Turning off of GluN2B subunits and turning on of CICR in hippocampal LTD induction after developmental GluN2 subunit switch
The authors have no conflict of interest to declare.
ABSTRACT
NMDA receptors (NMDARs) are essential for the induction of synaptic plasticity that mediates activity-dependent refinement of neural circuits during development. GluN2B subunits of NMDARs are abundant at synapses in the immature hippocampus and begin to be replaced by GluN2A subunits with the help of casein kinase 2 activity in the second postnatal week, the critical period for the GluN2 subunit switch (Sanz-Clemente et al. (2000) Neuron 67:984–996). However, the physiological role of GluN2B subunits in the hippocampus during this critical period has not been elucidated. Here, we report that GluN2B subunits mediate the induction of long-term depression (LTD) in the CA1 region of the hippocampus only until this period. Ifenprodil and Ro25-6981, selective inhibitors of NMDARs containing GluN2B subunits, blocked LTD in postnatal Day 11–14 (P11–14) rat hippocampal slices but not in P18–22 hippocampus. Just a few days after P14, synaptic NMDAR currents became narrower than those at P11–14, and calcium influx through NMDARs must be reduced. We found that calcium-induced calcium release (CICR) through ryanodine receptors starts to support the induction of NMDAR-dependent LTD at P18–22. Intracellular application of thapsigargin and ryanodine, inhibitors of Ca2+-ATP pumps on internal stores and ryanodine receptors, respectively, did not at all affect LTD in the hippocampus at P11–14 but completely blocked LTD in the P18–22 hippocampus. Therefore, calcium influx through NMDAR with GluN2B subunits is sufficient to induce LTD at P11–14, after which CICR compensates for the decrease in calcium influx during LTD induction. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Developmental synaptic plasticity is expressed in immature neural tissues and mediates activity-dependent remodeling of neural connections that have once been generated according to genetic information. Among them, long-term potentiation (LTP) and long-term depression (LTD) are the major forms of synaptic plasticity in the CA1 region of the hippocampus. Both require calcium influx through NMDARs at postsynaptic sites and different molecules downstream of calcium signaling (Bear and Malenka, 1994), the expression of some of which changes during development. For example, the expression level of calcium/calmodulin-dependent protein kinase (CaMK) IIα, which mediates LTP induction in the mature hippocampus, is low in 2-week-old rodents (Kelly et al., 1987). However, LTP is already fully expressed in the immature hippocampus. We have reported that LTP in the neonatal hippocampus is mediated by protein kinase (PK) A (Yasuda et al., 2003), whose expression at synapses reaches mature level only by 2 weeks after birth (Kelly, 1982). Thus, molecular mechanisms underlying synaptic plasticity switch as the brain matures, and it is possible that the calcium source for plasticity induction may differ during development. NMDAR subunit composition switches during development (Barria and Malinow, 2002; Philpot et al., 2001), and the switch is facilitated by the phosphorylation of GluN2B subunits by casein kinase (CK) 2 after the second postnatal week, when CK2 expression level is elevated in the developing hippocampus (Sanz-Clemente et al., 2010); thus, this is the critical period for the replacement of GluN2B subunits by GluN2A subunits. The shortening of NMDAR-mediated excitatory postsynaptic currents (ESPCs) is associated with this switch (Monyer et al., 1992), but the consequence of this shortening has not been described. Here, we report the effect of the GluN2 subunit switch on LTD induction through the regulation of NMDAR EPSC kinetics during and after the second postnatal week.
We also discuss the mechanisms compensating for LTD induction when NMDAR EPSCs become faster after the second postnatal week. We examined the involvement of calcium-induced calcium release (CICR) through ryanodine receptors in LTD induction after this period, because (1) CICR is associated with calcium influx through NMDARs activated by synaptic activity (Emptage et al., 1999) and (2) calcineurin and FK-binding proteins (FKBPs), a key enzyme for LTD induction in the hippocampus and the cerebral cortex and its binding proteins, respectively, bind to calcium store channels and affect each other (Snyder et al., 1998). We present evidence that CICR starts to participate in LTD induction at approximately 20 days after birth, when GluN2B subunits of NMDARs cease to contribute to LTD induction, suggesting that CICR through ryanodine receptors compensates for the reduction of calcium influx through NMDARs that results from the subunit switch of NMDARs from GluN2B subunits to GluN2A subunits during development.
MATERIALS AND METHODS
Electrophysiology
Animal use and all experimental procedures were approved by the Ethical Committee for Animal Experiments of Gunma University. Hippocampal slices were cut from postnatal 11- to 22-day-old Sprague-Dawley rats (Japan SLC, Inc., Hamamatsu, Japan). Briefly, the slices were cut in ice-cold oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid containing (in mM) 119 NaCl, 2.5 KCl, 26.2 NaHCO3, 1 NaH2PO4, 4 CaCl2, 4 MgSO4, and 11 glucose (pH 7.4) and incubated for at least 2 h. The slices were visualized in a submersion-type recording chamber mounted on an upright microscope (BX51WI; Olympus, Tokyo, Japan) and perfused with oxygenated recording solution (at 30 °C) including 100 μM picrotoxin. Field potential and whole-cell voltage-clamp recordings were made from neurons in the CA1 region using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale). Recording electrodes for voltage-clamp experiments were filled with a solution containing (in mM) 135 CsMeSO4, 10 HEPES, 0.2 EGTA, 8 NaCl, 5 QX-314 Cl, 4 Mg-ATP, and 0.3 Na3GTP (pH 7.2 with CsOH, osmolality adjusted to 280–290 mOsm). Field excitatory postsynaptic potentials (fEPSPs) and EPSCs were evoked with a stimulating glass electrode containing normal recording solution placed in the stratum radiatum. LTD of fEPSPs was induced by applying 1-Hz 15-min or 5-Hz 3-min stimulation at least 40 min after starting recording, because it takes 30–40 min for ifenprodil to fully block NMDAR EPSCs (Fig. 3) (Miwa et al., 2008). In voltage-clamp experiments, baseline synaptic responses at 0.05 Hz were obtained at −70 mV, and a 1-Hz 10-min or a 5-Hz 2-min train of pulses was paired with depolarization at −40 or −50 mV to induce LTD. Series and input resistances were continually monitored throughout an experiment. NMDAR EPSCs were isolated by adding 100 μM picrotoxin and 10 μM NBQX to the extracellular solution and recorded at +40 mV through an electrode filled with a solution containing (in mM) 102 CsMeSO4, 10 HEPES, 8 NaCl, 5 QX-314 Cl, 5 TEA Cl, 10 BAPTA, 4 Mg-ATP, and 0.3 Na3GTP (pH 7.2 with CsOH, osmolality adjusted to 281 mOsm) (Morishita et al., 2007; Yoshimura et al., 2003). Decays of averaged NMDAR EPSCs were fitted to a double exponential: I (t) = Aexp(−t/τf) + Bexp(−t/τs), where I is the amplitude of NMDAR EPSCs, A and B are the peak amplitudes of fast and slow components, respectively, and τf and τs are the decay time constants, respectively, using Igor software (WaveMetrics, Inc., Lake Oswego) (Carmignoto and Vicini, 1992; Philpot et al., 2001). Then, a weighted time constant (τw) was calculated as τw = (A × τf + B × τs)/(A + B) to analyze the kinetics of NMDAR EPSCs during and after the critical period for the GluN2 subunit switch (Philpot et al., 2001; Rumbaugh and Vicini, 1999; Yoshimura et al., 2003).
Statistical Analyses
Results are reported as mean ± SEM. The statistical significance of differences between the two groups was analyzed using Student's t test in Excel. For multiple comparisons, one-way ANOVA with Tukey–Kramer test in EZR (Easy R) software was used (Kanda, 2013).
Drugs
Ifenprodil, Ro25-6981, NBQX, QX-314 Cl, and MPEP were from Tocris Bioscience (Bristol, United Kingdom). Ryanodine was from Calbiochem (La Jolla) or Wako Pure Chemical Industries (Osaka, Japan). Thapsigargin and TEA Cl were from Wako. Other chemicals were from Sigma (St. Louis) or Wako.
RESULTS
GluN2B Subunits Mediate LTD Induction Only Until Second Postnatal Week
LTD induced by low-frequency stimulation in the CA1 region of the immature (postnatal Days 12–22) hippocampus is dependent on NMDARs and is triggered by elevated intracellular calcium (Malenka and Bear, 2004; Mulkey and Malenka, 1992). LTD induction does not require the activation of GluN2B subunits in the hippocampus older than the third postnatal week (Morishita et al., 2007); however, the role of GluN2B subunits in LTD induction has not yet been clarified during the second postnatal week when GluN2B subunits are more predominant. Initially, we examined the effects of ifenprodil, a GluN2B-selective antagonist, on induction of NMDAR-dependent LTD in the CA1 region during and after this period. Ifenprodil (3 μM) did not affect LTD induced by 1-Hz stimulation for 15 min in the hippocampus at postnatal days 18–22 (P18–22), only a few days after the period when developmental GluN2 subunit switch starts (Figs. 1B,C; control, 72.3 ± 2.5% of baseline 50 min after LTD induction, n = 14; ifenprodil, 73.2 ± 3.0%, n = 13). However, ifenprodil significantly blocked LTD at P11–14 (Figs. 1A,C; control, 63.2 ± 2.6%, n = 16; ifenprodil, 83.4 ± 3.0, n = 13; P < 0.00005; unpaired Student's t test), indicating that GluN2B subunits are involved in LTD induction only until the second postnatal week. We also examined the effects of Ro25-6981, another potent and selective GluN2B inhibitor, on LTD. Ro25-6981 (5 μM) significantly inhibited LTD at P11–14 (Figs. 2A,D; control, 61.2 ± 1.7%, n = 15; Ro25-6981, 85.5 ± 3.2%, n = 15; P < 0.000005). Ro25-6981 reduced LTD in the P15 − 17 hippocampus, immediately after the second postnatal week, although the reduction was smaller than that at P11–14 (Figs. 2B,D; control, 61.8 ± 4.5%, n = 9; Ro25-6981, 76.8 ± 1.8%, n = 8; P < 0.05). After this transition period, Ro25-6981 did not have any effects on LTD (P18–22, Figs. 2C,D; control, 75.4 ± 2.5%, n = 14; Ro25-6981, 73.7 ± 3.3%, n = 15). These data suggest that GluN2B subunits contribute to LTD induction until the second postnatal week, that involvement of GluN2Bs in LTD induction is reduced immediately after the transition period, and that GluN2Bs are not needed for LTD induction after P18.
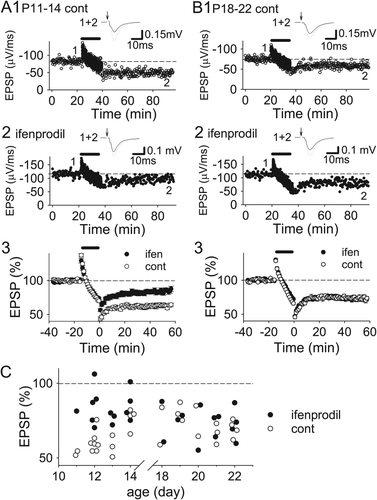
Involvement of GluN2B subunits in LTD induction in hippocampus only until second postnatal week. (A) Examples (A1 and 2) and summary (A3) of the effects of ifenprodil (3 μM) on LTD in the P11–14 hippocampus (control, n = 16; ifenprodil, n = 13). (B) Examples (B1 and 2) and summary (B3) of the effects of ifenprodil (3 μM) on LTD in the P18–22 hippocampus (control, n = 14; ifenprodil, n = 13). (C) Relationship of the magnitude of LTD with the age of hippocampal slices.
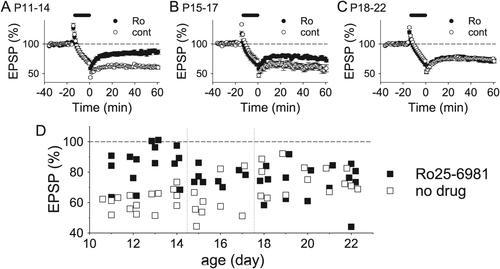
Another inhibitor confirms importance of GluN2B subunits in LTD induction at P11–14. Summary of the effects of Ro25-6981 (Ro, 5 μM) on LTD in the P11–14 (control, n = 15; Ro, n = 15) (A), the P15–17 (control, n = 9; Ro, n = 8) (B), and the P18–22 hippocampus (control, n = 14; Ro, n = 15) (C). (D) Relationship of the magnitude of LTD with the age of hippocampal slices.
We, therefore, next assessed the contribution of GluN2B subunits to synaptic NMDAR currents in the P11–14 and P18–22 hippocampus. Ifenprodil reduced NMDAR-mediated EPSCs by approximately 45% at P11–14 and by approximately 25% at P18–22 (Figs. 3A–C; P11–14, 55.6 ± 5.3% of baseline 40 min after the start of wash-in, n = 14; P18–22, 76.4 ± 7.8%, n = 15; P < 0.05). Furthermore, NMDAR EPSCs at P11–14 had slower weighted decay time constants than NMDAR EPSCs at P18–22 (Fig. 3D; P11–14, 194.4 ± 6.2 ms, n = 26; P18–22, 135.0 ± 3.7 ms, n = 40; P < 0.0000000005, unpaired Student's t test), suggesting that synaptic NMDARs contain much more GluN2B subunits at P11–14 than those 4 days after this period. The decay time constants of NMDAR EPSCs were significantly reduced by ifenprodil in both the P11–14 and the P18–22 hippocampus (Fig. 3E; P11–14, P < 0.0005; P18–22, P < 0.00005; paired Student's t test), confirming that ifenprodil effectively inhibits NMDARs with GluN2B subunits, whose currents passing through their channels have slower decay constants. Although calcium permeability through NMDAR channels may be similar between the P11–14 and the P18–22 hippocampus because the fractional calcium current passing through GluN1/GluN2B is not significantly different from that passing through GluN1/GluN2A (Schneggenburger, 1996), NMDAR EPSCs with a longer duration at P11–14 can cause a larger calcium influx than those at P18–22 and, therefore, contribute to LTD induction only at P11–14.
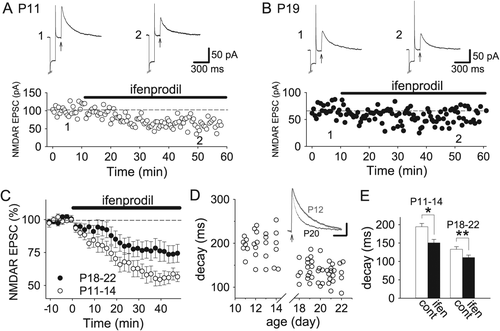
Decrease in GluN2B-mediated NMDAR EPSCs after second postnatal week. Examples (A and B) and summary (C) of the effects of ifenprodil on NMDAR EPSCs in the P11–14 (n = 14) and the P18–22 hippocampus (n = 15). (D) Relationship of the weighted time constants of NMDAR EPSC decay with the age of slices (P11–14, n = 26; P18–22, n = 40). Scale bars, 18.8 pA for P12 and 20 pA for P20, 200 ms. (E) Summary of the effects of ifenprodil on the weighted decay time constants of NMDAR EPSCs in the P11–14 (n = 14) and the P18–22 hippocampus (n = 15). *P < 0.0005, **P < 0.00005.
Overexpression of GluN2A does not affect LTD induced by 1-Hz stimulation: however, it abolishes LTD induced by 3- to 5-Hz stimulation in the hippocampus (Cui et al., 2013). Therefore, changes in GluN2A/GluN2B ratio could influence the threshold for LTD induced by 3–5 Hz stimulation. It is possible that the stimulation threshold for LTD induction is shifted at P11–14 and that GluN2B activity is required for LTD only at these ages. To examine this possibility, we applied 5-Hz stimulation for 3 min, which can evoke a greater increase in intracellular calcium concentration than 1-Hz stimulation and investigated whether GluN2B is still needed for LTD induced by 5-Hz stimulation in the P11–14 hippocampus. Ro25-6981 (5 μM) blocked LTD induced by 5-Hz stimulation (Supporting Information Fig. S1; control, 70.3 ± 3.5%, n = 11; Ro25-6981, 98.4 ± 2.5%, n = 10; P < 0.000005), suggesting that GluN2B is also required for LTD induced by 5-Hz stimulation at P11–14.
LTD induced by 1-Hz stimulation for 15 min at P18–22 is also NMDAR-dependent because 50 μM D-APV suppressed LTD (Fig. 4B; 96.5 ± 2.4%, n = 10, significantly different from control at P < 0.000005, Tukey–Kramer test). However, a 25% reduction in NMDAR EPSC did not affect LTD, because 0.5 μM D-APV, which attenuated NMDAR EPSCs by about 25% similarly to 3 μM ifenprodil at these ages (Figs. 3C and 4A; 0.5 μM D-APV, 78.0 ± 3.9%, n = 8; 1 μM D-APV, 63.8 ± 4.5%, n = 7), did not reduce LTD in the P18–22 hippocampus (Fig. 4B; control, 73.2 ± 1.4%, n = 12; 0.5 μM D-APV, 72.5 ± 3.4%, n = 14). Therefore, it is possible that GluN2B subunits are not required for LTD after the second postnatal week because calcium influx induced by the remaining 75% of NMDAR EPSCs is still sufficient for triggering LTD. Another possibility is that induction of NMDAR-dependent LTD may be assisted by other calcium sources that do not participate at the second postnatal week. We also found that LTD induced by 5-Hz stimulation was not inhibited by 50 μM D-APV at P18–22 (Fig. 5A; control, 73.9 ± 3.6%, n = 11: D-APV, 77.4 ± 3.0%, n = 10). These results suggest that NMDARs are required in LTD induced by 1-Hz stimulation but that NMDARs are not necessarily required in LTD induced by stimulation at higher frequencies at P18–22 and that induction of LTD at these ages involves other calcium sources. Therefore, we investigated other calcium sources that induce LTD at P18–22.
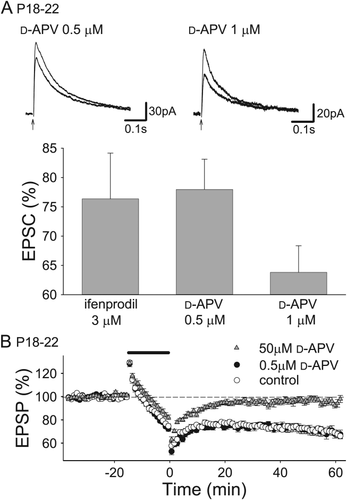
Approximately 25% reduction of NMDAR EPSCs does not affect LTD induction in P18–22 hippocampus. (A) Examples and summary of the effects of low-dose D-APV on NMDAR EPSCs in the P18–22 hippocampus (0.5 μM D-APV, n = 8; 1 μM D-APV, n = 7). Note that 0.5 μM D-APV reduced NMDAR EPSCs by approximately 25%, to the same extent as ifenprodil did. Data of the effects of ifenprodil on NMDAR EPSCs are from Figure 3. (B) Summary of the effects of 0.5 μM and 50 μM D-APV on LTD in the P18–22 hippocampus (control, n = 12; 0.5 μM D-APV, n = 14; 50 μM D-APV, n = 10).
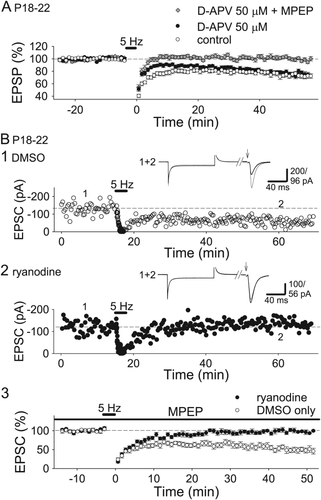
LTD induced by 5-Hz stimulation requires mGluR5s or NMDARs and ryanodine receptors in P18–22 hippocampus. (A) Summary of the effects of D-APV and D-APV plus MPEP on LTD induced by 5-Hz stimulation in P18–22 hippocampus (control, n = 11; D-APV, n = 10; D-APV + MPEP, n = 12). (B) Examples (1 and 2) and summary (3) of the effects of ryanodine loaded through a recording electrode on NMDAR-dependent LTD induced by 5-Hz stimulation in the presence of MPEP in the P18–22 hippocampus (DMSO only, n = 10; ryanodine, n = 10).
CICR Through Ryanodine Receptors Assists Induction of NMDAR-Dependent LTD Only After Second Postnatal Week
We examined the possibility of involvement of CICR through ryanodine receptors, because synaptically activated NMDARs can induce CICR through ryanodine receptors in a single spine (Emptage et al., 1999). When calcium in internal calcium stores at postsynaptic sites was depleted by loading neurons with thapsigargin (50 μM), an inhibitor of Ca2+-ATP pumps on internal stores, through a recording electrode, LTD was suppressed in the P18–22 hippocampus (Figs. 6B,C; control, 50.7 ± 5.5% of baseline 40 min after pairing, n = 9; thapsigargin, 102.2 ± 5.2%, n = 10; P < 0.000005, unpaired Student's t test). Thapsigargin did not have any effects on LTD in P11–14 slices (Figs. 6A,C; control, 54.0 ± 5.0%, n = 9; thapsigargin 43.9 ± 4.9%, n = 10). Therefore, calcium release from internal stores mediates LTD only after the second postnatal week.
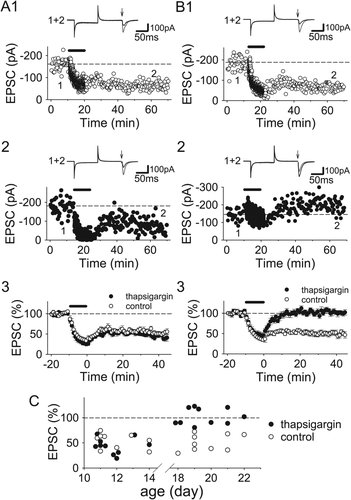
Calcium release from internal calcium stores is involved in LTD induction after second postnatal week. (A) Examples (A1 and 2) and summary (A3) of the effects of thapsigargin (50 μM) loaded in CA1 neurons through recording electrodes on LTD in the P11–14 hippocampus (control, n = 9; thapsigargin, n = 10). (B) Examples (B1 and 2) and summary (B3) of the effects of thapsigargin on LTD in the P18–22 hippocampus (control, n = 9; thapsigargin, n = 10). (C) The magnitudes of LTD are plotted against the age of slices.
Next, we examined the effects of ryanodine, a selective ryanodine receptor inhibitor, on LTD induction at P11–14 and P18–22. LTD induced by 1-Hz LFS was inhibited by ryanodine (200 μM) loaded in postsynaptic neurons through a recording pipette in P18–22 slices (Figs. 7B,C; control, 53.6 ± 4.9%, n = 14; ryanodine, 103.5 ± 6.3%, n = 11; P < 0.000005). However, ryanodine did not affect LTD induction at all in the P11–14 hippocampus (Figs. 7A,C; control, 44.3 ± 3.6%, n = 10; ryanodine, 45.5 ± 3.2%, n = 11). Thus, ryanodine receptor-mediated CICR does not contribute to LTD 2 weeks after birth and rapidly starts to be involved in LTD induction after developmental GluN2 subunit switch starts.
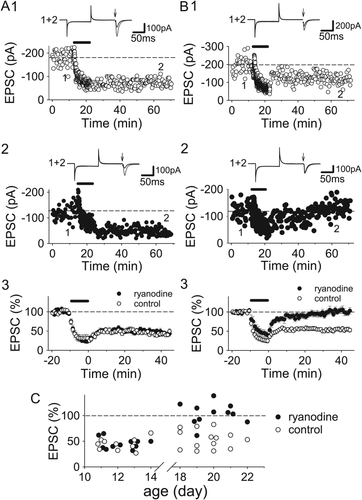
CICR through ryanodine receptors mediates LTD induction after second postnatal week. (A) Examples (A1 and 2) and summary (A3) of the effects of intracellular loading of ryanodine (200 μM) through recording electrodes on LTD in the P11–14 hippocampus (control, n = 10; ryanodine, n = 11). (B) Examples (B1 and 2) and summary (B3) of the effects of ryanodine on LTD induction in the P18–22 hippocampus (control, n = 14; ryanodine, n = 11). (C) The magnitudes of LTD are plotted against the age of slices.
NMDARs or group 1 metabotropic glutamate receptors (mGluRs) are involved in LTD induced by 5-Hz stimulation depending on experimental conditions (Oliet et al., 1997). Therefore, we also examined the effects of MPEP, an inhibitor of mGluR5, a subunit of group 1 mGluRs, on LTD induced by 5-Hz stimulation at P18–22. Although robust LTD was still induced by pairing 5-Hz stimulation for 2 min with depolarization at −50 mV in the presence of MPEP (5 μM) (Fig. 5B1, 3), application of both D-APV and MPEP blocked LTD induced by 5-Hz stimulation in the P18–22 hippocampus (Fig. 5A; D-APV + MPEP, 101.7 ± 2.5%, n = 12; significantly different from control at P < 0.000001 and from D-APV only at P < 0.00005; Tukey–Kramer test). Therefore, activation of either NMDARs or mGluR5s by 5-Hz stimulation is sufficient for inducing LTD at P18–22 under our experimental conditions. Group 1 mGluR-dependent LTD is reported to be independent of postsynaptic calcium in the hippocampus (Fitzjohn et al., 2001); thus, we tested the effects of ryanodine in the presence of MPEP to investigate the involvement of CICR in NMDAR- and calcium-dependent LTD induced by 5-Hz stimulation in the P18–22 hippocampus. Intracellular loading of ryanodine inhibited LTD induced by 5-Hz stimulation (Fig. 5B; DMSO only, 58.1 ± 7.1%, n = 10; ryanodine, 98.6 ± 3.7%, n = 10), suggesting that NMDAR-dependent LTD induced by 5-Hz stimulation also requires CICR at P18–22 and that significant changes in the threshold for LTD induction are less likely.
Thus far, we have reported that LTD is largely mediated by GluN2B subunits and CICR is not needed at P11–14 and that LTD requires CICR and GluN2B subunits are not important at P18–22. These findings raise a question of whether LTD expressions are different between these two time periods. To address this question, we induced LTD and measured the paired-pulse ratios (PPRs) of fEPSPs before and 50 min after LTD induction. Any significant changes in PPRs were not associated with LTD in the P11–14 or the P18–22 hippocampus (Supporting Information Fig. S2; P11–14, n = 9; P18–22, n = 10), suggesting that involvement of presynaptic changes in LTD expression is minimal for these two time periods.
DISCUSSION
Here, we show that synaptic NMDARs possessed much more GluN2B subunits, that NMDAR-EPSCs were wider at P11–14, when developmental GluN2 subunit switch starts, than those after this period, and that, therefore, calcium influx through NMDARs was sufficient to induce LTD at P11–14. Four days after this period, NMDAR EPSCs became narrower, and calcium influx associated with NMDAR EPSCs was not sufficient to induce LTD, which was compensated for by CICR through ryanodine receptors (Fig. 8).
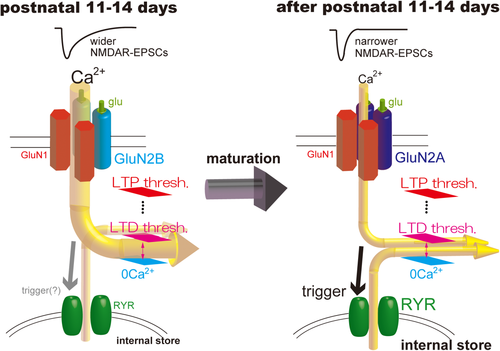
CICR is involved in induction of NMDAR-dependent LTD immediately after second postnatal week. NMDAR-mediated EPSCs are wider, and the associated calcium influx may be sufficient for reaching the threshold for LTD induction at P11–14, when the developmental GluN2 subunit switch starts to be facilitated (left). Calcium release through ryanodine receptors may happen at these ages; however, it is not needed for LTD induction. After the second postnatal week, NMDAR EPSCs become narrower and LTD induction requires calcium release through ryanodine receptors (right). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Role of GluN2B in LTD Induction in Hippocampus
The role of GluN2B subunits in synaptic plasticity in the hippocampus has been controversial. A critical role of GluN2B subunits in LTD induction in the hippocampus in P2–3 mice has been reported (Kutsuwada et al., 1996). A hypothesis was suggested that GluN2B subunits preferentially mediate LTD induction and GluN2A subunits favor LTP induction in the hippocampus in 3- to 4-week-old rats (Liu et al., 2004). In contrast, GluN2B-selective antagonists were found to exert minimal effects on LTD induction in 3-week-old rats in other laboratories (Morishita et al., 2007). Here, we found that ifenprodil and Ro25-6981, which are GluN2B-selective inhibitors, did not inhibit LTD in the P18–22 rat hippocampus but blocked LTD induced by 1- and 5-Hz stimulations on postnatal 11–14 days (Figs. 1 and 2, and Supporting Information Fig. S1), which exactly correspond to the critical period for the GluN2 subunit switch (Sanz-Clemente et al., 2010). At P11–14, we found that NMDAR-EPSCs were more sensitive to ifenprodil (Figs. 3A–C) and much wider (Figs. 3D,E) than those at P18–22, suggesting that calcium influx through NMDARs must be much greater at the second postnatal week and it can induce LTD in the hippocampus without an additional increase in calcium concentration from other calcium sources.
Involvement of CICR in LTD Induction Only After Developmental GluN2 Subunit Switch
CICR is triggered by NMDARs and elevates calcium concentration in dendrites and a single spine (Alford et al., 1993; Emptage et al., 1999) and has been reported to mediate LTD in the hippocampus in rodents older than postnatal 20 days (Futatsugi et al., 1999; Kumar and Foster, 2005; Nishiyama et al., 2000). We found that CICR through ryanodine receptors at postsynaptic sites was not involved in LTD induction in the immature animals at P11–14 (Fig. 7) (Sanz-Clemente et al., 2010). The mRNA expression levels of ryanodine receptor types 1 and 3 (RyR1 and RyR3) are maximum at P7 and that of RyR2 at P7 is almost the same as that in adults in the CA1 region of the mouse hippocampus (Mori et al., 2000). Therefore, the possibility that ryanodine receptors do not mediate LTD induction at P11–14 because of insufficient expression of ryanodine receptors is less likely. Suppose there is a threshold of calcium concentration for CICR, NMDARs might not trigger CICR and do not induce LTD in the presence of ifenprodil at P11–14 because ifenprodil inhibits NMDAR EPSCs to a greater degree (Fig. 3), and calcium influx through NMDARs must be less at P11–14 than at P18–22 in the presence of ifenprodil. However, this is not likely because intracellular application of thapsigargin and ryanodine did not affect LTD at P11–14 (Figs.6 and 7), indicating that calcium from internal stores and activation of ryanodine receptors are not needed for LTD induction at the second postnatal week. Ryanodine receptors start to mediate LTD induction after the levels of GluN2B subunits start to decrease at synapses and NMDAR EPSCs became narrower, suggesting that ryanodine receptors replace decreasing levels of GluN2B subunits that compensate for the lack of calcium influx after developmental GluN2 subunit switch. Ryanodine receptors are associated through FKBP with calcineurin, which is activated by calcium/calmodulin and induces LTD (Malenka and Bear, 2004; Mulkey et al., 1994; Snyder et al., 1998). Therefore, ryanodine receptors, rather than NMDARs, may effectively activate calcineurin and may trigger LTD even if they do not completely compensate for reduction of calcium influx when GluN2B subunits decrease at synapses after developmental GluN2 subunit switch.
Synaptic Plasticity Continues in the Hippocampus During Development
In the barrel cortex, reduction of GluN2B subunits very early in development causes loss of ability to exert LTP without changes in NMDAR EPSC kinetics (Barth and Malenka, 2001). In the rat visual cortex, the end of LTP induction is associated with the decline in GluN2B subunits after the critical period for binocular plasticity (Yoshimura et al., 2003), although synaptic plasticity remains to be induced after the critical period in the mouse visual cortex (Jiang et al., 2007) and other cortical areas in the adult brain (Thomases et al., 2014). In hippocampal synapses, LTP continues to be induced for longer periods, although the underlying protein kinases downstream of calcium switch during development (Yasuda et al., 2003). The magnitude of NMDAR-dependent LTD in the CA1 region declines during development (Dudek and Bear, 1993; Kemp et al., 2000), but LTD is still induced in the 8-week-old hippocampus (Ku et al., 2008). Recent studies suggest that synapse elimination is associated with long-term retention of newly generated synapses during consolidation of new memory, and LTD-like mechanisms are hypothesized to underlie the synapse elimination (Caroni et al., 2014). Therefore, LTD could still be important in learning and memory in adults. Involvement of CICR in LTD induction after developmental GluN2 subunit switch is also a mechanism by which hippocampal plasticity is maintained during development, presumably because the hippocampus utilizes its plasticity to function when animals reach maturity.
Although we have focused on the supportive roles of CICR through ryanodine receptors in induction of NMDAR-dependent LTD in the developing hippocampus, our results do not exclude involvement of other calcium sources in LTD induction. We found that LTD was still induced by 5-Hz stimulation in the presence of 50 μM D-APV or MPEP and was inhibited following application of both 50 μM D-APV and MPEP in the P18–22 hippocampus (Fig. 5), suggesting that mGluR5s can induce LTD at these ages. mGluR5s, which are group 1 mGluRs, can mobilize calcium through intracellular IP3 receptors and trigger LTD (Choi et al., 2005; Reyes-Harde and Stanton, 1998; but see Fitzjohn et al., 2001). Different from NMDAR-dependent LTD, mGluR-dependent LTD is also found in the mature hippocampus (Gladding et al., 2009; Kumar and Foster, 2007; Otani and Connor, 1998); therefore, mGluRs may be more critical in LTD induction particularly in the adult brain. Interestingly, 1-Hz stimulation induces LTD by activation of T-type voltage-gated calcium channels (VGCCs) and CICR through ryanodine receptors in the dentate gyrus of the hippocampus (Wang et al., 1997). VGCCs are also involved in LTD induction in the CA1 region (Christie et al., 1996; Normann et al., 2000); therefore, VGCCs could induce LTD through activation of CICR also in the CA1 region. Thus, multiple calcium sources may support LTD induction after the second postnatal week, which corresponds to the critical period for the GluN2 subunit switch when GluN2Bs dominantly induce LTD.
Acknowledgments
The authors thank Dr. Pablo Castillo for helpful comments on the manuscript.