock ownership: BeiGene
A. Tedeschi
Consultant or advisory role: BeiGene, AstraZeneca, AbbVie, Janssen
Honoraria: BeiGene, AstraZeneca, AbbVie, Janssen