Object-place-context learning impairment correlates with spatial learning impairment in aged Long–Evans rats
Abstract
The hippocampal formation is vulnerable to the process of normal aging. In humans, the extent of this age-related deterioration varies among individuals. Long–Evans rats replicate these individual differences as they age, and therefore they serve as a valuable model system to study aging in the absence of neurodegenerative diseases. In the Morris water maze, aged memory-unimpaired (AU) rats navigate to remembered goal locations as effectively as young rats and demonstrate minimal alterations in physiological markers of synaptic plasticity, whereas aged memory-impaired (AI) rats show impairments in both spatial navigation skills and cellular and molecular markers of plasticity. The present study investigates whether another cognitive domain is affected similarly to navigation in aged Long–Evans rats. We tested the ability of young, AU, and AI animals to recognize novel object-place-context (OPC) configurations and found that performance on the novel OPC recognition paradigm was significantly correlated with performance on the Morris water maze. In the first OPC test, young and AU rats, but not AI rats, successfully recognized and preferentially explored objects in novel OPC configurations. In a second test with new OPC configurations, all age groups showed similar OPC associative recognition memory. The results demonstrated similarities in the behavioral expression of associative, episodic-like memory between young and AU rats and revealed age-related, individual differences in functional decline in both navigation and episodic-like memory abilities.
1 INTRODUCTION
The hippocampal formation is a key structure of the medial temporal lobe (MTL) system that supports spatial navigation and episodic memory (Scoville & Milner, 1957; Teng & Squire, 1999). By integrating allocentric, spatial information based on self-motion cues from the medial entorhinal cortex (MEC) and egocentric, sensory information about the external world from the lateral entorhinal cortex (LEC), the hippocampus creates context-specific representations necessary for the recall of individual events in the form of cognitive maps (Knierim, 2015; O'Keefe & Nadel, 1978; Tolman, 1948). The extent of age-related impairment in learning and memory has been linked to alterations of anatomy and physiology in the hippocampus and its major cortical input structure, the entorhinal cortex (Hirni et al., 2016; Jack et al., 1997). With age, the human entorhinal cortex shrinks in volume, leading to a loss of dentate gyrus input that contributes to disrupted pattern separation and a reduction of feedforward inhibition that leads to proximal CA3 hyperactivity (Fouquet et al., 2012; Lee et al., 2021; Mueller & Weiner, 2009; Yassa, Lacy, et al., 2011; Yassa, Mattfeld, et al., 2011). In addition, in the prodromal phase of late onset, sporadic Alzheimer's disease pathology in the brain includes deposition of amyloid-beta (Aβ) with pathological tau that is highly localized in the entorhinal cortex, where the earliest neuron loss occurs (Braak & Braak, 1995; Gómez-Isla et al., 1996; Hyman et al., 1984; Kordower et al., 2001; Olsen et al., 2017).
Similar deterioration of the hippocampal formation has been observed in one extensively studied rodent model of normal aging. As outbred Long–Evans rats age, deficits in spatial navigation develop to varying extent across individuals. Approximately half of aged rats show preserved spatial memory in the Morris water maze (aged memory-unimpaired, AU), while the other half exhibit memory impairments (aged memory-impaired, AI) (Gallagher et al., 1993). The severity of the spatial learning deficits has been related to the magnitude of change in different markers of hippocampal integrity, such as decreases in synaptic densities, weakened synaptic plasticity, and a rigidity of spatial firing patterns of hippocampal neurons (Gallagher & Rapp, 1997; Rosenzweig et al., 2003; Wilson et al., 2006). Additionally, AI rats with impaired hippocampus-dependent spatial memory exhibit alterations in multiple biomarkers of LEC, but not MEC, neuronal plasticity that parallel the early LEC deterioration in humans with age-related memory impairment, including reduced expression of reelin, loss of synaptophysin, and increased tau phosphorylation in layer II neurons (Stranahan, Haberman, et al., 2011; Stranahan, Salas-Vega, et al., 2011).
However, it remains unknown whether aged Long–Evans rats exhibit preservation of or concurrent impairment in episodic-like associative memory, another functional domain that relies on the hippocampal formation. In this study, we investigated the ability of young, AU, and AI rats to form episodic-like memories in a modified novel object-place-context (OPC) recognition paradigm. The standard OPC recognition paradigm consists of two sample phases of distinct OPC configurations followed by a test phase. It uses the rats' innate preference to explore novelty to probe for episodic-like memory, the ability to integrate information about objects (“what”), their locations (“where”), and the contexts in which the events take place (“occasion specifier”), similar to the temporal context used in the traditional “what-where-when” depiction of episodic-like memory (Eacott & Norman, 2004). LEC or hippocampal lesions spare young rats' non-associative object or place recognition memory but abolish entirely their ability to distinguish between objects of familiar and novel OPC configurations in the standard OPC recognition paradigm (Chao et al., 2016; Eacott & Norman, 2004; Langston & Wood, 2010; Vandrey et al., 2020; Wilson, Langston, et al., 2013; Wilson, Watanabe, et al., 2013).
Here, we report that Morris water maze performance of aged Long–Evans rats strongly predicted their associative recognition memory on the OPC recognition paradigm. Furthermore, AI rats were able to establish such associative memory but only at a later timepoint compared to young and AU rats. The findings suggest parallel functional impairments in spatial navigation and associative, episodic-like memory in aged, Long–Evans rats and reveal the potential of elucidating circuit mechanisms underlying age-related functional decline across cognitive domains.
2 MATERIALS AND METHODS
2.1 Subjects and behavioral characterization
Forty-two male Long–Evans rats (9 young; 20 AU; 13 AI) were used in this study. Young rats were obtained from Charles River Laboratories and tested on the Morris water maze at 4–6 months of age. Male retired breeders were obtained at 9 months of age from the same source and individually housed at Johns Hopkins University until 22–26 months of age for behavioral assessment on the water maze. All rats were housed in the same vivarium and had ad libitum access to food and water during a 12/12 h light/dark cycle. All animal care and housing conformed to the National Institutes of Health standards using protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
The rats were characterized for spatial learning and memory abilities in the water maze as previously described (Branch et al., 2019; Gallagher et al., 1993; Lee et al., 2022). They were trained on three trials per day for 8 days in a water maze (circular pool surrounded by curtains with large contrasting cues) to locate a hidden escape platform that remained at the same location. On every sixth trial, the escape platform was unavailable for the first 30 s. These trials served as probes to assess the rats' search proximity to the platform location. The performance of the rats was evaluated through their learning indices (LI), calculated as the sums of weighted proximity scores across probe trials (Gallagher et al., 1993). The classification of aged rats into AU and AI was based on an a priori LI cutoff at 240, which represents two standard deviations above the mean established through normative data of young animals collected over many years, with lower scores reflecting more accurate searches (Branch et al., 2019; Gallagher et al., 1993). On the day following the completion of the 8-day water maze characterization, rats were given six trials to locate a visible platform to screen for nonspecific task impairments in locomotor skills and escape motivation. Any rats that failed to swim directly to the visible platform were excluded. Rats with health issues, such as pituitary tumors or cloudy eyes, were also excluded. For this study, the rats were selected from multiple independent runs to generate a sample of young and aged rats with a wide distribution of LI. The experimenter performing the OPC tests was blind to the LI scores of the selected rats.
2.2 Experimental procedure
After the behavioral assessment on the water maze, the rats underwent object-based recognition memory tests during the light phase of the cycle. A square box (67 × 67 cm2) and a cylinder (76 cm diameter) were used in combination with floors of gray smooth texture and striped rough texture to create four different contexts. Black curtains surrounded the behavioral arena. A stuffed toy (36 × 60 × 2 cm3) was fixed on the west side of the curtains as a spatial cue in the global framework. The objects used were easily cleanable household objects made from plastic, metal, and ceramic. The size of the objects was no smaller than 5 cm in any dimension and no larger than 15 cm. Exploration of the objects was assessed via an overhead video recorder linked to a monitor. Based on data from six pilot young rats, objects with similar exploration times and of similar size and complexity were selected as matched pairs to minimize object preference biases.
Each rat underwent 2 days of handling, 2 days of habituation to the arena, and 2 days of habituation to distinct objects within the arena. Objects presented during habituation were not used for testing. Recognition memory tests followed. A novel-object recognition (NOR) test first took place as a control to confirm each rat's preference for novelty in general (Figure 1a). In this task, the sample phase involved two duplicates of the same object placed in the centers of the NE and NW quadrants of an environment. After 3 min of exposure to these objects, the rat was removed and a novel object was introduced into the same environment at one of the two locations, along with a duplicate of the sample-phase object. After a 3-min intersession interval, the rat was placed back in the environment and allowed to explore freely for 3 min.
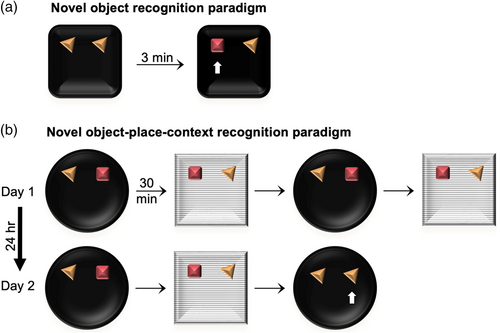
The NOR task was followed by two instantiations of a 2-day OPC test protocol. The standard OPC task protocol in prior literature (Chao et al., 2016; Eacott & Norman, 2004; Langston & Wood, 2010; Vandrey et al., 2020; Wilson, Langston, et al., 2013; Wilson, Watanabe, et al., 2013) utilized a single sample phase for each context followed by a test phase. However, our pilot data on aged rats showed decreased locomotion and spontaneous exploration in the sample phases. Because the amount of differential exploration in the test phase of the standard OPC task is weaker than in the NOR task even in young rats (Eacott & Norman, 2004; Langston & Wood, 2010; Vandrey et al., 2020), we were concerned about the ability to detect age-related differences with the standard protocol. We thus modified the standard OPC protocol by introducing multiple repeats of the two sample phases across 2 days, to encourage the initial formation of stronger OPC associations in the sample phases and to minimize false negatives due to poor sample-phase exploration. On day one, four sample phases took place (Figure 1b). In the first sample phase, two different objects were placed in the middles of two adjacent quadrants in context 1. The rat was placed in the environment and allowed to explore freely for 3 min. After a 30-min intersession interval, the second sample phase followed with duplicates of the two objects switching locations in context 2, in which the floor and wall shape were different from context 1. This sequence of OPC configurations was repeated for the third and fourth sessions of Day 1 and for the first and second sessions of Day 2. The test phase followed the second session of Day 2 and involved two duplicates of one of the sample-phase objects at both quadrants in context 1. The expectation was that rats with intact memory would explore the object located in a novel place-context configuration more than the object in the familiar place-context configuration.
To eliminate any leftover odor cues that may bias a rat's choice of exploration, duplicates of the same objects were used in different phases during testing. 70% ethanol was sprayed onto the objects and the apparatus after each phase. Each session lasted for 3 min. Two days after the second day of the protocol, the same 2-day protocol was repeated with new objects and contexts (one of the contexts was the same as that used in the NOR test). The identity of the test object and context, order of object and context presentation in the sample phases, and side of presentation were counterbalanced across rats, as is shown in Figure S1.
2.3 Data analysis
Object exploration was scored manually by two people based on the overhead camera videos of the test environments. Scorers were blind to the age status and LI of the rats and to which object was the novel object/configuration. Object exploration was defined as the period when a rat's nose was in close proximity (≤2 cm) to a given object with its nose directed toward it. The time the rat spent climbing or rearing on top of the object was excluded. The reliability of manual video scoring was checked through blind re-scoring of a subset of videos for each task by the same observer and through additional scoring of all videos by another observer. If the scoring difference was more than 15% of the original score for a given video, the video was re-scored by both observers until a consensus (difference <15%) was reached. If a rat's total object exploration time did not reach 5 s during either the NOR sample or test phase, data from the rat was removed from the dataset (Vandrey et al., 2020). If a rat's average total object exploration time across OPC sample phases or during the OPC test phase did not reach 5 s for one of the two novel OPC recognition tests, data from the rat was removed from the analysis of average OPC recognition performance. We also implemented an exclusion threshold of 10 s, but this increased threshold did not alter main findings of this paper (data not shown). The observed object exploration times during test phases were converted into discrimination ratios (DR = (time at novel object − time at familiar object)/(time at novel object + time at familiar object)). Side bias indices for exploration phases with two identical objects (i.e., the NOR sample phase and the OPC test phases) were calculated as (time at left object – time at right object)/(time at left object + time at right object). Deviations of OPC-test side biases from NOR-sample side biases were calculated as the perpendicular distance from individual rats' datapoints on a scatter plot to the diagonal line, or |. In addition, the same set of videos were labeled automatically by DeepLabCut to quantify the cumulative distance traveled by the animal within a session (Mathis et al., 2018).
Distributions of the data were described as mean ± standard error of the mean. Normality of the data was confirmed with the Shapiro–Wilk test. Pearson correlations were calculated between object exploration task performance measured by DR and water maze performance quantified by LI among all aged rats to test for linear relationships between associative recognition memory and spatial deficits. The same Pearson correlation analysis was performed between test-phase object exploration times and water maze performance to test for linear relationships between locomotion levels and spatial memory deficits. Depending on the normality of the data distributions, one-sample t-tests or Wilcoxon sign rank tests were performed to determine whether the average DR for young and aged rats were significantly different from chance (0). Two sample t-tests or Wilcoxon rank sum tests between young and aged rats were performed to determine whether task performance and object exploration times showed statistically significant differences.
A linear mixed-effects model was constructed to quantify the impact of various factors on aged rats' discrimination ratios in the novel OPC recognition tests. The main variables of interest were LI, test number (OPC1 or OPC2), and the interaction between LI and test number. LI was z-scored to be easily interpretable so that the resulting coefficient estimate reflected the change in DR per standard-deviation change in LI. Other covariates in the constructed model included rat cohort number, test object identity, novel object location, test context, object bias within the test context, and side bias. For each round of testing, object bias refers to a rat's average bias index for the test object against the non-test object across all sample phases in the context subsequently used for the test session. For a given sample phase, the object bias index is calculated as (time spent exploring test object − time spent exploring non-test object)/(time spent exploring test object + time spent exploring non-test object). Side bias refers to the rat's side bias index during the sample phase of NOR, where two identical objects were placed in the experimental apparatus. For each test, the side bias index is calculated as (time spent exploring the object on the side where the novel OPC configuration would be − time spent exploring the object on the side where the familiar OPC configuration would be)/(time spent exploring the object on the side where the novel OPC configuration would be + time spent exploring the object on the side where the familiar OPC configuration would be). A positive index suggests that the rat's side bias is toward the side of the novel OPC configuration during the novel OPC recognition test. A negative index suggests a bias toward the side opposite of the novel OPC configuration. Rat identity was included as a random-effect variable. Following the linear mixed-effects model, Type III ANOVA based on Satterthwaite approximation was used to test the statistical significance of each fixed-effect term.
3 RESULTS
3.1 Hippocampus-dependent spatial memory declined to varying degrees with age
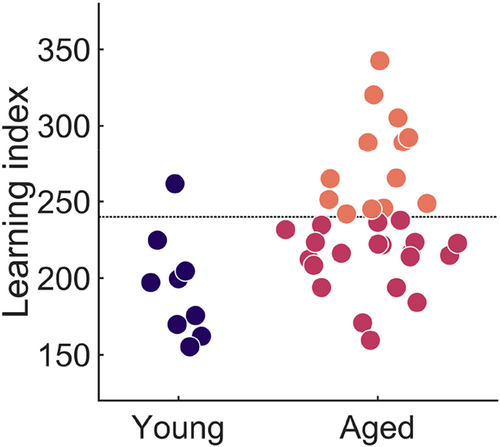
The Morris water maze task assesses the rat's ability to learn to navigate to a specific location and relies on intact hippocampal function (Morris, 1984; Redish & Touretzky, 1998). The learning index (LI), a weighted sum of proximity scores across multiple probe trials, quantified the performance of the rats, with lower scores reflecting a more accurate search (Gallagher et al., 1993). An LI of 240 serves as a cutoff for the classification of aged rats into memory-unimpaired and memory-impaired categories (see Section 2; Branch et al., 2019; Gallagher et al., 1993). The present study included nine young rats, all except one of which had a LI below 240, and 33 aged rats (Figure 2). The performance of young rats on the Morris water maze was different from that of aged rats (t(40) = −2.873, p = .007). The aged rats showed a large spread of LI values, with some performing on the water maze as well as the young rats and others performing much more poorly.
3.2 Object recognition memory and novelty preference remained intact among all rats
After the water maze characterization, rats were first tested on a standard non-associative object recognition task (Ennaceur & Delacour, 1988) to determine whether age-related spatial memory impairment predicted disrupted object recognition or discrimination under the procedures used in the current experiment. All young rats explored the novel objects more than the familiar objects during the test phase, demonstrating intact object recognition memory and novelty preference (discrimination ratios: 0.342 ± 0.059; t(8) = 5.762, p = 4.233e−04; Figure 3a). Similar to the young, aged rats preferentially explored the novel objects (discrimination ratios: 0.410 ± 0.035, t(32) = 11.676, p = 4.490e−13). There was no statistically significant difference in task performance between young and all aged rats (t(40) = −0.923, p = .362). In addition, among aged rats, there was no statistically significant correlation between LI and discrimination ratios (Pearson's r = 0.086, p = .635).
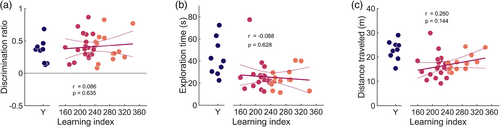
Total test-phase object exploration times differed significantly between young and aged rats (total exploration times: (young) 43.11 ± 5.60 s, (aged) 25.46 ± 2.14 s; Wilcoxon signed-rank test: T = 299, Z = 3.221, p = .001; Figure 3b). This difference was accompanied by shorter distances traveled by aged rats (total distance traveled: (young) 22.86 ± 1.35 m, (aged) 16.67 ± 0.76 m; Wilcoxon signed-rank test: T = 301, Z = 3.280, p = .001; Figure 3c). However, LI was not significantly correlated with object exploration time (Pearson's r = −0.088, p = .628) or total travel distance (Pearson's r = 0.260, p = .144) during the NOR test phase.
Total sample-phase object exploration times also differed significantly between young and aged rats (total exploration times: (young) 41.56 ± 5.16 s, (aged) 25.11 ± 2.17 s; Wilcoxon signed-rank test: T = 290, Z = 2.945, p = .003; Figure S2A). This difference was accompanied by shorter distances traveled by aged rats during the sample phase (total distance traveled: (young) 21.52 ± 1.50 m, (aged) 17.44 ± 0.76 m; Wilcoxon signed-rank test: T = 273, Z = 2.422, p = .016; Figure S2B), in accordance with a previous finding of a general reduction in locomotor activity with age in open fields (Gage et al., 1984). Among aged rats, higher LI was associated with more distance traveled in the open field during the NOR sample phase (Pearson's r = 0.474, p = .005).
3.3 Spatial memory deficit predicted associative OPC recognition memory impairment in aged rats
After establishing unimpaired, non-associative object recognition memory and novelty preference in both young and aged rats, each rat underwent two OPC recognition tests with a new object pair and two new contexts. Averaging DR values across both OPC tests, young rats preferentially explored objects with the novel OPC configuration and had average discrimination ratios (DR) significantly greater than chance (DR: 0.164 ± 0.055; t(8) = 2.986, p = .017; Figure 4a) demonstrating OPC-integrated associative recognition memory. Aged rats showed near-chance average DR across tests (DR: 0.066 ± 0.033; Wilcoxon signed-rank test: T = 345, Z = 1.901, p = .057), but there was no statistically significant difference in average DR between young and aged rats (Wilcoxon signed-rank test: T = 239, Z = 1.749, p = .080). However, within the group of aged rats, average DR on the two OPC recognition tests was significantly correlated with water maze performance (Pearson's r = −0.387, p = .026). Thus, aged rats' spatial navigation skills predicted their ability to express episodic-like, associative memory.
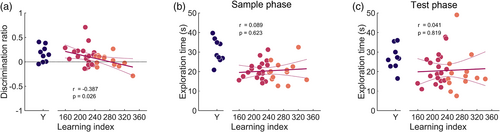
Young rats had greater mean object exploration times than aged rats during both sample and test phases (sample phase: young = 30.56 ± 2.04 s, aged = 20.83 ± 0.83 s, t(38) = 5.177, p = 7.640e−06; test phase: young = 27.08 ± 2.07 s, aged = 21.28 ± 1.52 s, Wilcoxon signed-rank test: T = 254.5, Z = 2.252, p = .024; Figure 4b,c, Figure S3A–C). Aged rats' LI was not significantly correlated with total sample-phase object exploration times (Pearson's r = 0.089, p = .623) or test-phase object exploration times (Pearson's r = 0.041, p = .819), strongly suggesting that the relationship between LI and DR was not an artifact of differences in object exploration time across rats. In addition, there was a significant age-related difference in total distance traveled between young and aged rats (young = 24.12 ± 0.82 m, aged = 16.01 ± 0.68 m; t(38) = 6.012, p = 5.489e−07) but no statistically significant correlation between LI and total travel distances among aged rats in the test phases (Pearson's r = 0.311, p = .088; data not shown).
3.4 Associative recognition performance improved on the second OPC test in all rats
To check for cross-test performance replicability, individual rats' performance on the two OPC recognition tests were analyzed independently. In the first OPC test, both young and aged rats showed near-chance performance (young = 0.066 ± 0.064, t(8) = 1.030, p = .333; aged = 0.010 ± 0.063, t(31) = 0.155, p = .878; Figure 5a), with no statistically significant difference between the groups (t(39) = 0.451, p = .654). However, within the aged group, linear regression analysis established a strong correlation between individual aged rats' water maze LI and OPC recognition DR (Pearson's r = −0.576, p = 4.50e−04). Contrary to our expectations, aged rats with high LI scores (indicating poor learning) on the water maze did not have DR scores around 0 on the OPC recognition test (which would indicate no object preference); instead, they had negative DR scores, indicative of preferential explorations of the objects with the familiar OPC configuration. Between young and aged rats, average test-phase object exploration times were statistically different (young = 27.89 ± 2.22 s, aged = 19.06 ± 1.29 s; t(39) = 3.270, p = .002; Figure 5b), but no statistically significant relationship was found between LI and total object exploration times of aged rats (Pearson's r = −0.018, p = .922).
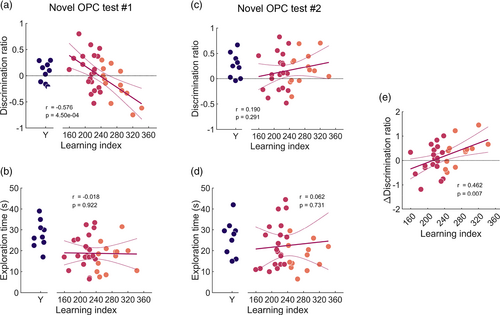
In the second OPC test, both young and aged rats had discrimination ratios significantly greater than chance (young: DR = 0.263 ± 0.081; t(8) = 3.247, p = .012; aged: DR = 0.151 ± 0.059, t(31) = 2.545, p = .016; Figure 5c), demonstrating successful OPC associative memory. There was no statistically significant difference in DR between the two age groups (t(39) = 0.932, p = .357). There was no statistically significant relationship between water maze performance and associative recognition memory for the aged rats (Pearson's r = 0.190, p = .291). In contrast to the first OPC test, rats from both age groups performed similarly well the second time they went through the OPC recognition paradigm. In addition, young and aged rats explored objects for similar amounts of time during the test phase (young = 26.28 ± 2.85 s, aged = 22.58 ± 2.45 s; Wilcoxon signed-rank test: T = 241, Z = 1.623, p = .105; Figure 5d). LI of aged rats again exhibited no statistically significant relationship with their test-phase object exploration times (Pearson's r = 0.062, p = .731).
Across the first and second OPC tests, young and aged rats both exhibited increases in discrimination ratios that nearly reached statistical significance (young: t(16) = −1.918, p = .073; aged: t(64) = −1.955, p = .055). Among aged rats, the correlation coefficients between learning indices and discrimination ratios were significantly different between the first and second OPC tests (Z(33) = −3.828, p = 1.290e−4; Lee & Preacher, 2013). Further analysis between LI and the change in DR across tests among aged rats revealed a statistically significant correlation (Pearson's r = 0.462, p = .007; Figure 5e; object exploration times by test across sample and test phases are shown in Figure S3D–F). Thus, the performance improvement in the aged rats is driven primarily by the greater improvement in performance of the high-LI, aged rats across tests.
3.5 The LI–DR relationships among aged rats are not explained by confounding factors
An unexpected and puzzling result was that on the first OPC test, the AI rats with the highest LIs preferentially explored the familiar, rather than the novel, OPC-configured objects (i.e., the DR for these rats was negative). This exploration bias cannot be explained simply by a general familiarity preference of AI rats, as all rats explored the novel objects more than the familiar objects in the initial NOR experiment (Figure 3). Alternatively, certain objects or contexts might be more salient for individual rats and facilitate episodic-like memory formation. Cohort differences or differential object exploration based on inherent side biases may also lead to the unexpected pattern of performance. We thus conducted a series of post hoc analyses in an attempt to garner preliminary insight into this result.
We first constructed a linear mixed-effects model for aged rats. Task-related, fixed-effect terms included OPC test number, test object identity, novel OPC-configured object location, and test context. Rat-related, fixed-effects terms included z-scored LI, the interaction between z-scored LI and OPC test number, cohort number, average bias toward the test object across the sample phases in the test context, and side bias. Side bias was estimated by comparing the differential exploration of the two identical objects in the NOR sample phase with that in the OPC tests, as the NOR sample phase was similar to the two OPC test phases (i.e., two identical objects). Rat identity was included in the model as a random-effect term. The model confirmed that both LI and the interaction between LI and test number contributed significantly to aged rats' discrimination ratios on the novel OPC recognition paradigm, when all of the other potential confounding factors were controlled for (Table 1). The model also revealed a significant contribution of side bias to the DR, but further analysis confirmed that the side bias did not account for the negative DRs of AI rats in the first OPC test (Figures S4–S6).
| Variable | Estimate (95% CI) | t- or F-statistics | df | p-Value |
|---|---|---|---|---|
| Z(LI) | −0.239 (−0.386, −0.092) | 10.70 | 1, 44 | .002 |
| Test | 7.804 | 1, 44 | .231 | |
| Z(LI) × test | 9.208 | 1, 44 | .004 | |
| Cohort | 0.384 | 6, 44 | .885 | |
| Object | 0.540 | 6, 44 | .774 | |
| Location | 1.304 | 1, 44 | .260 | |
| Context | 1.845 | 3, 44 | .153 | |
| Object bias | −0.078 (−0.486, 0.330) | 0.148 | 1, 44 | .702 |
| Side bias | 0.429 (0.0371, 0.821) | 4.866 | 1, 44 | .033 |
- Note: A linear mixed-effects model was constructed to estimate and test the effects of LI, trial, and other covariates on aged rats' DR in the novel OPC recognition tests. Fixed-effects terms are listed under Variable. Coefficient estimates and 95% confidence intervals for each continuous fixed-effects term are shown under Estimate. For discrete fixed-effects terms, coefficient estimates were calculated for individual levels separately and are not shown. t-statistics for continuous fixed-effects terms and F-statistics for discrete fixed-effects terms (with two or more degrees of freedom) are under t- or F-statistics. p-values in boldface are statistically significant at p < 0.05. df, degrees of freedom.
4 DISCUSSION
4.1 Parallel functional deficits in spatial navigation and episodic-like memory among aged rats
Given the known vulnerability in hippocampal and LEC cellular physiology in aged Long–Evans rats, the current experiment sought to relate the known impairment in spatial learning and memory ability with the functional integrity of MTL-dependent, non-navigational associative memory. Using a spontaneous object exploration paradigm, we tested young and aged rats on an episodic-like, OPC recognition task. As Eacott and Norman (2004) argued, this task is a model of episodic memory, as it combines an integrated memory of what, where, and which (as opposed to the classic “what, where, and when” characterization of episodic-like memory). Several lesion studies have established the dependence of this task on the LEC and hippocampus (Chao et al., 2016; Eacott & Norman, 2004; Langston & Wood, 2010; Vandrey et al., 2020; Wilson, Langston, et al., 2013; Wilson, Watanabe, et al., 2013). We found that aged rats exhibited varying degrees of functional impairment on this task that paralleled the extent of their deficit on the Morris water maze (see also Robitsek et al., 2008). The observation demonstrates that the age-related decline in MTL-associated cognition is not limited to spatial learning but is accompanied by a compromised ability to express episodic-like, associative memory.
4.2 Improvement in associative memory expression in AI rats
By separately analyzing the data from the first and second OPC recognition tests, we observed that AI rats showed significant performance improvement the second time they experienced the OPC recognition paradigm. Previously, aging and memory impairments have been associated with the emergence of rigidity in hippocampal spatial representations and slower rates of learning in terms of spatial task acquisition and place cell map formation (Foster, 1999; Lee et al., 2022; Rapp & Amaral, 1992; Wilson et al., 2003, 2004). The late expression of OPC memory among AI rats compared to young and AU rats provides a piece of evidence in support of concurrent age-related functional deficits in spatial navigation and episodic-like memory.
Interestingly, rather than improving from chance-level to above-chance performance, these rats switched from preferentially exploring the objects in the familiar OPC configuration on the first test to preferentially exploring the objects in the novel OPC configuration on the second test. The initial below-chance performance of AI rats is unlikely a result of an inherent preference for familiarity in general. In the standard non-associative object recognition task, all rats preferentially explored the novel objects regardless of age and age-related spatial memory impairment. This agrees with previous studies demonstrating intact novel object recognition memory at short delay intervals in aged rats (Bergado et al., 2011; Burke et al., 2010; Cavoy & Delacour, 1993; Gámiz & Gallo, 2012; Lukaszewska & Radulska, 1994) and in rats with LEC lesions (Wilson, Langston, et al., 2013; Wilson, Watanabe, et al., 2013). Through post hoc analysis, the use of side bias was also ruled out as the sole explanatory factor for negative DRs, although the mixed-effects model revealed that side bias was a statistically significant factor.
Importantly, the negative DR scores did not persist across the two tests. The same AI rats successfully established OPC associative memory the second time they experienced this same paradigm. One possibility is that the AI rats might have taken longer than the AU rats to become familiarized with background cues in the testing environment, which perhaps distracted them from forming strong representations of the local OPC information. Alternatively, AI rats might have taken longer than AU rats to acquire a schema of the OPC protocol (Tse et al., 2007), which might have diminished their ability to create the complex OPC configural associations. Although the object pair and contexts changed, the constancy of apparatus location within the experimental room and temporal structure of the task might have facilitated assimilation of experiences.
A potential explanation for the below-chance performance (i.e., the apparent preference for the familiar OPC configuration) in the first OPC test is that the AI rats initially associated object and place information into memory but either failed to recognize or encode context changes or failed to form more complex, three-way OPC associations. In this case, a recency effect might cause the rats to refer to the sample session immediately preceding the test session for novelty evaluation, even though this sample session is in a different context than the test session. As Figure 1 shows, if one ignores the context, the object-place pairing that is changed in the test session relative to the preceding sample session is in the location that would be considered the familiar OPC configuration for that context. Thus, the preferential exploration of the familiar OPC-configured object in the test phase might be an expression of successful recognition memory based on object-place associations, rather than OPC associations, and result in negative discrimination ratios. Testing these hypotheses would be a fruitful aim for future investigation.
4.3 Potential information-processing deficit of the LEC–hippocampus pathway
Long–Evans rats have demonstrated divergent age-related network-level alterations. On a T-maze task that required successive switches between competing hippocampal place and striatal response navigation strategies, the performance of AU rats with preserved spatial memory differed from both young and AI rats (Pereira et al., 2015). Whereas young rats were biased toward place strategies and AI rats were biased toward response strategies, AU rats displayed no bias for either type of learning and acquired both strategies equally rapidly, suggestive of diminished competition between the MTL and cortico-striatal memory systems.
Similarly, adaptive changes in the MTL system may impact different streams of information processing to different extents, leading to differential impairments in various MTL-dependent cognitive tasks. Within the MTL, the entorhinal cortex is the major cortical input to the hippocampus and is composed of two subdivisions, MEC and LEC, that contain distinct cytoarchitecture as well as different functional cell types (Leitner et al., 2016; Ohara et al., 2019; Ray et al., 2014; Varga et al., 2010). MEC has many spatially modulated neurons, including grid cells, head direction cells, border cells, speed cells, and object-vector cells (Hafting et al., 2005; Høydal et al., 2019; Kropff et al., 2015; Sargolini et al., 2006; Solstad et al., 2008). In contrast, LEC has minimal movement-related theta and consists of cells tuned to local sensory information, egocentric bearing to spatial reference points, and sequences of events (Bitzenhofer et al., 2022; Deshmukh & Knierim, 2011; Tsao et al., 2013, 2018; Wang et al., 2018). Receiving convergent inputs from the postrhinal cortex and perirhinal cortex, LEC holds the richest set of association connections in the rat cerebral cortex (Bota et al., 2015; Doan et al., 2019). It is thought that the MEC–hippocampus stream of information processing supports self-motion-based path-integration computation, whereas the LEC–hippocampus stream represents the specific content and temporal order of an ongoing experience (Knierim, 2015).
AU and AI rats exhibited distinct performance patterns across the two OPC tests. Previous studies have established prominent cellular and molecular deficits of AI rats in the LEC, but not MEC, and hippocampus (Gallagher & Rapp, 1997; Rosenzweig et al., 2003; Stranahan, Haberman, & Gallagher, 2011; Stranahan, Salas-Vega, et al., 2011; Wilson et al., 2006). We speculate that the establishment of episodic-like, associative recognition memory in the OPC task requires more engagement of the LEC–hippocampus stream of information processing than the MEC–hippocampus pathway. Future studies with region-specific manipulations in the MTL system will yield insight on the roles of MEC, LEC, and hippocampus in this paradigm.
4.4 Caveats
Due to the repeated nature of the OPC sample phases in the current experimental design, the use of sequence-based, object-place memory, as opposed to OPC memory, would allow rats to perform equally well. Specifically, if a rat remembered the sequence of alternating left–right locations of the objects, then identifying a mismatch in the expected object-place alternations could lead to similar positive discrimination ratios during the OPC test phase. Thus, the use of OPC versus object-place sequence memory in the two OPC recognition tests cannot be dissociated in this study. However, regardless of whether individual rats used an OPC strategy or an object-place-temporal sequence strategy, the rats exhibiting late expression of associative recognition memory were the same ones that showed hippocampus-dependent spatial memory impairment and belonged to the age group with altered markers of neuronal plasticity in the LEC (Gallagher et al., 1993; Stranahan, Haberman, et al., 2011; Stranahan, Salas-Vega, et al., 2011).
Another caveat is that inherent side biases of individual rats could only be probed in three exploration phases—the NOR sample phase and the two OPC tests—in the present study. The role that side bias could play was not taken into account when we designed the modified novel OPC recognition protocol. Therefore, whether a rat used solely object identity during OPC recognition tests was based on one reference session, the NOR sample phase, in the current data analysis. In future experiments, having identical objects during habituation and comparing side preferences across multiple exploration phases may help establish a better baseline of side-bias indices.
Finally, different retention delays were used in the NOR and novel OPC recognition paradigm, complicating a direct comparison of memory strength across the two tasks. However, our main objective for conducting the 3-min-delay NOR test was to simply confirm in a task that has minimal mnemonic demands that aged rats can discriminate the objects and express exploration preference for novelty similar to young rats. Both of these abilities would be necessary to demonstrate mnemonic performance in the more difficult OPC task. We do not make any claims based on this paper about how young or aged rats differ in their strength or longevity of memory across the NOR and OPC tasks, given the multiple differences in the designs of the two tasks.
5 CONCLUSION
Much effort has been spent on understanding circuit-level changes in the aging MTL system that contribute to impaired spatial navigational skills in rats. Importantly, the hippocampal formation is also critical for episodic-like, associative memory. The present study demonstrates that with age, the LEC–hippocampus-dependent ability to integrate what, where, and which (when) information declines in parallel with MEC–hippocampus-dependent deficits in the spatial domain among Long–Evans rats. Interestingly, AI rats with hippocampal and LEC impairment were able to express associative episodic-like recognition memory on the second novel OPC recognition test, after extended experience in the spontaneous exploration paradigm. The study opens the opportunity to examine changes in the information processing capabilities of distinct MTL regions that contribute to concurrent functional decline across cognitive domains.
AUTHOR CONTRIBUTIONS
Yuxi Chen: Conceptualization, methodology, investigation, formal analysis, visualization, writing—original draft. Audrey Branch: Conceptualization, methodology, writing—review & editing. Cecelia Shuai: Investigation, writing—review & editing. Michela Gallagher: Conceptualization, funding acquisition, writing—review & editing. James Knierim: Conceptualization, methodology, funding acquisition, Writing—review & editing.
ACKNOWLEDGMENTS
We would like to thank Robert McMahan and Jala Atufa for assistance in running the Morris water maze experiment. We thank Dr. James Ainge for valuable advice on conducting the object recognition experiments, Dr. Scott Zeger for help in the statistical analysis, and Dr. Lucas Glover for helpful discussion. Supported by grant P01 AG009973 from the National Institute on Aging of the U.S. Public Health Service and the Johns Hopkins Science of Learning Institute.
CONFLICT OF INTEREST STATEMENT
Dr. Gallagher is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to discovery and development of therapies to treat cognitive impairment. She has a financial interest in the company.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.