TREATMENT AND OUTCOMES OF PATIENTS WITH NK/T-CELL LYMPHOMA TREATED WITH MODIFIED (m)SMILE AND INTENSITY-MODULATED RADIOTHERAPY (IMRT), A SINGLE CENTER EXPERIENCE
Introduction: Extranodal NK/T cell lymphoma (ENKTL) is a rare subtype of lymphoma, poorly responsive to anthracycline-based chemotherapy. Treatment and prognosis are largely driven by stage, and the PINK and PINK-E scores appear to further refine it. Outcomes for patients with ENKTL have improved with L-asparaginase containing regimens into combined modality approaches. Based on the reported efficacy of SMILE (Yamaguchi, Cancer Sci, 2008), in 2009, we adopted a modified (m)SMILE (dexamethasone, methotrexate, ifosfamide, peg-asparaginase, etoposide) regimen given every 3 weeks, followed by radiotherapy (RT) as our standard approach. Main differences from the SMILE are: I) shorter course of chemotherapy, II) the use of peg-asparaginase (most common dose was 1500 U/m2), and III) lower dose and Intensity-Modulated Radiotherapy (IMRT) consolidation.
Methods: We retrospectively analyzed 28 patients with ENKTL treated at our institution with mSMILE from 10/2009–3/2019. Early-stage patients were planned to receive 2 cycles of mSMILE+IMRT, advanced stage 3-4 cycles of mSMILE+/-IMRT. We collected patient characteristics and estimated survival with the Kaplan-Meier method.
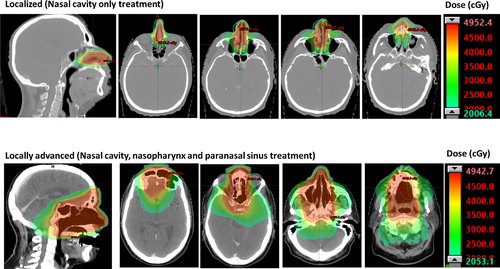
Results: Patient characteristics were: age median 52y (24-69); female 53%; Asian 25%, other races 75%. Response post mSMILE was 93% (26/28, CR 68%) and response post IMRT was 95% (23/24, CR 87.5%). After a median follow-up of 31 months (range 5-105) 17 patients are alive (61%). Among early-stage patients (IE)/low PINK-E (n=13), overall survival (OS) was 100% at the median follow up, and progression-free survival (PFS) was 92%. However, there were two subsequent events: one death due to relapse and one death from unrelated causes in remission. Intermediate and high-risk stage/PINK-E patients fared similarly, with an OS of 43% and a PFS of 33.3% at the median follow-up. Four pts (14%) had chemotherapy dose reductions, 9 pts (32%) experienced G3-4 non-hematologic toxicity, all pts had hematologic toxicity, for 21/28 (75%) G3-4. Twenty-four pts, (86%) received RT as part of their treatment course. 22/24 received IMRT consolidation post 1-2 mSMILE 16 with receiving 4500 cGy. Of these 22, 2 pts received IMRT consolidation composite with total body irradiation conditioning for allo-transplant to total doses of 3947 cGy. An example of IMRT field design is shown in the figure.
Keywords: extranodal lymphomas; L-asparaginase; T-cell lymphoma (TCL).