Prenatal stress diminishes gender differences in behavior and in expression of hippocampal synaptic genes and proteins in rats
Abstract
The study determined whether there were gender differences in the expression of hippocampal genes in adult rats in association with dissimilarity in their behavior, and how these were affected by prenatal stress. Pregnant Wistar rats were subjected to varied stress once daily on days 14–20 of gestation. Adult female offspring of control rats showed significantly less anxiogenic behavior in the elevated plus maze and better discrimination between a novel and familiar object than males in the object recognition test. These gender differences in behavior were markedly attenuated by prenatal stress. Using Affymetrix DNA chip technology on hippocampal extracts prepared from littermates of the offspring used for behavioral tests, we found that 1,680 genes were differentially expressed in control males and females. The gender difference in gene expression was decreased to 11% (191 genes) by prenatal stress. In both sexes, processes like the translational machinery, mitochondrial activity, and cation transport were downregulated compared to controls, but there was a greater suppression of genes involved in vesicle trafficking, regulation of synaptic plasticity, and neurogenesis in females than in males. This was compensated by a higher expression of other components of vesicle trafficking, microtubule-based processes, and neurite development. Prenatal stress decreased the expression of 19 Rab proteins in females and five Rabs in males, but a compensatory increase of Rab partner proteins and effectors only occurred in females. Exposure to stress decreased the expression of synaptic proteins, synaptophysin, and synaptopodin in prenatally stressed males and females and increased those of PSD-95 and NR1 subunit of the N-methyl-D-aspartic acid (NMDA) glutamate receptor only in females. The study provides an unbiased view of key genes and proteins that act as gender dependent molecular sensors. The disruption of their expression by adverse early life stress may explain the alterations that occur in behavior. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Prenatal stress has been shown to alter several types of behavior in humans and rodents of both sexes. In rats, prenatal stress induces anxiety both in males and females while spatial learning deficits appear to be more prevalent in males (Zagron and Weinstock, 2006; Zuena et al., 2008). The behavioral alterations depend on the duration and intensity of the stress and result from exposure of the developing fetal brain to excess levels of maternal stress hormones as they can be prevented by maternal adrenalectomy and restored by corticosterone (COR) administration (Barbazanges et al., 1996; Zagron and Weinstock, 2006). In the majority of studies in rats (reviewed in Weinstock, 2008), maternal stress was applied during the last week of gestation so that COR reaches the fetal brain at a critical time during the development of cortical and limbic areas (Bayer et al., 1993). It could, therefore, alter their programming (Matthews, 2000) and impair glutamate-mediated synaptic plasticity (Son et al., 2006; Yaka et al., 2007).
Sexual dimorphic behavior results from gender differences in synaptic connections and neurotransmitter activity (Devries, 1990). Numerous studies support the view that such dimorphic behavior is influenced considerably by the organizational effect of adrenocortical and gonadal steroids. This occurs during the last week of gestation and extends until about 10 days postpartum (MacLusky and Naftolin, 1981; Matsumoto, 1991; Rhees et al., 1999; Segovia et al., 1999; Sakuma, 2009). Normal male sexual dimorphic behavior is dependent on the presence of appropriate levels of testosterone, brain estrogen receptors, and the activity of aromatase in critical brain areas on days 18 and 19 of gestation (McEwen et al., 1977). Prenatal stress during the last week of gestation reduced testosterone levels (Ward and Weisz, 1984), aromatase activity (Weisz et al., 1982), and neurotransmitter release (Reznikov and Nosenko, 1995). Prenatal stress also abolished the gender difference in neurogenesis in the hippocampus, which is higher in control males than in females (Zuena et al., 2008) as well as those in brain structure and function (Weinstock, 2007; Mandyam et al., 2008). These neurochemical and structural alterations may also explain the changes induced by prenatal stress in sexual dimorphic behaviors like sweet preference (McGivern et al., 1986), exploration in novel environments (Masterpasqua et al., 1976) juvenile play (Ward and Stehm, 1991) and in spatial learning in the Morris water maze (Zuena et al., 2008). The effects of prenatal stress on sexual dimorphic behavior could also arise from the exposure of the fetus to excess maternal COR during the last week of gestation, since administration of glucocorticoids during gestation, like prenatal stress, decreases aromatase activity in key brain areas (Reznikov et al., 1999).
Neuronal activity and synaptic input regulate the development and maintenance of dendritic structure (Cline, 2001). Prenatal stress was found to reduce spine density and dendritic length in the anterior cingulate and orbitofrontal cortex of male and female rats (Murmu et al., 2006) and hippocampus of males (Martinez-Tellez et al., 2009). Spine density is regulated by many genes and proteins like synaptopodin, in addition to those of the cytoskeleton (Matus, 2000). Therefore, the behavioral alterations induced by prenatal stress may be due to changes in the expression of genes and proteins resulting from exposure of the fetus to excess maternal stress hormones. This suggestion was supported by the finding of a significant downregulation of genes that participate in synaptic activity, processes of neurotransmitter release, and axonal growth in the hippocampus of juvenile prenatally stressed (PS) female rats (Bogoch et al., 2007). Furthermore, the association between behavior and altered gene expression was demonstrated by the restoration of a significant proportion these hippocampal synaptic genes to control levels by neonatal handling which prevented the appearance of anxiety.
The aim of the present study was to determine the effect of prenatal stress on two behaviors that show differences in males and females, entry in open arms of the elevated plus maze EPM (Johnston and File, 1991), and exploration of novel objects (Sutcliffe et al., 2007), which depend on intact function of the ventral (Deacon et al., 2002) and dorsal hippocampus (Mumby et al., 2002; Li and Chao, 2008), respectively. The object recognition test (ORT) was chosen in preference to the Morris water maze because it does not impose an additional stress on the rats which can further influence behavior in the two sexes. In association with any change in sexual dimorphic behavior, we compared the effect of prenatal stress on the expression of hippocampal genes in male and female littermates. We also determined how postnatal stress of exposure to the Elevated plus maze (EPM) affects the expression of key synaptic proteins in the hippocampus.
MATERIALS AND METHODS
Animals
The study protocol was approved by the joint ethics committee (IACUC) of the Hebrew University and Hadassah Medical Center for Animal Welfare, which is an AAALAC International Accredited Institute. Female pathogen-free (SPF) Wistar rats weighing 280–300 g (Harlan Biotech, Jerusalem, Israel) on day 1 of pregnancy (detected by the presence of a vaginal plug) were randomly allocated to stress (18 rats) and control (18 rats) groups and housed singly in the animal house at an ambient temperature of 22 ± 1°C and a 12 h diurnal light cycle, (lights on 07:00 h, off 19:00 h). Food and water was provided ad libitum and the cages were changed twice weekly.
Maternal Treatment
Experiments were performed in the animal facility during the light phase at an ambient temperature of 21–22°C. On days 13–22 of gestation, rats were stressed once daily by one of three different stressors. On days 13, 16, and 20, they were restrained for 45 min in transparent cylinders, 10 cm in diameter and 15 cm long. On days 14, 17, and 21, rats were exposed to the forced swim test by placing them individually for 15 min into a Plexiglas cylinder, 20 cm in diameter, 60 cm high containing 30 cm of water at a temperature of 25°C. On days 15 and 19, rats were put for 30 min onto an elevated platform 20 cm diameter 60 cm above the floor. We have previously shown that these stressors elevated maternal plasma COR fivefold to sixfold (Murmu et al., 2006). The time period during gestation in which the rats were stressed was chosen because this is when key areas of the limbic systems develop (Bayer et al., 1993) and when sexual dimorphic behavior is initiated (MacClusky and Naftolin, 1981). Control pregnant females were left undisturbed in their home cages. The length of gestation was 22 days.
Pup Treatment
After birth, all pups were weighed and litters were culled to eight pups (while trying to maintain equal numbers from each gender as far as possible). The pups were weaned at the age of 21 days and housed in groups of two to three from different litters, according to sex and prenatal treatment. All experiments were performed on male and female offspring aged 2–3 months from the same litters. Not more than two pups of each gender per litter were used for any behavioral experiment and only one per litter for gene analyses to reduce litter and environmental effects. The behavior was recorded by an observer who was unaware of the treatment the rats had received.
Anxiogenic Behavior
The EPM test is based on the conflict between the natural desire to explore a novel situation and the fear of open spaces (Handley and Mithani, 1984). Behavior of rats was tested in the EPM in 11–12 control (C) and PS rats of each gender as previously described (Poltyrev and Weinstock, 2004). The test was carried out in dim light in a room next to that in which the rats were housed. The rats were exposed individually to the maze for 5 min and the time they spent in the open and closed arms and the number of entries into these arms was recorded by means of a computer program which registered each movement. An entry into an open arm was considered only if all four paws were inside the arm.
Object Recognition Test
The ORT for rodents is a nonspatial nonaversive memory test (Ennaceur and Delacour, 1988). It is based on the fact that rats typically respond to environmental changes by preferential exploration of novel objects over those that are familiar. Nine to ten C and PS rats of each gender were familiarized with the test arena, a box made of dark brown Plexiglas 80 cm width, 80 cm length and 50 cm high, by placing them in it individually for five min on each of four successive days. On the fifth day, two identical objects about 15 cm high were placed in the arena and the time taken by the rats to explore each object during a period of 2 min was recorded by means of a computer program. One hour later, the objects were replaced by one that was identical to the first two and by a different one and the time taken to explore each of them was again recorded. This time interval was chosen between trials because it was reported to detect a gender differences in object discrimination and in the time exploring a novel (N) and familiar (F) object (Sutcliffe et al., 2007). The following parameters were calculated for each group; total time exploring both objects in trial 1 (T1) and trial 2 (T2); the difference in time spent exploring a novel and familiar object in trial 2 (N-F); discrimination index (DI), object/total exploration time of both objects, (N-F/N + F).
RNA Isolation and GeneChip Processing
We applied the Affymetrix DNA chip technology to hippocampal extracts from control male and female littermates and from those that had experienced identical maternal stress to determine whether the alteration in behavior can be explained by changes at the molecular level. Three rats from each group of C and PS male and female rats were decapitated, the hippocampi rapidly dissected out, snap frozen in liquid nitrogen, and stored at −70°C. Total hippocampal RNA was prepared with Triazol and subsequently cleaned by the RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's protocols. RNA quality and quantity were measured by the Thermo Scientific NanoDrop 1,000 spectrophotometer with A260/A280 of 1.85–2.1. The mRNA reverse transcription, labeling, hybridization, and other processing were performed in the Center for Genomic Technologies at the Hebrew University of Jerusalem using standard Affymetrix protocols (Santa Clara, CA). cRNA was hybridized to Affymetrix rat gene 1.0st arrays. The cRNA from each individual animal was hybridized to one GeneChip. The data were normalized using robust multiarray averaging (RMA) algorithm (Affymetrix expression console software). A probe set had to obtain a minimal intensity value of four to be considered as detected above background expression leaving 14,400 active genes.
Gender Differences in Hippocampal Gene Expression
The Rat Gene 1.0 ST Array (Affymetrix) includes 27,342 genes. Of these genes, 14,400 were expressed in all samples. The expression of 1,680 of these genes was differentially regulated between adult control male and female rats; 687 genes showed a greater expression in females than in males and 983 were more strongly expressed in males than in females (Fig 3a). We analyzed these sets for foremost annotations using gene ontology (GO) (Camon et al., 2004). The major cell processes that showed a relatively higher expression in females than in males are concerned with apoptosis and lipid metabolism (Table 1). In males, higher expression was found for processes involved in translation regulation, protein and membrane trafficking, and synaptic transmission (Table 1).
| Process | No. of genes | P value |
|---|---|---|
| (a) Genes showing higher expression in males than in females (983 out of 1680 genes) | ||
| Regulation of translational initiation | 8 | 3.4 E – 5 |
| Protein-RNA complex assembly | 11 | 9.3 E – 5 |
| Protein transport | 30 | 5.9 E – 4 |
| Protein folding | 15 | 7.1 E – 4 |
| Exocytosis | 10 | 2.4 E – 3 |
| Intracellular protein transport | 21 | 5.3 E – 3 |
| Glutamate signaling pathway | 5 | 5.6 E – 3 |
| Nuclear transport | 10 | 8.7 E – 3 |
| Synaptic transmission | 20 | 9.6 E – 3 |
| (b) Genes showing higher expression in females than in males (687 out of 1,680 genes) | ||
| Negative regulation of apoptosis | 15 | 3.19 E – 4 |
| Membrane lipid metabolic process | 12 | 4.50 E – 4 |
| Regulation of programmed cell death | 24 | 1.10 E – 3 |
| Sterol metabolic process | 7 | 6.48 E – 3 |
Prenatal stress reduced the number of genes that were differentially expressed in males and females from 1,680 to 191 (Fig. 3a). Throughout this study, we maintained the same parameters for determination of the significant gene sets: P > 0.05 and filtration by a >1.2-fold change for upregulation or downregulation. The major categories of cellular processes of the differentially expressed genes in control rats (based on 1,680 genes, Fig. 3b) and after prenatal stress (based on 191 genes Fig. 3c) are shown. Several categories of cellular processes including transcription regulation, cation transport, and G-protein signaling are common to the two sets despite a huge difference in gene amounts in each set. All the data were submitted according to the MIAME protocol to the gene expression omnibus (GEO) and ARRAY repositories. The data is archived in series: GSEXXX.
Real-Time Reverse Transcriptase PCR
Total RNA was isolated as described above. Samples were treated for 15 min with 10 U of RNase-free DNase (Qiagen) at 25°C prior to the reverse transcriptase reaction. cDNA was generated with the M-MLV Reverse Transcriptase using 1 μg of total RNA according to the manufacturer protocol (Promega). Conditions for PCRs were optimized in a gradient cycler with regard to Taq DNA polymerase, annealing temperature, and primer concentration. Quantitative real-time reverse transcriptase reaction (qRT-PCR) was performed using Absolute Blue QPCR SYBR Green ROX Mix (Thermo Scientific) containing all reagents and buffers required for quantitative RT-PCR on a Real-Time PCR machine (StepOnePlus, Applied Biosystems). The amount of the gene target was measured relative to that of the control samples as a calibrator. Threshold cycle (Ct) values were used to calculate fold changes by the 2−ΔΔCt method with L19 ribosomal gene (housekeeping gene of the large subunit of the ribosome, endogenous control). Each reaction was repeated at least three times and the significance of observed changes was assessed by Student t-test. We used RNA taken from other rats of the same groups together with that of the GeneChip experiments. All reactions were performed on the same cDNA preparation and reaction products were designed to range between 90 and 150 nucleotides. Each set of oligonucleotides was tested three times. Comparisons were made only between PS and C rats of the same gender. The oligonucleotide primers that were used are listed with their “forward” and “reverse” sequences.
Protein Isolation and Analysis
Secondary antibodies used to detect monoclonal and polyclonal antibodies were Goat α mouse and Goat α rabbit, respectively. β-tubulin (Sigma) was used in all experiments for normalization, as it was unchanged in all experimental groups. The luminescence signal was recorded and analyzed using ImageJ software. Western blots were quantified and normalized according to the levels in C-naïve rats of the same gender. To examine the effect of acute stress on the expression levels of presynaptic and postsynaptic proteins, three rats from each group of male and female C and PS rats were decapitated 2 h after the exposure to the EPM. The hippocampus was rapidly dissected out from each rat, snap frozen in liquid nitrogen, and stored at −70°C for later use in protein separation. Hippocampi were also removed from three each of naïve C and PS male and female littermates of the rats tested for anxiogenic behavior and stored at −70°C for later use in protein separation. They were then were homogenized in homogenization buffer containing 320 mM sucrose, 10 mM Tris-HCl, pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), protease inhibitor cocktail (Sigma), and the phosphatase inhibitors 1 mM NaVO4 and 5 mM NaF. Samples were resuspended in solubilization buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris-HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, protease inhibitor cocktail (Sigma), and the phosphatase inhibitors 1 mM NaVO4 and 5 mM NaF. SDS-sample buffer was added to each vial and the samples were boiled for 5 min prior to loading onto gels. Equal amounts of protein (40 μg) were used in each lane, separated by means of 8–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane. The membranes were incubated in blocking buffer (5% nonfat milk powder in 0.5% v/v Tween 20, 10 mM Tris-HCl, pH 7.6, 150 mM NaCl) for 1 h at room temperature prior to incubation with the primary antibodies at a concentration recommended by the vendor. Following horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) at 1:5,000 dilution, detection using the ECL Plus kit (Amersham Biosciences) was used. Major synaptic proteins were detected by means of a set of primary antibodies to synaptophysin (Sigma, Israel), synaptopodin (S9442 Sigma), and PSD-95 (Upstate Biotechnology). Antibodies to glutamate receptors NR1 (NMDAR1, M207, Sigma) and GluR1 (AMPA subunit 1, BIOMOL International) are monoclonal and polyclonal, respectively.
Data Analysis
Differences between the four groups of rats in behavioral parameters in the EPM and ORT were analyzed by Analysis of variance (ANOVA) for factors such as gender and prenatal treatment (stress or control); Duncan's post hoc test was applied when appropriate and a difference of P < 0.05 was considered to be statistically significant. All data are expressed as the mean value ± Standard error of the mean (SEM).
Robust multichip average (RMA) normalization was applied according to published protocols (Irizarry et al., 2003). The RMA preprocessed data were imported into Partek Pro 6.0 genomics suite (St. Charles, MO). A clustering procedure was executed to merge similar gene expression vectors by measuring the Euclidian distance of a gene expression vector from all other gene expression vectors. The most similar samples were merged first using an average clustering protocol and resulted in a dendogram. The length of the edges of the dendogram shows the relative similarity of the samples to each other with the shortest edge being the most similar in the gene expression patterns.
Significance of the difference of genes between C and PS groups was tested by a nonparametric t-test. Only genes that showed a difference in expression greater than 1.2-fold (equivalent to <0.833 for downregulation) and a difference between groups of P < 0.05 were considered statistically significant and were further analyzed. Functional categorization by GO (Camon et al., 2004) is available for about 60% of the genes and enabled us to reveal the cellular and biochemical functions of the genes that showed significant changes in expression as a result of prenatal stress. Functional annotation was performed by means of the statistical model implemented in Database for Annotation, Visualization and Integrated Discovery (DAVID) (Dennis et al., 2003).
Differences in protein levels in four groups of rats of the same gender were analyzed by ANOVA for factors prenatal treatment and stress (exposure to the EPM). Duncan's post hoc test was applied when appropriate and a difference of P < 0.05 was considered to be statistically significant. As Western blots were measured for each gender separately, they were quantified and normalized according to the value of C-naïve rats of the same gender. Therefore, it was not possible to compare differences in the effect of prenatal stress in the two genders.
RESULTS
Effect of Prenatal Stress on Behavior in the EPM
The effect of the varied form of maternal stress on behavior in the EPM revealed a highly significant effect of gender in the time spent in open arms (F(1,39) = 15.11, P < 0.0001; Fig. 1) and in the number of entries into open arms relative to total arms entries (F(1,39) = 10.35, P < 0.005) as females spent much more time than males entering into and exploring the open arms of the maze. This difference was abolished by prenatal stress. There were no differences between the groups in the time spent in closed arms or in the center of the maze. Prenatal treatment did not have a significant effect on the time in open arms in males but there was a reduction in females that reached a low level of significance (P = 0.1).
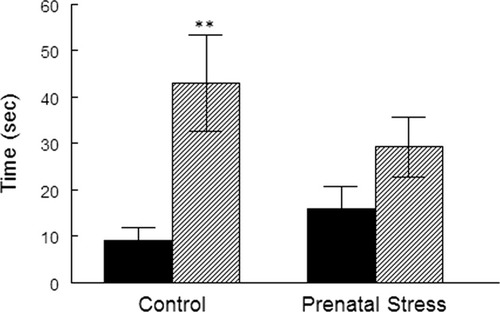
The effect of prenatal stress on anxiogenic behavior in the EPM. Data shown as mean ± SEM. Black columns: males; hatched columns: females. Significantly different from males, **P < 0.01. Prenatal stress attenuated the gender difference in anxiogenic behavior.
Effect of Prenatal Stress on Exploration and Memory in the ORT
ANOVA for T1 and T2 in the ORT revealed a significant effect of gender (F(1,36) = 8.93, P < 0.005) and (F(1,36) = 8.97, P < 0.005), respectively and in the time exploring novel and familiar (N-F) object (F(1,36) = 7.88, P < 0.005). Females spent significantly more time than males exploring the objects (Fig. 2a) and showed better discrimination between them (Fig. 2b). Prenatal treatment had no effect on any of these parameters, but there was a significant prenatal treatment x gender interaction for T1, (F(1,36) = 4.59, P < 0.05), T2, (F(1,36) = 10.19, P < 0.005), and T (N-F) (F(1,36) = 6.30, P < 0.025). Prenatal stress increased exploration and discrimination in males and tended to reduce it in females. The DIs of the groups were; C males, −0.01 ± 0.15 (N.S.); C females, 0.36 ± 0.12 (P < 0.01); PS males, 0.28 ± 0.08 (P < 0.01); PS females, 0.28 ± 0.08 (P < 0.01), showing that all groups except C males discriminated between the objects. Thus, prenatal stress abolished the sex difference both in the total time of exploration and the ability to discriminate between the new and familiar object.
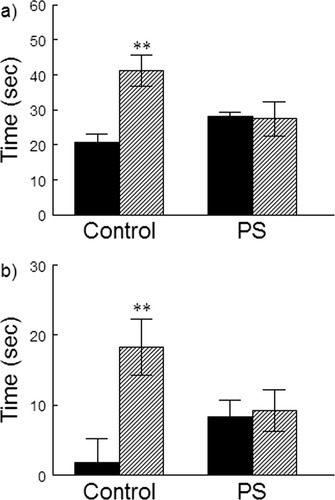
The effect of prenatal stress on exploration of, and discrimination between, novel objects in the ORT. (a) Total time spent in exploration of both objects in Trial 2. Legend as for figure 1. C females spent significantly more time than C males exploring the new and familiar object (**P < 0.01). The difference between males and females was abolished by prenatal stress. (b) Difference in time spent exploring novel and familiar object. C females showed significant discrimination between the novel and familiar objects (**P < 0.01). C males did not show significant object discrimination. The difference between males and females was abolished by prenatal stress.
Gender Differences in Hippocampal Gene Expression
The Rat Gene 1.0 ST Array (Affymetrix) includes 27,342 genes. Of these genes, 14,400 were expressed in all samples. The expression of 1,680 of these genes was differentially regulated between adult control male and female rats; 687 genes showed a greater expression in females than in males; and 983 were more strongly expressed in males than in females (Fig. 3a). We analyzed these sets for foremost annotations using GO (Camon et al., 2004). The major cell processes that showed a relatively higher expression in females than in males are concerned with apoptosis and lipid metabolism (Table 1). In males, higher expression was found for processes involved in translation regulation, protein and membrane trafficking, and synaptic transmission (Table 1).
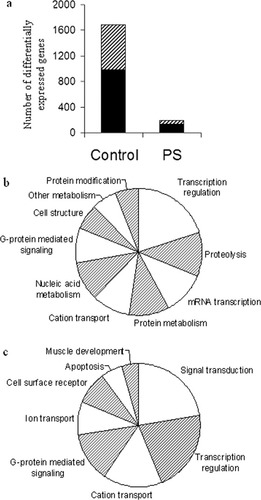
Gender difference in expression of hippocampal genes is markedly reduced by prenatal stress. (a) Differential expression of hippocampal genes in C male and C female rats and its alteration by prenatal stress. Black columns: Higher expression in males; Hatched columns: higher expression in females. (b) Major cell processes that were differentially expressed in the hippocampus of C male and C female rats. Processes are represented by 1,680 genes. 1,193 genes (70%) have GO annotations. Categories that are associated with <5% of the annotated genes are excluded. (c) Major cell processes represented that were differentially expressed in the hippocampus of PS male and PS female rats. Processes are represented by 191. One hundred sixteen genes (60%) have GO annotations. Categories that associated with <5% of the annotated genes are excluded.
Prenatal stress reduced the number of genes that were differentially expressed in males and females from 1,680 to 191 (Fig. 3a). Throughout this study, we maintained the same parameters for determination of the significant gene sets: P > 0.05 and filtration by a >1.2-fold change for upregulation or downregulation. The major categories of cellular processes of the differentially expressed genes in control rats (based on 1,680 genes, Fig. 3b) and after prenatal stress (based on 191 genes Fig. 3c) are shown. Several categories of cellular processes including transcription regulation, cation transport, and G-protein signaling are common to the two sets despite a huge difference in gene amounts in each set.
Differences in Hippocampal Gene Expression Induced by Prenatal Stress
About 5,600 genes were differentially expressed between C and PS females (using the threshold of significance, see Materials and Methods), of which 42% were overexpressed and 58% underexpressed. Far fewer genes (about 1,400) were differentially expressed in C and PS males; 27.5% were overexpressed and 72.5% underexpressed (Tables 2 and 3). An unsupervised clustering procedure of the expression of genes in all subjects (12 rats) used in the analysis showed a complete separation of C males and females but an overlap in those subjected to prenatal stress (Fig. 4). This supported the observation that the robust difference in gene expression in C male and female rats is no longer seen in those that experienced prenatal stress.
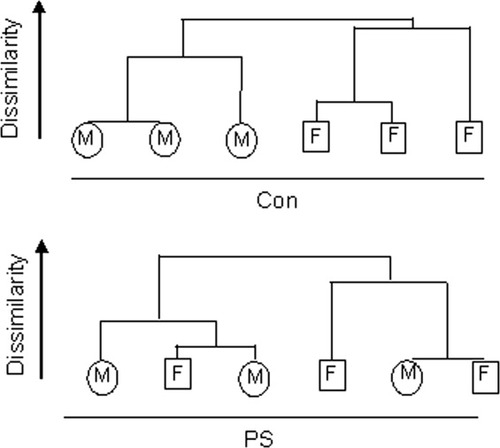
Unsupervised clustering of all samples. The dendogram shows the most similar samples that are merged first (at the bottom of the scheme). The length of the connecting lines of the dendogram is a proxy for the relative similarity of the samples to each other, with the shortest line being the most similar in the gene expression profiles. A complete separation of C males and females is seen which is lost after prenatal stress.
| Process | Females (3,277 out of 5,653) | Males (1,039 out of 1,432) | ||
|---|---|---|---|---|
| No. of genes | P value | No. of genes | P value | |
| Translation machinery | ||||
| Ribosome | 32 | 2.6 E – 18 | 52 | 1.2 E – 18 |
| Protein biosynthesis | 55 | 3.76 E – 10 | 31 | 1.2 E – 14 |
| Mitochondrial activity | ||||
| Mitochondrial membrane | 38 | 3.26 E – 8 | 25 | 1.5 E – 3 |
| Cytochrome-C oxidase activity | 12 | 7.56 E – 5 | 7 | 7.8 E – 5 |
| Oxidative phosphorylation | 6 | 5.16 E – 2 | 21 | 7.5 E – 5 |
| Vesicle trafficking | ||||
| Vesicle mediated transport | 80 | 2.26 E – 3 | 24 | 1.7 E – 4 |
| Transmission of nerve impulse | 82 | 6.6 E – 3 | – | – |
| Clathrin-coated vesicle | 28 | 1.2 E – 2 | – | – |
| Synaptic transmission | 72 | 1.5 E – 2 | 27 | 5.7 E – 2 |
| Secretory pathway | 44 | 7.5 E – 2 | – | – |
| Developmental signaling | ||||
| Forebrain development | 12 | 4.4 E – 2 | 6 | 1.9 E – 2 |
| Neuron differentiation | 69 | 8.3 E – 5 | – | – |
| MAP kinase phosphatase activity | 7 | 7.9 E – 4 | – | – |
| Neurogenesis | 72 | 1.5 E – 3 | – | – |
| Regulation of synaptic plasticity | 8 | 2.2 E – 3 | – | – |
| Neuropeptide signaling pathway | – | – | 7 | 7.7 E – 2 |
| Structural & Membrane Components | ||||
| Myelin sheath | 5 | 2.8 E – 2 | – | – |
| Actin filament based processes | 35 | 2.9 E – 2 | 4 | 4.6 E – 2 |
| Cation transporter activity | 86 | 7.5 E – 3 | 24 | 5.5 E – 2 |
| Maintenance | ||||
| Ubiquitin cycle | – | – | 14 | 3.7 E – 2 |
| Process | Females (2,376 out of 5,653) | Males (393 out of 1,432) | ||
|---|---|---|---|---|
| No. of genes | P value | No. of genes | P value | |
| Vesicle Trafficking | ||||
| Vesicle mediated transport | 80 | 3.4 E – 8 | – | – |
| Exocytosis | 28 | 1.2 E – 5 | – | – |
| Secretory pathway | 52 | 1.6 E – 5 | – | – |
| Structural & Membrane Components | – | – | ||
| Microtubule based processes | 40 | 2.4 E – 7 | – | – |
| Neurite development | 32 | 9.2 E – 3 | – | – |
| Maintenance | – | – | ||
| Ubiquitin cycle | 46 | 5.2 E – 6 | – | – |
| Anti-apoptosis | – | – | 7 | 4.2 E – 2 |
| Response to stress | 6 | 5.7 E – 2 | 24 | 5.0 E – 2 |
Despite the relatively large number of genes that are differentially expressed in C males and females, there is an overlap between many of the biological processes that were downregulated by prenatal stress. These include the following: (1) protein synthesis, specifically those comprising the ribosomes; (2) mitochondrial activity; (3) synaptic vesicle biogenesis and exocytosis. However, the regulation of neurogenesis and of neuron differentiation was downregulated selectively in females. Also in females, several prominent processes were upregulated by prenatal stress. These include vesicle transport in general, with exocytosis and that of synaptic vesicles in particular, together with genes of the microtubule assembly. The latter showed a significantly lower representation in C females than in males. On the other hand, prenatal stress caused a negligible upregulation in gene expression in males (Table 3).
Validation of Difference in Gene Expression Using Real-Time RT-PCR
We have shown that the results obtained from the DNA-chip experiment are a powerful tool for pinpointing the relevant pathways and cellular processes that are sensitive to the experimental paradigm. In general, the expression level that is measured by the GeneChip is consistent with that obtained by direct measurement of each gene (Draghici et al., 2006), but animal variability cannot be ignored. Therefore, we used real-time RT-PCR on individual rats to quantify and substantiate the observed changes in gene expression. Seven genes representing different aspects of synaptic vesicle and the release machinery were tested (Table 4). These included three of the genes that were downregulated in males and females, presumably affecting identical pathways, and an additional gene that was exclusively affected by prenatal stress in females. The selected genes were all implicated in shaping the synapse during learning and memory. The genes that were proposed to be similarly affected in females and males are (by their gene symbols and RefSeq identifier) Doc2b (NM_031142), ARC (NM_019361), and Rab3a (NM_013018). In addition, Nlgn3 (NM_134336) and Rims4 (NM_170666) were downregulated only in females. The results of this analysis are shown in Table 4. All five genes that were found in the gene array to be downregulated by prenatal stress in females also showed a decreased expression in real-time RT-PCR and two of the genes (Doc2, ARC) also showed reduced expression in males. Rab3a did not show reduced expression in PS males. Note that based on the DNA Chip results Rab3a was only slightly below the predetermined threshold (0.795).
| Genes down regulated by prenatal stress in gene array (P < 0.05) | Relative expression in real-time RT-PCRa | ||||
|---|---|---|---|---|---|
| Gene | RefSeq | Females | Males | Females | Males |
| Doc2b | NM_031142 | 0.609 | 0.688 | 0.50 ± 0.08 | 0.49 ± 0.13 |
| ARC | NM_019361 | 0.538 | 0.557 | 0.52 ± 0.12 | 0.43 ± 0.15 |
| Nlgn3 | NM_134336 | 0.766 | (0.862) | 0.47 ± 0.10 | 1.2 ± 0.2 |
| Rab3a | NM_013018 | 0.746 | 0.795 | 0.57 ± 0.04 | 1.1 ± 0.11 |
| Rims4 | NM_170666 | 0.694 | – | 0.37 ± 0.07 | 0.77 ± 0.06 |
| Snap25 | NM_030991 | 1.87 ± 0.28 | 0.96 ± 0.06 | ||
- a Relative to control (=1) of the same gender. Bold numbers denote significant change in gene expression. In parentheses, expression levels not meeting the threshold value for down regulated gene. (–) unchanged.
It is to be expected that following prenatal stress there would be a compensating effect that would result in over expression of genes that shape the synapse. Therefore, we checked the expression of Snap25 (NM_030991), a major presynaptic SNARE protein that participates in membrane dynamics, presynaptic trafficking, and synaptic vesicle fusion (Chen et al., 2001). The Snap25 gene was upregulated in PS females but not in PS males in the DNA chip experiment. Snap25 showed an increase of 1.87 ± 0.28 relative to control in the real time RT-PCR in PS females. Vesicle trafficking and biogenesis was shown as one of the processes that were affected by prenatal stress in males and females (Table 4). Therefore, we tested the effect on Rabs, a large set of proteins that execute intracellular trafficking (Zerial and McBride, 2001).
Alteration of the Expression of Rab Proteins by Prenatal Stress
Rabs are family of small GTPases that are involved in exocytosis and priming of synaptic vesicles (i.e., Rab3a), in regulated endocytosis (Rab5), motor binding, SNAREs function, and intracellular trafficking (Stenmark, 2009). Therefore, changing the relative quantities of different Rabs is expected to alter the delicate balance of membrane trafficking processes. In females, the number of downregulated Rabs was greater than expectation (Fisher test, P < 0.05) (Table 5). Most compartments involved in the set of Rabs that were downregulated, participate in exocytosis (Rab3a) (Stahl et al., 1996) and in the endocytotic pathway (Bucci et al., 1992). On the other hand, some Rabs that participate in organelle fusion, delivery of vesicles to the plasma membrane (Rab27b) (Gomi et al., 2007), and intracellular trafficking (Rab30) were upregulated in females suggesting some compensating mechanism. Only five Rabs showed a decreased expression in PS males, of which three were also downregulated in females. It is noteworthy that these three genes are fundamental to synaptic vesicle priming and fusion (Rab3a) and to the recycling of endosomes (Rab15, Rab35). In addition, two different isoforms of Rab5 (Rab5b and Rab5c) that are functionally redundant were downregulated in males and females (Table 5).
| Gene | RefSeq | Isoa | Female (5,653) | Male (1,432) | Function |
|---|---|---|---|---|---|
| Rab2 | NM_212547 | L | ↓ | IC, CGN ER to Golgi transport | |
| Rab3 | NM_013018 | A | ↓ | ↓ | Exocytosis in neurons and neuro-endocrine cells |
| Rab3 | NM_080580 | D | ↓ | Exocytosis in neurons and neuro-endocrine cells | |
| Rab4 | NM_017355 | B | ↓ | Fast EE recycling to PM | |
| Rab5 | NM_001079936 | B | ↓ | PM to endosome, fusion of CCV | |
| Rab5 | NM_001105840 | C | ↓ | PM to endosome, fusion of CCV | |
| Rab6 | NM_053366 | A | ↓ | Intra-Golgi retrograde transport | |
| Rab10 | NM_017359 | ↓ | Vesicle translocation | ||
| Rab11 | NM_032617 | B | ↓ | Slow endocytic recycling through RE | |
| Rab13 | NM_031092 | ↓ | Assembly of TJ | ||
| Rab15 | NM_198749 | ↓ | ↓ | Trafficking from EE to RE | |
| Rab22a | NM_001108966 | ↓ | Trafficking between the TGN and EE | ||
| Rab24 | NM_001015023 | ↓ | Regulates formation of autophagosomes | ||
| Rab26 | NM_133580 | ↓ | Localized in the mature granule membranes | ||
| Rab27 | NM_053459 | B | ↑ | Regulate exocytotic events, Melanosome to PM | |
| Rab30 | NM_001015012 | ↑ | Localized to the Golgi stacks | ||
| Rab34 | NM_001012140 | ↓ | Regulation of lysosomal morphology | ||
| Rab35 | NM_001013046 | ↓ | ↓ | Slow endocytic recycling through RE | |
| Rab36 | NM_001109589 | ↓ | Localized at the Golgi body |
- a Iso, isoform; ↓ = down regulation; ↑ = upregulation.
- TGN, trans-Golgi network; CGN, Cis-Golgi network; ER, endoplasmic reticulum; IC, intermediate compartment; PM, plasma membrane; EE, early endosome; RE, recycling endosome; CCV, clathrin-coated vesicles.
| Genes | Forward | Reverse |
| L19 | CTGAAGGTCAAAGGGAATGTG | GGACAGAGTCTTGATGATCTC |
| SNAP25 | ATATGGCCCTAGACATGGG | CAGCATCTTTGTTGCACGTT |
| ARC | GAGAGCTGAAAGGGTTGCAC | GCCTTGATGGACTTCTTCCA |
| RIMS4 | GTTCAGTGACTTCCTGGGGA | CGCTCCTGTAAACCGATCTC |
| Doc2b | ATTACGGGATCACGGATGAG | GTGTGGTTGGGTTTCAGCTT |
| Rab3a | CGCAATGACAAGAGGATCAA | GGGCATTGTCCCATGAGTAA |
| Nlgn3 | GACGGGGATGAAGATGAAGA | GTTGCCTGTTCCTTCCATGT |
Effect of Acute Stress on Prenatally Stressed Rats
The prevalent functional category that was downregulated by prenatal stress in the hippocampus is associated with the structure and function of the synapse. We, therefore, tested the hypothesis that an acute stress would expose the deficiency in gene expression that was seen in the resting state. For this purpose, we measured the expression of a number of presynaptic and postsynaptic proteins that have a fundamental role in the organization and function of the synapse; (i) synaptophysin, the most abundant synaptic vesicle membrane protein; (ii) synaptopodin, an organizer that affects synapse shape and plasticity through its interaction with the cytoskeleton; (iii) PSD95, the post synaptic density (PSD) organizer; (iv) GluR1 and NR1, representatives of the most prominent glutamate receptors. The levels of these proteins were compared in naïve C and PS rats with those 2 h after acute stress induced by exposure to the EPM. The data are shown in Figure 5. In naïve rats, prenatal stress significantly decreased the expression of GluR1 in females (P < 0.001) and NR1 in males. Exposure to the EPM decreased the expression of synaptophysin selectively in PS males and females and that of synaptopodin in C females (P < 0.05) and in PS males (P < 0.05) and females (P < 0.01). The expression of NR1 was selectively reduced in C males (P < 0.05). In C and PS females only, the expression of PSD95 (P < 0.01) and of NR1 (P < 0.05) was significantly increased by stress exposure.
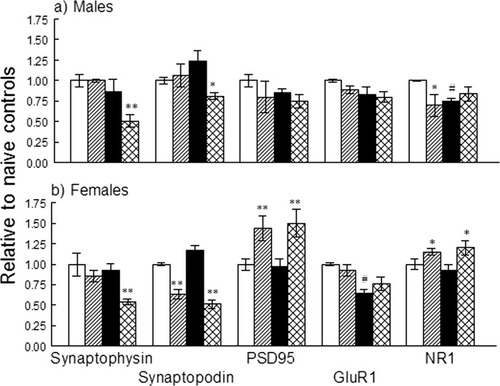
The effect of exposure to EPM (acute stress) on protein expression in PS and C rats. White columns: naïve controls; Black columns: naïve PS rats; hatched columns: C rats exposed to EPM; cross-hatched columns: PS rats exposed to EPM. Data represent mean expression relative to naïve C rats± SEM. Significantly different from naïve rats of same prenatal treatment group, *P < 0.05; **P < 0.01. #Significantly different from naïve C rats, P < 0.05.
DISCUSSION
The present study examined the effect of varied stress on two behaviors, which show sexual dimorphism in rats, anxiety in the EPM (Johnston and File, 1991) and exploration and discrimination of novelty in the context of the ORT (Sutcliffe et al., 2007). As the hippocampus plays a role both in the execution of anxiogenic behavior (Deacon et al., 2002) and in object recognition (Mumby et al., 2002) we also compared gene expression in the hippocampus of male and female rats and determined how this was affected by prenatal stress. We confirmed the difference in behavior of control Wistar male and female rats previously reported in other rat strains (Johnston and File, 1991; Sutcliffe et al., 2007) and found that the gender difference was markedly attenuated by prenatal stress. We also report for the first time that 1,680 hippocampal genes show a differential expression between male and female rats and that is decreased to 11% (191 genes) by prenatal stress. The substantial disparity in the gene profile of males and females may reflect a difference in the rate of maturation of the hippocampus that is gender dependent and is seen in the greater number of mature neurons in adult males than in females (Mandyam et al., 2008).
The direction and degree of effect of prenatal stress on anxiogenic behavior depends on the intensity and duration of maternal stress and the age and gender of the rat (Weinstock, 2008). It also depends on the level of anxiety in controls. Male rats of the Wistar strain show large differences in exploratory activity and anxiety in the EPM. By selective breeding, two substrains were produced that showed either high anxiety (HAB) or/and low anxiety (LAB) in this test (Landgraf and Wigger, 2002). Prenatal stress decreased anxiety in male HAB rats and increased it in LAB rats (Bosch et al., 2006). In the current study on an unselected population of Wistar rats, the behavior of C males resembled that of the HAB rats cited above. On the other hand, the lower level of anxiety seen in C females was reminiscent of the behavior of LAB males. Our finding accords with that of others who showed that females display lower anxiogenic behavior than males in a variety of tests, including that in the EPM (Johnston and File, 1991; Slotten et al., 2006). This may be influenced by their estrogen levels according to the stage of the estrous cycle (Mora et al., 1996; Marcondes et al., 2001) and might explain why the effect of prenatal stress in females was not as robust as in previous studies both from our group and others (Fride and Weinstock, 1988; Zagron and Weinstock, 2006; Richardson et al., 2006). Although plasma estrogen levels were not measured in PS rats in this study or in others in which their behavior in the EPM was assessed, estradiol administration has been shown to have a different effect on behavior in PS and C rats (Walf and Frye, 2007). This suggests that prenatal stress may alter the sensitivity of hippocampal receptors to estradiol or other neuroactive steroids. Irrespective of the stage of the estrous cycle, prenatal stress abolished the difference in anxiogenic behavior in the two sexes as shown in other studies (Fride and Weinstock, 1988; Ordyan and Pivina, 2004).
To our knowledge, the effect of prenatal stress on exploration and discrimination of novel objects in the ORT has not been previously examined. We found that females but not males were able to discriminate between a novel and familiar object if the interval between the two trials is 60 min. Males are able to show object discrimination after a time interval of 30 min or less (Shoham et al., 2007; Sutcliffe et al., 2007). The enhanced memory consolidation of females may be due to the influence of estradiol on hippocampal estrogen receptors (Fernandez et al., 2008). As indicated above, prenatal stress may be able to alter the balance between the effect of estradiol and other neuroactive steroids in the hippocampus thereby influencing memory consolidation. On the other hand, prenatal stress increased object recognition and exploratory activity in males to resemble that in females.
The decrease in sexual dimorphic behavior induced by prenatal stress may have resulted from a reduction in and/or timing of the testosterone peak in males (Ward and Weisz, 1984) and in the activity of aromatase in males and females, as shown for prenatal immobilization stress (Weisz et al., 1982). The change in testosterone and enzyme activity could have occurred because of the fivefold to sixfold increase induced by stress in maternal circulating COR during the last week of gestation, as comparable increases in circulating glucocorticoid induced by steroid administration to pregnant rats also reduced brain aromatase activity in the offspring (Reznikov et al., 1999).
We postulate that the divergence in the behavior of C males and females in the above tests could be reflected in the difference in the expression of a large number of genes (1,680 above the significant threshold). We found that the biological processes of axonogenesis, synaptogenesis, and synapse maturation (that are subdivided to synaptic transmission, vesicle mediated transport, and microtubule cytoskeleton) appear to be relatively more active in adult males than in females. On the other hand, genes that showed a higher expression in females than in males were mainly concerned with maintenance and neurogenesis. In accordance with the attenuation of the gender difference in behavior, prenatal stress reduced by 89% the number of genes differentially expressed in the two sexes. In both males and females, a decrease was seen in processes like the translational machinery (subdivided to ribosomes and protein biosynthesis), mitochondrial activity, and cation transport that are usually associated with stress and changes in environmental conditions. In PS females, there was a greater suppression of genes involved in vesicle trafficking, regulation of synaptic plasticity, and neurogenesis. We postulate that the higher expression of components of vesicle trafficking, microtubule-based processes, and neurite development seen only in females is a compensatory mechanism for the large number of genes that were repressed by prenatal stress.
Prenatal stress altered the expression of a sizeable number of genes of the Rab family. In females, the expression of 19 genes was significantly decreased (P < 0.05), and include Rab3a, Rab5b, Rab14, Rab6a, Rab10, Rab35, Rab24, Rab22a. This was associated with a compensatory increase of Rab partner proteins and effectors, Rab3ip and Rab3Gap2 for Rab3 and RAb6ip2 for Rab6. The expression of only five Rab proteins was significantly reduced in PS males and there were no signs of compensation by over expression of any of the Rabs or their effectors.
It is understandable that many of the genes that changed their expression as a result of prenatal stress may be localized to discrete areas in the hippocampus (Thompson et al., 2008). For example, Doc2b is strongly expressed in the dentate gyrus (DG) and CA1 area, and TGFβ in the CA3 area. In spite of this specific location, TGFβ and Doc2 were both downregulated in a whole hippocampal extract in PS females to a level of 0.618 and 0.687, respectively. It may, therefore, be expected that changes in gene suppression would be even more significant in subregions of the hippocampus.
The foregoing discussion focused on processes that are affected by prenatal stress based on gene expression profiles. However, there could be a discrepancy between the levels of transcripts and proteins in the cells. Regulation at the level of RNA turnover, translational efficiency, and miRNAs expression may alter the accessibility of the encoded protein products. We, therefore, tested the change that occurred in response to stress in the expression of dynamic key proteins in the synapse including synaptophysin, synaptopodin, and PSD95. All the proteins tested in our study have been shown to vary considerably in their expression between healthy and diseased brains (Burns and Augustine, 1995). Synaptopodin plays a role in activity-dependent enlargement of dendritic spines in hippocampal neurons (Okubo-Suzuki et al., 2008) and also functions as a linker with dendritic calcium stores (Vlachos et al., 2009) and between the translocation of PSD95 (as a representative of other PSD proteins) and subunits of AMPA receptors (as represented by NR1). As described above for Rabs and their effectors in PS females, the suppression of synaptopodin is compensated by a higher expression of its associated genes, RyR2 (2.52-fold), Snap25 (1.87-fold), Myo5b (2.27-fold), and Kif5b (2.2-fold). Kif5b has been implicated in activity-dependent plasticity (Cai et al., 2007) and mediates axonal transport of active zone components that are essential for presynaptic assembly. Myo5b mediates cargo transport including the glutamate receptor GluR1, and reduces the surface expression of GluR1 but not NR1 in developing hippocampal neurons (Lise et al., 2006). These changes may provide a compensating effect for the translocation of glutamate receptors. From this analysis, it is evident that a molecular balance for dendrite formation, glutamate receptors and presynaptic components is severely affected by prenatal stress in both sexes. The alterations in gene expression were confirmed by measurement of that of the proteins in response to stress. Exposure of controls to the EPM decreased the expression of NR1 in males and synaptopodin in females. This was accompanied only in females by an enhanced expression of PSD95 and NR1. In PS rats of both sexes, the additional stress decreased the expression of synaptophysin and synaptopodin and caused a compensatory increase in the expression of PSD95 and NR1 only in females. The rapid changes in proteins as a result of EPM stress may be attributed mainly to an alteration in their subcellular localization. These data reinforce the findings from the gene arrays and show that females are also able to compensate for the reduction in the expression of key synaptic proteins by strengthening that of proteins acting at both sides of the synapse. This compensation does not occur in males.
In conclusion, we have provided an unbiased view of the key proteins and genes that act as gender-dependent molecular sensors. The disruption of the expression of these genes and proteins by adverse early life experience may explain the alterations that occur in behavior.
Acknowledgements
The able technical assistance of Mrs. Gilat Sacks is gratefully acknowledged. Yoel Bogoch is a fellow of the Sudarsky Center for Computational Biology.




