Glycosylated Cell-Penetrating Poly(disulfide)s: Multifunctional Cellular Uptake at High Solubility
Abstract
The glycosylation of cell-penetrating poly(disulfide)s (CPDs) is introduced to increase the solubility of classical CPDs and to achieve multifunctional cellular uptake. With the recently developed sidechain engineering, CPDs decorated with α-d-glucose (Glu), β-d-galactose (Gal), d-trehalose (Tre), and triethyleneglycol (TEG) were readily accessible. Confocal laser scanning microscopy images of HeLa Kyoto cells incubated with the new CPDs at 2.5 μm revealed efficient uptake into cytosol and nucleoli of all glycosylated CPDs, whereas the original CPDs and TEGylated CPDs showed much precipitation into fluorescent aggregates at these high concentrations. Flow cytometry analysis identified Glu-CPDs as most active, closely followed by Gal-CPDs and Tre-CPDs, and all clearly more active than non-glycosylated CPDs. In the MTT assay, all glyco-CPDs were non-toxic at concentrations as high as 2.5 μm. Consistent with thiol-mediated uptake, glycosylated CPDs remained dependent on thiols on the cell surface for dynamic covalent exchange, their removal with Ellman's reagent DTNB efficiently inhibited uptake. Multifunctionality was demonstrated by inhibition of Glu-CPDs with d-glucose (IC50 ca. 20 mm). Insensitivity toward l-glucose and d-galactose and insensitivity of conventional CPDs toward d-glucose supported that glucose-mediated uptake of the multifunctional Glu-CPDs involves selective recognition by glucose receptors at the cell surface. Weaker but significant sensitivity of Gal-CPDs toward d-galactose but not d-glucose was noted (IC50 ca. 110 mm). Biotinylation of Glu-CPDs resulted in the efficient delivery of streptavidin together with a fluorescent model substrate. Protein delivery with Glu-CPDs was more efficient than with conventional CPDs and remained sensitive to DTNB and d-glucose, i.e., multifunctional.
Introduction
Cell-penetrating poly(disulfide)s (CPDs) have been introduced recently to overcome limitations of conventional guanidinium-rich cell-penetrating peptides (CPPs,1-14 e.g. 1, Figure 1) with regard to cytotoxicity and endosomal capture on the one hand and to achieve bifunctional cellular uptake on the other.15-17 In CPDs 2, simple conjugates of arginine and lipoic acid, the peptide backbone of CPPs 1 is replaced by a poly(disulfide). This is of interest because reductive depolymerization by glutathione should inactivate CPDs 2 as soon as they arrive in the cytosol, thus minimizing toxicity. Moreover, dynamic covalent thiol-disulfide exchange with thiols on the cell surface15-27 should provide access to mechanistically new pathways to enter into cells. However, the preservation of guanidinium cations in the sidechains of CPDs 2 should maintain the counterion-mediated uptake mechanism of CPPs.1-1428 Without going into details, the strong proximity effects in repulsion-driven ion pairing result in stable but labile pairing with anions at the cell surface, translocation through transient micellar pores, and release into the cytosol by ion exchange with internal polyanions follow. Kinetic competition by endocytosis can be overcome with CPP activators.28 CPDs are thus bifunctional, entering cells by a combination of thiol-mediated and counterion-mediated uptake. The biotinylated CPDs 3 have been shown to deliver quantum dots, artificial metalloenzymes, and functional peptides into the cytosol of various cells.2930 Elegant strategies for the efficient delivery of antibodies and nanoparticles with CPDs have been reported as well.31-33
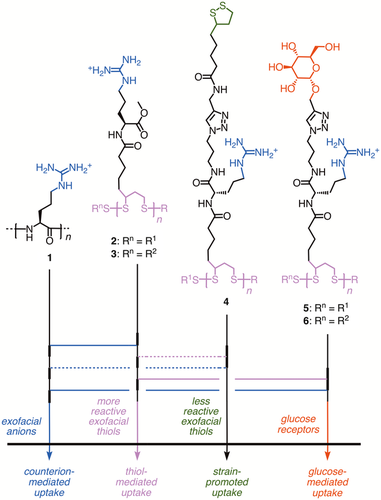
To integrate the recently introduced strain-promoted cellular uptake,34-36 the sidechains of CPDs 4 were equipped with strained disulfides.15 Whereas high activities were indeed found at low concentrations, CPDs 4 suffered from poor solubility at higher concentrations. Similar, although less pronounced solubility problems were already observed with the original CPDs 2 at high concentrations, particularly in conjugation with some but not all proteins. To increase solubility of functional systems, PEGylation37-42 and the attachment of trehalose43-45 have received much attention. Glycosylation with other carbohydrates such as glucose and galactose has been successful as well,46-49 although direct applications to CPPs are remarkably rare and fully developped only recently in a breakthrough report from the Deming group.50-52 Glycosylation was particularly interesting to solubilize CPDs because of the promise to integrate a third pathway to enter into cells, i.e., to achieve multifunctional cellular uptake. Particularly glucose receptors, mostly GLUT1, have been shown to mediate the efficient uptake of fluorescent model substrates attached to glucose.5354 In the following we report design, synthesis, and evaluation of CPDs that are decorated with glucose (Glu), galatose (Gal), trehalose (Tre), and triethyleneglycol (TEG). Glu-CPDs 5 and 6 excel with highest activity, multifunctionality, unproblematic solubility, and compatibility with the delivery of proteins to cytosol and nucleus.
Results and Discussion
The new CPDs were synthesized by sidechain engineering from CPD 7 as common precursor (Scheme 1). This strategy is important to obtain comparable results based on polymer backbones of identical length and dispersity. Monomer 8 and the red-fluorescent initiator 9 were prepared following reported procedures.15 Lipoic acid was used in racemic form because uptake of CPDs has been shown to be independent of the absolute configuration at this stereogenic center. Polymer 7 was obtained by ring-opening disulfide-exchange polymerization55-57 of monomer 8 triggered by initiator 9 and terminated by iodoacetamide 10.15 GPC Analysis of CPDs 7 confirmed a very low polydispersity of PDI = 1.03 ± 0.04 and a relatively short length with n = 19 monomers per polymer, resulting in an Mw = 9.5 ± 1.2 kDa.15 The alkynated d-glucose 11 was obtained from d-glucose 12 by acid catalyzed glycosidation with alkyne 13 following reported procedures.58-62 Temporary benzoyl protection was necessary to isolate pure α anomer 11 (Scheme S1). Previous reports have indicated that glucose-mediated uptake is nearly independent of the configuration at the anomeric center.53 Applicability of CuAAC (copper(I)-catalyzed alkyne-azide cycloaddition)1563-65 was confirmed first by reacting alkyne 11 with monomeric azide 8 in H2O/THF 9:1 in the presence of sodium ascorbate, CuSO4, and tris(benzyltriazoylmethyl)amine (TBTA) at room temperature. The expected triazole product was obtained in intact form as the main product (Figure S3). CuAAC of alkyne 11 with azide polymer 7 was realized under identical conditions. The sidechain-modified63-65 product was analyzed by reductive depolymerization and quantification of the obtained monomer mixture by RP-HPLC (Figure S6). The yield of sidechain modification in Glu-CPD 5 calculated to 75%.
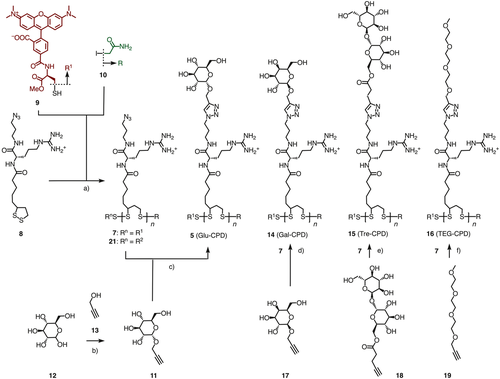
CPDs 14 – 16 were prepared in similarly high yield following the same procedure (Scheme 1). The necessary alkyne substrates 15 – 19 were readily accessible following literature procedures (Figure S1, Scheme S2).58-62 The original CPDs 2 were prepared for comparison, following the reported procedures optimized to obtain polymers of comparable properties (PDI = 1.22 ± 0.15, n = 19, Mw = 9.3 ± 1.4 kDa).1617
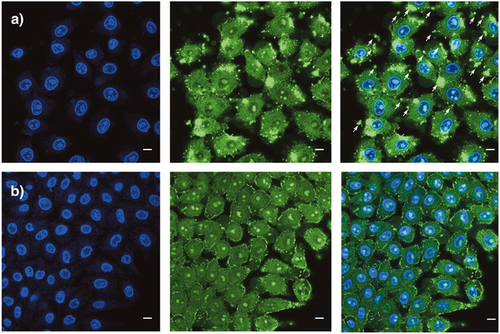
Cellular uptake was determined in HeLa Kyoto cells following routine procedures. Confocal laser scanning microscopy (CLSM) images were recorded after incubation with 2.5 μm CPDs at 37 °C in Leibovitz's medium, together with Hoechst 33342 to label the nucleus (shown in blue, Figure 2). At these high concentrations, the classical CPDs 2 showed excellent uptake together with much precipitation outside the cells (Figure 2,a). These fluorescent precipitates were not fully removed during conventional washing procedures prior to microscopy (Figure 2,a, arrows). In clear contrast, Glu-CPDs 5 showed equally efficient uptake into cytosol, nucleus, and particularly nucleoli, but without any trace of precipitate (Figure 2,b). Similar images were obtained for Gal-CPDs 14 and Tre-CPDs 15. TEG-CPDs 16, however, showed much precipitation and little uptake and were not further used (Figure S11). This result was surprising considering much precedence37-42 for effective solubilization by TEGylation: At least in the context of CPDs, glycosidation was clearly more effective.
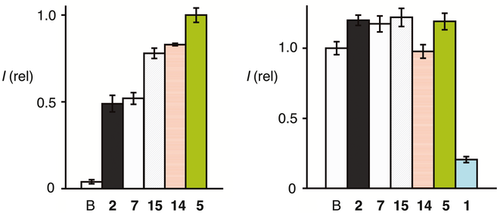
Uptake efficiencies were quantified by flow cytometry. After 15 min of incubation at 2.5 μm, highest counts were observed for HeLa Kyoto cells exposed to Glu-CPDs 5 (Figure 3). Gal-CPDs 14 and Tre-CPDs 15 were not much less active, whereas the performance of non-glycosylated CPDs 2 and 7 was significantly weaker. According to the MTT assay, none of the new CPDs was cytotoxic, also at the high 2.5 μm concentrations used to probe solubility under extreme conditions (Figure 3). Polyarginine, a model CPP 1, was used as a positive control to assure that the assay is functional.1617
The best performing Glu-CPDs 5 were studied in more detail (Figure 4). According to flow cytometry analysis, incubation at 4 °C strongly decreased activity. The observed decrease was comparable with that for the original CPDs 2. Such a decrease is commonly interpreted as support for the involvement of energy-dependent uptake mechanisms, i.e., endocytosis. However, CPDs 2 and several analogs that operate by thiol-mediated uptake have previously been shown to be essentially insensitive to inhibitors of different forms of endocytosis, including chlorpromazine (clathrin-mediated), wortmannin (macropinocytosis), methyl-β-cyclodextrin (caveolae), etc.161731-36 The origins of the sensitivity of CPD-mediated uptake to temperature are thus presumably different, possibilities include changes in membrane fluidity and phase separation, thiol reactivity, and so on. The presence of 10% serum decelerated cellular uptake of Glu-CPDs 5 slightly. After 4 h of incubation, identical activities were obtained with and without serum (Figure S15).
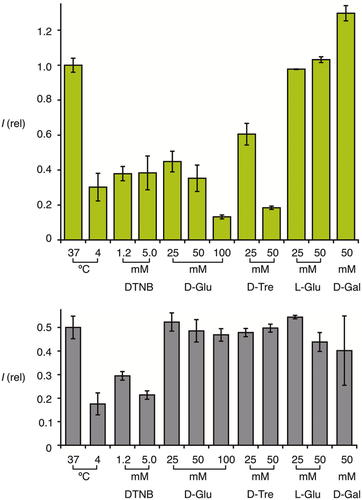
Preincubation of cells with 1.2 or 5.0 mm Ellman's reagent (DTNB) strongly reduced the uptake efficiency of Glu-CPDs 5 (Figure 4). This inhibition was again as with the original CPDs 2. DTNB inhibition is a hallmark of thiol-mediated uptake because it oxidizes exofacial thiols into disulfides, thus inhibiting the thiol-disulfide exchange leading to the dynamic covalent attachment of the CPDs to the cell surface.16-27 Sensitivity to DTNB thus supported that thiol-mediated uptake occurs also with Glu-CPDs 5.
In the presence of increasing concentrations of d-glucose, the uptake activity of Glu-CPDs 5 decreased with an IC50 around 20 mm (Figures 4 and 5 ). This glucose sensitivity was in clear contrast to the original CPDs 2 (Figures 4 and 5♢). However, both Glu-CPDs 5 and the original CPDs 2 were insensitive toward l-glucose and d-galactose (Figure 4). This stereoselective inhibition by d-glucose supported that the recognition of Glu-CPDs 5 but not the original CPDs 2 by glucose receptors at the cell surface contributes to cellular uptake. Similar sensitivity of Glu-CPDs 5 but not CPDs 2 to d-trehalose confirmed that the d-glucose unit in d-trehalose is also recognized by glucose receptors. Interestingly, the inhibition of Glu-CPDs 5 by d-glucose and DTNB was over-additive (Figure 4). This could indicate that the two pathways are coupled and might suggest that glucose receptors are a target of CPDs.
). This glucose sensitivity was in clear contrast to the original CPDs 2 (Figures 4 and 5♢). However, both Glu-CPDs 5 and the original CPDs 2 were insensitive toward l-glucose and d-galactose (Figure 4). This stereoselective inhibition by d-glucose supported that the recognition of Glu-CPDs 5 but not the original CPDs 2 by glucose receptors at the cell surface contributes to cellular uptake. Similar sensitivity of Glu-CPDs 5 but not CPDs 2 to d-trehalose confirmed that the d-glucose unit in d-trehalose is also recognized by glucose receptors. Interestingly, the inhibition of Glu-CPDs 5 by d-glucose and DTNB was over-additive (Figure 4). This could indicate that the two pathways are coupled and might suggest that glucose receptors are a target of CPDs.
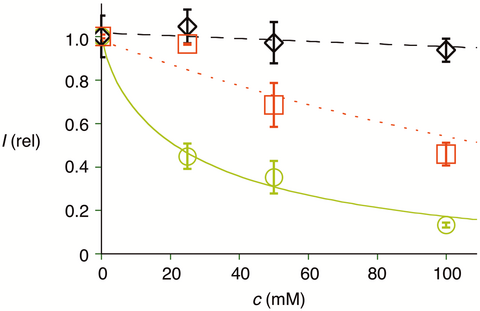
 ) and CPDs 2 (♢) into HeLa cells as a function of the concentration of d-glucose, and of Gal-CPDs 14 (
) and CPDs 2 (♢) into HeLa cells as a function of the concentration of d-glucose, and of Gal-CPDs 14 ( ) as a function of the concentration of d-galactose in the medium.
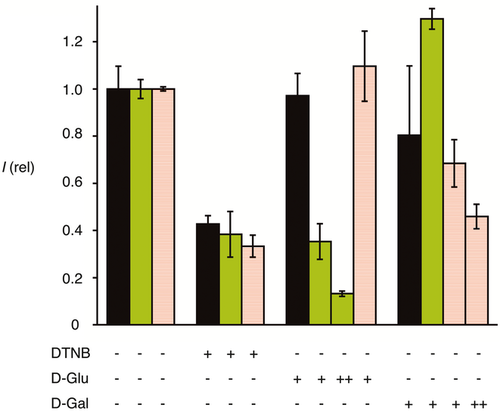
) as a function of the concentration of d-galactose in the medium.Corroborative support for multifunctional cellular uptake by Glu-CPDs 5 was obtained from the complementary Gal-CPDs 14. According to flow cytometry, uptake activity of Gal-CPDs 14 was only slightly below Glu-CPDs 5 and clearly above the non-glycosylated originals 2 (Figure 2). Like all CPDs, Gal-CPDs 14 were non-toxic to HeLa Kyoto cells at 2.5 μm in the MTT assay (Figure 2). Also like all other CPDs, Gal-CPDs 14 were efficiently inhibited by DTNB, thus operating by thiol-mediated uptake (Figure 6). Unlike Glu-CPDs 5, however, Gal-CPDs 14 were not inhibited by d-glucose (Figure 6). Also unlike Glu-CPDs 5, Gal-CPDs 14 were inhibited by d-galactose, whereas the original CPDs 2 were insensitive to both d-glucose and d-galactose (Figure 6). This orthogonality of Glu-CPDs 5 and Gal-CPDs 14 provided corroborative support for multifunctional uptake by both, combining counterion-mediated and thiol-mediated uptake with carbohydrate-mediated uptake. The inhibition of Gal-CPDs 14 by d-galactose was with an IC50 ca. 110 mm clearly less efficient than that Glu-CPDs 5 by d-glucose (IC50 ca. 20 mm, Figure 5 vs. Figure 5
vs. Figure 5 ). This difference confirmed that the poorly characterized receptors involved in galactose-mediated uptake54 are clearly less powerful than the well-established glucose receptors accounting for glucose-mediated uptake.5354
). This difference confirmed that the poorly characterized receptors involved in galactose-mediated uptake54 are clearly less powerful than the well-established glucose receptors accounting for glucose-mediated uptake.5354
Compatibility of glycosylated CPDs with protein delivery was probed with the most promising Glu-CPDs. Biotinylated Glu-CPDs 6 were prepared from the biotinylated, red-fluorescent initiator 20. This target molecule was readily accessible following previously reported procedures.2930 Ring-opening disulfide-exchange polymerization of monomer 8 and terminator 10 afforded azide CPDs 21. The obtained polymers were characterized by PDI = 1.17 ± 0.03, n = 19, Mw = 9.8 ± 1.7 kDa. The azide groups along their backbone were reacted with Glu-alkyne 11 to afford red-fluorescent Glu-CPDs 6 with 78% yield of sidechain glycosylation. The green-fluorescent biotin conjugate 22 was prepared following previously reported procedures (Scheme S3).2930
Cellular uptake of streptavidin 23 by biotinylated Glu-CPDs 6 was determined for 1:1 complexes (as with the original CPDs, complexes with more than one Glu-CPD per streptavidin did not give better results; not shown). The remaining three binding sites of tetramer 24 were saturated with a ligand 22 as fluorescent model substrate for cellular uptake by CPD-streptavidin complexes. As a control, the corresponding complex 25 was prepared from the previously reported biotinylated version of original, non-glycosylated CPDs 3 (Scheme 2).
Protein delivery with Glu-CPD complexes 24 was determined by incubation of HeLa Kyoto cells at concentrations of 625 nm for 6 h at 37 °C. The CLSM images obtained after routine washing procedures revealed excellent uptake into cytosol, nucleus, as well as nucleoli (Figure 7,b – 7,f). Besides an overall strong co-localization (Figure 7,d), the fluorophore of the Glu-CPDs 5, displayed in green, showed increased localization in nucleoli (green, Figure 7,e vs. 7,f), whereas leftovers of the fluorophore of model substrate 22, displayed in red, were visible at the cell membrane (red, Figure 7,d). The loss of some model substrate 22 already at the cell surface suggested that the disulfide in 22 also exchanged with exofacial thiols to produce a covalent product that, having lost the CPD, cannot enter into the cell (red, Figure 7,d). Dominant green-displayed and little red-displayed fluorescence from nucleoli (Figure 7,e vs. 7,f) supported efficient reduction of the disulfide in 22 by glutathione to release most of the fluorescent model substrate in the cytosol, as desired (Figure 7,f). Residual red-displayed fluorescence from the nucleoli (red dots within blue circles in Figure 7,c) implied that the cytosolic reduction of the disulfide in 22 does not reach completion before some intact, or almost intact, Glu-CPD complexes 24 can escape into the nucleus.
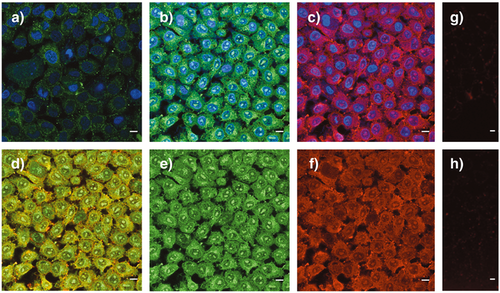
Protein delivery with Glu-CPD complexes 24 was much more efficient than with the original CPD complexes 25, which showed quite poor activity under these conditions (Figure 7,a vs. 7,b). Complexes 25 with longer CPDs have been shown previously to reach the nucleoli as well.29 The failure of the shorter CPDs in complexes 25, used in this study to maximize comparability with Glu-CPD complexes 24, to do so was consistent with increasingly dominant depolymerization in the cytosol with decreasing polymer length.66 This suggested that the cytosolic depolymerization of Glu-CPD complexes 24, shielded by a sweet surface, might be slower than that of the unshielded CPD complexes 25, slow enough for fragments to escape to the nucleus for multivalent ion pairing with noncoding RNA in the nucleoli. Different intracellular localization of 24 and 25 is thus the consequence of different overall activity, it is unlikely that specific compartments could be targeted by this sidechain modification.
Inhibition of protein delivery with Glu-CPD complexes 24 by DTNB and glucose was remarkably efficient (Figure 7,g and 7,h). These findings confirmed compatibility of Glu-CPDs with efficient protein delivery at preserved multifunctionality, that is an operational combination of counterion-, thiol- and glucose-mediated uptake.
Conclusions
In this report, we introduce Glu-CPDs, that is cell-penetrating poly(disulfide)s decorated with glucose along their scaffold, for delivery of cargos including proteins to cytosol and nucleus with so far highest efficiency. The ‘sweet’ Glu-CPDs excel with maximal solubility and unique multifunctionality. Namely, counterion-mediated uptake known from CPPs28 are combined with thiol-mediated uptake of CPDs1617 and glucose-mediated uptake involving specific recognition by glucose receptors at the cell surface53 to open several doors at the same time and thus maximize efficiency. Relevance of counterion-mediated uptake and the bypassing of endosomal capture through endocytic pathways has been demonstrated previously, the latter by insensitivity to inhibitors of endocytosis, and was thus not further reconfirmed.161731-36 Support for existence and relevance of thiol- and glucose-mediated uptake was obtained by uptake inhibition with Ellman's reagent and d-glucose (and d-trehalose but neither l-glucose nor d-galactose).
The present study confirms the value of the recently introduced sidechain engineering strategy.15 Glu-CPDs were identified as the best among a collection of polymers with identical backbone but decorated with glucose, trehalose, galactose, and TEG (interestingly, solubilization with TEG was ineffective). This sidechain engineering on an identical backbone is important to assure comparability.
Recommendations whether or not the new glu-CPDs should now be preferred for practical applications to deliver substrates of free choice, from QDs2930 to fluorescent probes,67 would be premature. The here reported method is most valuable for comparative screening for the most effective sidechain functionalization, but the accessible polymer backbones are relatively short (n = 19), and not all sidechains are glycosylated (yields of 70 – 80%).15 The most potent original, non-glycosylated CPDs, which allowed for the unprecedented delivery of quantum dots into the cytosol, could be grown as long as n = 49.2930 To produce completely glycosylated CPDs of similar length, polymerization protocols with monomers that are already equipped with glucose will have to be developed. If successful, the ability of these longer and completely glycosylated CPDs to improve on the unique advantages of the established CPDs 2, particularly the cytosolic delivery of giant substrates at low toxicity,2930 will then have to be evaluated first. If clearly superior, the Glu-CPDs identified in this study could indeed replace the original CPDs for general use, although their construction, from lipoic acid, arginine and glucose only, requires more synthetic effort. Studies along these lines are ongoing and will be reported in due course.
Experimental Section
Acknowledgements
We thank A. Roux and G. Molinard for advise, access to, and assistance with cell culture, the NMR, the bioimaging, and the MZ 2.0 platforms for services, and the University of Geneva, the Swiss National Centre of Competence in Research (NCCR) Chemical Biology, the NCCR Molecular Systems Engineering and the Swiss NSF for financial support.
Author Contribution Statement
P. M., E. B., N. S., and S. M. conceived this work, designed the experiments, discussed the results, and wrote the manuscript, P. M. and E. B. synthesized all compounds used, P. M. conducted the uptake experiments.




