Factors associated with cardiovascular events after simultaneous liver–kidney transplant from the US Multicenter Simultaneous Liver–Kidney Transplant Consortium
Abstract
Cardiovascular disease is a leading complication after both liver and kidney transplantation. Factors associated with and rates of cardiovascular events (CVEs) after simultaneous liver–kidney transplant (SLKT) are unknown. This was a retrospective cohort study of adult SLKT recipients between 2002 and 2017 at six centers in six United Network for Organ Sharing regions in the US Multicenter SLKT Consortium. The primary outcome was a CVE defined as hospitalization due to acute coronary syndrome, arrhythmia, congestive heart failure, or other CV causes (stroke or peripheral vascular disease) within 1 year of SLKT. Among 515 SLKT subjects (mean age ± SD, 55.4 ± 10.6 years; 35.5% women; 68.1% White), 8.7% had a CVE within 1 year of SLKT. The prevalence of a CVE increased from 3.3% in 2002–2008 to 8.9% in 2009–2011 to 14.0% in 2012–2017 (p = 0.0005). SLKT recipients with a CVE were older (59.9 vs. 54.9 years, p < 0.0001) and more likely to have coronary artery disease (CAD) (37.8% vs. 18.4%, p = 0.002) and atrial fibrillation (AF) (27.7% vs. 7.9%, p = 0.003) than those without a CVE. There was a trend toward older age by era of SLKT (p = 0.054). In multivariate analysis adjusted for cardiac risk factors at transplant, age (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.02, 1.11), CAD (OR, 3.62; 95% CI, 1.60, 8.18), and AF (OR, 2.36; 95% CI, 1.14, 4.89) were associated with a 1-year CVE after SLKT. Conclusion: Among SLKT recipients, we observed a 4-fold increase in the prevalence of 1-year CVEs over time. Increasing age, CAD, and AF were the main potential explanatory factors for this trend independent of other risk factors. These findings suggest that CV risk protocols may need to be tailored to this high-risk population.
INTRODUCTION
Simultaneous liver kidney transplant (SLKT) is an important option for liver transplant candidates with end-stage renal disease (ESRD), sustained acute kidney injury (AKI) unlikely to recover after liver transplant, and those with inborn metabolic diseases to help mitigate the risk of posttransplant renal dysfunction.[1] SLKT has become common practice, with more than 700 performed in 2016; additionally, the proportion of liver transplants performed as SLKT has increased from 2.7% in 2000 to 9.3% in 2016.[2]
With increasing prevalence of SLKT, it is important to explore potential adverse outcomes from this surgery as it has implications on allocation of scarce organs as well as management of this population. Cardiovascular disease (CVD), which encompasses the diagnoses of coronary heart disease, stroke, heart failure, peripheral vascular disease (PVD), arrhythmias, and valvular heart disease, is a leading cause of morbidity and mortality after both liver transplant and kidney transplant.[3] Up to 30% of liver transplant recipients have a CVD complication within 1 year of transplant.[3] One study found that CVD was the most common cause of death with intact graft function after kidney transplant, with ischemic heart disease being the most common etiology of cardiovascular events (CVEs).[4] In contrast, the most common underlying cause of early (<1 year) CVEs in liver transplant recipients is nonischemic in origin.[5, 6] Thus, the prevalence and significance of CVD after liver transplant alone and kidney transplant alone has been well established.[7] However, factors associated with and rates and types of CVEs after SLKT are not well studied. Our study aims to investigate the prevalence of and factors that influence the development of CVEs after SLKT.
MATERIALS AND METHODS
The US Multicenter Simultaneous Liver–Kidney Consortium includes data of adult (age > 18 years) SLKT recipients and donors who had transplantation performed between February 2002 to June 2017 at six large transplant centers (Columbia University Irving Medical Center, Duke University, Northwestern Medicine, University of California San Francisco, University of Michigan, University of Washington) in six United Network for Organ Sharing regions. Institutional Review Board approval was obtained at each site, and de-identified data were uploaded to Research Electronic Data Capture based at the University of Michigan. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul.
Clinical characteristics, including recipient demographic information at time of listing and at time of transplant, and donor demographic information were collected. Recipient characteristics, including diagnoses of new onset hypertension, diabetes, body mass index (BMI), estimation of renal function with estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease (CKD) Epidemiology Collaboration creatinine equation, serum creatinine (Cr), and new diagnoses of renal failure, were documented up to 5 years after transplant. Diagnosis of pretransplant CVD risk factors (e.g., coronary artery disease [CAD], stroke) and risk equivalents (e.g., diabetes) were defined by International Classification of Diseases, Ninth and Tenth Revisions (ICD-9 and ICD-10) codes located on patient problem lists (Table S1). Use of aspirin, statins, or beta blockade was defined at the time of hospitalization for transplantation. Obesity was defined as BMI ≥ 30 kg/m2. Renal failure was defined as CKD stage 4, CKD stage 5, or sustained AKI necessitating treatment with renal replacement therapy and/or kidney transplant listing. “New onset” of hypertension, diabetes, renal failure, or obesity was determined based on the presence of the ICD-9 and ICD-10 code for hypertension, diabetes, renal failure, or BMI ≥ 30 kg/m2, respectively, at the time points of 6 months and 12 months after SLKT in participants who did not have these characteristics present at the time of SLKT listing. Data were collected on the number of hospitalizations and reason for hospitalizations at two different time points: within the first 6 months posttransplant and between 6 and 12 months posttransplant. Posttransplant CVEs requiring hospitalization were evaluated by ICD-9 and ICD-10 codes located in the top three discharge diagnoses for acute coronary syndrome (ACS), arrhythmias, heart failure, stroke, PVD, hypertensive urgency, valvular heart disease, or pulmonary embolism (PE) (Table S1).
CAD screening protocols at the participating centers are summarized in Table S2. In general, all centers performed electrocardiogram and resting echocardiogram in all patients. Centers then used a risk-based protocol to determine need for further noninvasive or invasive testing to identify CAD. In five of six centers, dobutamine stress echocardiography (DSE) was performed in patients with diabetes or with two or more CAD risk factors (e.g., age > 45, smoking, hyperlipidemia, hypertension, or family history of CAD). The University of Michigan performed DSE in all patients. All centers used a dedicated cardiologist or cardiology team to evaluate the need for invasive angiography in patients at high risk for CAD (Table S2). At the participating centers, it was considered prohibitive to accept the patient as an SLKT candidate with the following conditions: (1) decreased left ventricular systolic function (ejection fraction <45%); (2) decreased right ventricular function and/or significant right ventricular dilation; (3) uncontrolled pulmonary hypertension, defined as pulmonary arterial systolic pressure ≥ 35 mm Hg at rest despite maximal medical management; (4) significant uncorrectable structural valvular abnormalities (aortic stenosis, mitral stenosis, aortic regurgitation, tricuspid regurgitation); (5) uncorrectable CAD with induced ischemia on stress testing; (6) significant carotid disease, in particular if symptomatic; and (7) diffuse atherosclerotic disease involving multiple organs. Patients deemed to be at unacceptably high cardiovascular risk by the care team based on these criteria were clinically excluded from a transplant and thus were not included in our study sample because they did not undergo SLKT.
Immunosuppression protocols were evaluated at each of the six transplant centers. All centers used a similar immunosuppression protocol for SLKT with tacrolimus-based immunosuppression with mycophenolic acid and corticosteroids. In April 2015, Northwestern Medicine's SLKT immunosuppression protocol was revised to include induction with basiliximab on days 0 and 2 in addition to solumedrol, maintenance with tacrolimus and mycophenolic acid, and a corticosteroid taper to 5 mg indefinitely. All other centers used SLKT immunosuppression protocols similar to kidney transplant-alone immunosuppression protocols, with induction with thymoglobulin, basiliximab, and dacluzimab determined by the presence of panel-reactive antibodies and sensitization. Therapeutic tacrolimus trough levels were based on days after SLKT and maintained between 8 and 12 ng/mL in the first 90 days at all six centers.
Transplant eras were defined as 2002–2008, 2009–2011, and 2012–2017. From 2002 to 2008, the Model for End-Stage Liver Disease (MELD) score was adopted and used to guide liver allocation; from 2009 to 2011, SLKT listing criteria reflected the Organ Procurement and Transplantation Network (OPTN) version 1 policy derived at the 2008 consensus conference; the era of 2012–2017 reflects OPTN version 2-driven policy from the 2012 consensus conference.[8]
Statistical analysis
Clinical characteristics were described using frequency and percentages for categorical variables and means and SDs for continuous variables. The primary outcome was a hospitalization for a CVE within 1 year of SLKT. Patients who were missing information on hospitalization status at 6 and 12 months after transplant were excluded from the analysis (n = 55). Twenty-one variables were input into a univariate logistic regression model. Multivariate analysis using logistic regression was then performed. Covariates were chosen based on a priori clinical significance and association with CVE. Model 1 was adjusted for age, sex, and race; model 2 for age, sex, race, risk factors for CVE (hypertension, diabetes, obesity at time of listing, nonalcoholic steatohepatitis [NASH] etiology for liver transplant), prevalent pretransplant CVD (CAD, atrial fibrillation [AF], and congestive heart failure [CHF]), and pretransplant medication use (statin, aspirin, or selective beta blocker); model 3 for age, sex, race, new onset hypertension, diabetes, and obesity; and model 4 for age, sex, race, new onset hypertension, diabetes, obesity, and renal failure. Sensitivity analysis was also performed only among those without established CVD at the time of transplant and also excluding those who had a CVE within 30 days of SLKT. Results are presented using odds ratios (ORs) with 95% confidence intervals (CIs), and p values are reported. Analyses were performed with SAS 9.4.
RESULTS
Demographics
Among the 515 SLKT recipients identified, the mean age was 55.4 ± 10.6 years. Women comprised 35.5%, 64.3% self-identified as White, and 11.8% self-identified as Black (Table 1). The most common listing diagnosis for liver transplant was hepatitis C (31.3%) followed by alcohol (23.1%) and NASH (12.0%). At the time of transplant listing, 46.2% of SLKT recipients had an AKI and 53.8% had stage 4–5 CKD. Among the 155 patients with CKD, 55.5% had hypertension and 45.8% had diabetes, supporting these etiologies as the most likely underlying causes for CKD. History of CAD, CHF, stroke, AF, PVD, and PE was present in 20.0% (n = 103), 11.3% (n = 58), 10.3% (n = 53), 9.7% (n = 50), 7.0% (n = 36), and 1.4% (n = 7) of SLKT recipients at the time of transplant, respectively. Cardioprotective medications were used in 14.4% of SLKT recipients at the time of transplant listing: 35 were on aspirin, 23 were on a statin, and 31 were on a selective beta blocker. Among 103 patients with CAD, 66 (64.2%) patients had pretransplant angiography (two computed tomography coronary angiography and 64 invasive angiography). Of these, 14 patients had percutaneous coronary revascularization and seven had coronary artery bypass grafting, indicating the presence of significant CAD in 4.1% (21/515) of total SLKT recipients.
| Total population (N = 515) | CVE at 12 months (n = 47) | No CVE at 12 months (n = 468) | p valuea | |
|---|---|---|---|---|
| n, % or n (SD) | n, % or n (SD) | n, % or n (SD) | ||
| Recipient sex, male | 332, 64.5% | 31, 65.9% | 301, 64.3% | 0.82 |
| Recipient age | 55.4 (10.6) | 59.9 (7.35) | 54.9 (10.8) | <0.0001 |
| Recipient race | 0.93 | |||
| White | 331, 64.3% | 30, 66.7% | 301, 64.0% | |
| Black | 61, 11.8% | 4, 8.9% | 57, 12.1% | |
| Hispanic | 41, 8.0% | 4, 8.9% | 37, 7.9% | |
| Other | 82, 15.9% | 7, 15.6% | 75, 16.0% | |
| Listing diagnosis | 0.41 | |||
| Alcohol | 119, 23.1% | 11, 23.4% | 108, 23.1% | |
| HCV | 161, 31.3% | 18, 40.0% | 143, 30.4% | |
| Immune | 47, 9.1% | 5, 11.1% | 42, 8.9% | |
| NASH | 62, 12.0% | 5, 11.1% | 57, 12.1% | |
| Other | 126, 24.5% | 6, 13.3% | 120, 25.5% | |
| HCC at time of listing | 74, 14.4% | 5, 11.1% | 69, 14.8% | 0.50 |
| Cardiovascular comorbidities at time of listing | ||||
| HTN | 277, 53.8% | 27, 60.0% | 250, 53.4% | 0.40 |
| DM | 217, 42.1% | 18, 40.0% | 199, 42.4% | 0.75 |
| CAD | 103, 20.0% | 17, 37.8% | 86, 18.4% | 0.002 |
| Atrial fibrillation | 50, 9.7% | 13, 27.7% | 37, 7.9% | <0.0001 |
| Any other arrhythmia | 52, 10.1% | 13, 27.7% | 39, 8.3% | <0.0001 |
| CHF | 58, 11.3% | 10, 21.3% | 48, 10.3% | 0.03 |
| PVD | 36, 7.0% | 5, 10.6% | 31, 6.6% | 0.33 |
| Stroke | 53, 10.3% | 7, 14.9% | 46, 9.8% | 0.30 |
| PE | 7, 1.4% | 1, 2.1% | 6, 1.3% | 0.50 |
| Coronary angiography performed | 84, 16.3% | 14, 29.8% | 70, 15.0% | 0.011 |
| Revascularization before SLKT | 21, 4.1% | 0, 0.0% | 6, 1.3% | 1.00 |
| Renal dysfunction at time of listing | 480, 93.2% | 41, 100.0% | 439, 99.3% | 1.00 |
| Renal dysfunction type | 0.0007 | |||
| AKI | 133, 25.8% | 22, 75.9% | 111, 42.9% | |
| CKD | 115, 30.0% | 7, 24.1% | 148, 57.1% | |
| AKI treatment with RRT | 111, 21.6% | 9, 100.0% | 102, 92.7% | 1.00 |
| AKI type | 0.25 | |||
| Prerenal/HRS | 134, 26.0% | 14, 58.3 | 120, 64.2 | |
| ATN | 32, 6.2% | 2, 8.3 | 30, 16.0 | |
| Multiple | 45, 8.7% | 8, 33.3 | 37, 19.8 | |
| Serum Cr at listing | 3.7 (2.4) | 4.06 (2.8) | 3.66 (2.3) | 0.39 |
| HD at time of listing | 146, 28.3% | 9, 20.0% | 137, 29.6% | 0.17 |
| OPTN SLK criteria | 0.84 (0.37) | 0.95 (0.21) | 0.82 (0.38) | 0.0011 |
| MELD at listing | 28.2 (8.8) | 28.7 (7.9) | 28.2 (8.9) | 0.70 |
| MELD-Na at listing | 26.5 (8.0) | 27.4 (8.7) | 26.4 (7.9) | 0.69 |
| MELD at transplant | 30.3 (7.7) | 30.1 (7.4) | 30.3 (7.7) | 0.90 |
| MELD-Na at transplant | 30.6 (7.7) | 31.2 (6.6) | 30.5 (7.8) | 0.60 |
| BMI at listing | 29.0 (6.2) | 29.6 (6.4) | 28.3 (6.3) | 0.24 |
| Underweight (BMI < 18.5) | 24, 4.7% | 2, 4.4% | 22, 4.7% | 0.31 |
| Normal (BMI 18.5–24.9) | 152, 29.5% | 8, 17.8% | 144, 30.9% | |
| Overweight (BMI 25–30) | 161, 31.3% | 16, 35.6% | 145, 31.1% | |
| Obese (BMI ≥ 30) | 174, 33.8% | 19, 42.2% | 155, 33.3% | |
| Cardioprotective medication use at listing, any | 74, 14.4% | 7, 14.9% | 67, 14.3% | 0.97 |
| Aspirin | 35, 6.8% | 3, 6.4% | 32, 6.8% | 0.87 |
| Statin | 23, 4.5% | 2, 4.3% | 21, 4.5% | 0.91 |
| Selective beta blocker | 31, 6.0% | 4, 8.5% | 27, 5.8% | 0.52 |
| Immunosuppression during hospitalization | 0.72 | |||
| Tacrolimus | 487, 94.6% | 42, 93.3 | 445, 94.9 | |
| Cyclosporine | 27, 5.2% | 3, 6.7 | 24, 5.1 | |
| Cellcept | 471, 91.4% | 41, 91.1% | 430, 91.7% | |
| Induction used | 120, 23.3% | 9, 20.9% | 111, 23.7% | 0.68 |
| Induction type | 0.85 | |||
| Basiliximab | 84, 16.3% | 5, 62.5% | 79, 71.2% | |
| Thymoglobulin | 10, 19.4% | 1, 12.5% | 9, 8.1% | |
| Dacluzimab | 5, 1.0% | 2, 25.0% | 3, 20.7% | |
| Corticosteroids | 457, 88.7% | 45, 100.0% | 412, 93.0% | 0.10 |
- Note: CVE defined as a hospitalization for ICD-9 and ICD-10 codes for acute coronary syndrome, arrhythmias, heart failure, stroke, PVD, valvular heart disease, hypertensive urgency, or PE.
- Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; Cr, creatinine; CVE, cardiovascular event; DM, diabetes mellitus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HD, hemodialysis; HRS, hepatorenal syndrome; HTN, hypertension; ICD, International Classification of Diseases; MELD, Model for End-Stage Liver Disease; NASH, nonalcoholic steatohepatitis; OPTN, Organ Procurement and Transplantation Network; PE, pulmonary embolism; PVD, peripheral vascular disease; RRT, renal replacement therapy; SLK, simultaneous liver–kidney; SLKT, simultaneous liver–kidney transplant.
- a t test for continuous variables and chi-square or Fisher's exact test for categorical variables.
Within 1 year of SLKT, 45 participants (8.7%) had 66 CVEs; among them, nine (19.1%) had more than one CVE recorded during a hospitalization. The majority of participants (91.5%, n = 43) who had a CVE experienced it within the first 6 months after SLKT. Sixty CVEs occurred within the first 6 months of SLKT; 41.7% (n = 25) were defined as arrhythmia and of these, 12 were attributed to AF, 20.0% (n = 12) to CHF, 11.7% (n = 7 total; 4 reported as hemorrhagic) to stroke, 8.3% (n = 5) to ACS, 6.7% (n = 4) to PE, 5% (n = 3) to PVD, one to valvular heart disease, and one to hypertensive urgency. There were two unspecified CVEs documented in this time period. There were 11 CVEs reported between 6 and 12 months: four were defined as CHF, three as ACS, two as arrhythmias, one as ischemic stroke, and one was unspecified.
Participants who had a CVE at 1 year after SLKT were older (59.9 vs. 54.9 years, p < 0.0001) and more likely to have CAD (37.8% vs. 18.4%, p = 0.002), AF (27.7% vs. 7.9%, p < 0.0001), other arrhythmias (27.7% vs. 8.3%), and prior CHF (21.3% vs. 10.3%, p = 0.03) at time of listing compared to those without a CVE. Comparing groups with a CVE at 1 year to those without a CVE at 1 year, there were no significant differences in the prevalence of hypertension (60.0% vs. 53.4%), diabetes (40.0% vs. 42.4%), obesity (42.4% vs. 33.3%), stroke (14.9% vs. 9.8%), PE (2.1% vs. 1.3%), PVD (10.6% vs. 6.6%), or cardioprotective medication use (14.9% vs. 14.3%) at time of listing. There were also no significant differences in MELD score, MELD-Na score, indication for liver transplant, or rates of hemodialysis at time of listing.
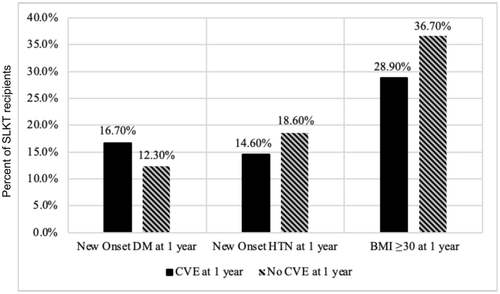
At 1 year after SLKT, there were no significant differences in the rate of new onset diabetes (16.7% vs. 12.3%), hypertension (14.6% vs. 18.6%), or obesity (28.9% vs. 36.7) (Figure 1). There were also no differences in the rate of postoperative hemodialysis or renal failure after SLKT (Table 2). There were also no significant differences in immunosuppression regimens used after SLKT (Table 1).
| CVE at 12 months (n = 47) | No CVE at 12 months (n = 468) | p value | |
|---|---|---|---|
| n, % | n, % | ||
| Postoperation HD (during hospitalization for initial transplant) | 9, 20.9% | 107, 22.8% | 0.78 |
| eGFR at 1 year | 49.8 (25.1) | 56.9 (22.1) | 0.09 |
| Renal failure within 1 year of transplant | 24, 54.5% | 194, 41.9% | 0.10 |
| Renal failure type | 0.77 | ||
| AKI | 12, 60.0% | 86, 56.6% | |
| Stage 4–5 CKD | 8, 40.0% | 66, 43.4% | |
| Renal failure treatment | 0.58 | ||
| RRT | 12, 100% | 58, 90.6% | |
| Kidney transplant listing | 0, 0% | 6, 9.4% | |
| Days to renal failure (initiation of RRT or listing for retransplant) | 808.1 (1167.8) | 1146.6 (1660.3) | 0.46 |
| Renal composite score, eGFR < 60 | 20, 62.5% | 186, 54.2 | 0.37 |
- Note: Renal failure defined as presence of renal failure at any time from 2002 to 2017 after transplant. CVE defined as a hospitalization for ICD-9 and ICD-10 codes for acute coronary syndrome, arrhythmias, heart failure, stroke, peripheral vascular disease, valvular heart disease, hypertensive urgency, or pulmonary embolism.
- Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; CVE, cardiovascular events; eGFR, estimated glomerular filtration rate; HD, hemodialysis; ICD, International Classification of Diseases; RRT, renal replacement therapy.
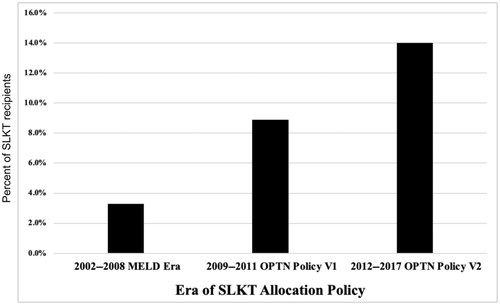
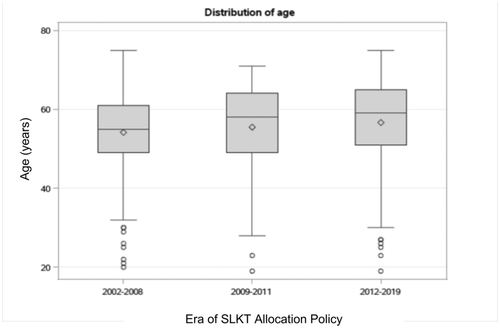
The prevalence of a CVE within 1 year of SLKT increased by era of transplant from 3.3% in 2002–2008 to 8.9% in 2009–2011 to 14.0% in 2012–2017 (p = 0.0005) (Figure 2). There was also a trend toward older age by era of SLKT (p = 0.054) (Figure 3). CAD prevalence did not differ by era, with 19.6% from 2002 to 2008, 27.8% from 2009 to 2011, and 17.4% from 2012 to 2019 (p = 0.11).
Univariate analysis
In univariate analysis, age (OR, 1.06; 95% CI, 1.02, 1.10), CAD (OR, 2.69; 95% CI, 1.41, 5.14), CHF (OR, 2.33; 95% CI, 1.08, 5.03), or AF (OR, 4.45; 95% CI, 2.14, 9.26) at time of listing were associated with a CVE at 1 year (Table 3). Hypertension, diabetes, obesity, stroke, PE, PVD, and use of cardioprotective medications at time of listing were not associated with CVE at 1 year after SLKT (Table 3). NASH as listing etiology for liver transplant was also not associated with CVE at 1 year after SLKT (OR, 0.91; 95% CI, 0.34, 2.39). New onset hypertension (OR, 0.75; 95% CI, 0.31, 1.84), diabetes (OR, 1.34; 95% CI, 0.6, 3.36), and obesity (OR, 1.64; 95% CI, 0.35, 3.09) were not associated with CVE at 1 year after SLKT.
| Unadjusted OR | p value | |
|---|---|---|
| Recipient age | 1.06 (1.02, 1.10) | 0.003 |
| Recipient female sex | 1.00 (0.53, 1.90) | 1.00 |
| Recipient Black race | 0.70 (0.24, 2.08) | 0.53 |
| HTN at listing | 1.31 (0.70, 2.44) | 0.40 |
| DM at listing | 0.91 (0.49, 1.69) | 0.75 |
| Obesity at listing (BMI > 30) | 1.47 (0.79, 2.73) | 0.23 |
| CAD at listing | 2.69 (1.41, 5.14) | 0.003 |
| Atrial fibrillation at listing | 4.45 (2.14, 9.26) | <0.0001 |
| Stroke at listing | 1.57 (0.66, 3.73) | 0.31 |
| Pulmonary embolism at listing | 1.63 (0.19, 13.87) | 0.65 |
| Peripheral vascular disease at listing | 1.64 (0.60, 4.46) | 0.33 |
| CHF at listing | 2.33 (1.08, 5.030 | 0.03 |
| Cardioprotective medication usea at listing | 1.02 (0.43, 2.38) | 0.97 |
| New onset DM at 1 year | 1.43 (0.6, 3.36) | 0.42 |
| New onset HTN at 1 year | 0.75 (0.31, 1.84) | 0.53 |
| New onset obesity after transplant (BMI > 30) | 1.04 (0.35, 3.09) | 0.94 |
| NASH cirrhosis etiology | 0.91 (0.34, 2.39) | 0.84 |
| Renal failure (after SLKT) | 1.66 (0.89, 3.10) | 0.11 |
| Renal composite score, eGFR < 60 | 1.25 (0.59, 2.68) | 0.56 |
- Note: CVE defined as a hospitalization for ICD-9 and ICD-10 codes for acute coronary syndrome, arrhythmias, heart failure, stroke, peripheral vascular disease, valvular heart disease, hypertensive urgency, or pulmonary embolism.
- Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CVE, cardiovascular event; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; ICD, International Classification of Diseases; NASH, nonalcoholic steatohepatitis; OR, odds ratio; SLKT, simultaneous liver–kidney transplant.
- a Cardioprotective medications include aspirin, statin, or selective beta blocker use.
Multivariate analysis
In multivariate analysis, age at time of transplant remained a significant factor (OR, 1.07; 95% CI, 1.03, 1.12) even after adjusting for sex, race, prevalent pre-SLKT CHF, AF, and CAD and CVD risk factors, including hypertension, diabetes, and obesity at time of listing, NASH as indication for liver transplant, and cardioprotective medicine use (e.g., aspirin, statin, beta blocker) at time of transplant[6] (Table 4). Notably, prevalent CAD at transplant was the most significant factor associated with CVE within 1 year of SLKT in the fully adjusted model (OR, 3.62; 95% CI, 1.60, 8.18) followed by AF (OR, 2.36; 95% CI, 1.14, 4.89). In sensitivity analysis excluding events that occurred within <30 days of transplant (n = 16), the strength of associations was attenuated but remained significant for age (OR, 1.04; 95% CI, 1.01, 1.09) and for CAD (OR, 1.57; 95% CI, 1.10, 3.69) but not for AF in the fully adjusted model. When patients with pretransplant history of prior CVD (n = 265) were excluded from the analysis, only age was associated with 1-year CVE (OR, 1.03; 95% CI, 1.01, 1.1).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Recipient age | 1.06 (1.02, 1.10) p = 0.003 | 1.06 (1.02, 1.11) p = 0.005 | 1.07 (1.02, 1.11) p = 0.006 | 1.06 (1.02, 1.11) p = 0.007 |
| Recipient female sex | 0.94 (0.49, 1.79) p = 0.84 | 0.86 (0.41, 1.80) p = 0.70 | 1.04 (0.48, 2.24) p = 0.93 | 1.01 (0.47, 2.20) p = 0.97 |
| Recipient Black race | 0.65 (0.22–1.95) p = 0.44 | 0.65 (0.20, 2.07) p = 0.47 | 0.43 (0.10, 1.92) p = 0.27 | 0.45 (0.10, 2.05) p = 0.31 |
| HTN at time of listing | 1.25 (0.61, 2.55) p = 0.79 | |||
| DM at time of listing | 0.63 (0.31, 1.29) p = 0.39 | |||
| Obesity at time of listing | 1.49 (0.76, 2.93) p = 0.34 | |||
| CAD at time of listing | 3.62 (1.60, 8.18), p = 0.002 | |||
| CHF at time of listing | 1.57, (0.65, 3.79) p = 0.32 | |||
| Afib at time of listing | 2.36 (1.14, 4.89) p = 0.02 | |||
| Cardioprotective medication at listinga | 0.68 (0.26, 1.75), p = 0.57 | |||
| NASH cirrhosis etiology | 0.56 (0.17, 1.85) p = 0.33 | |||
| New onset DM at 1 year | 1.65 (0.58, 4.75) p = 0.35 | 1.76 (0.61, 5.08) p = 0.30 | ||
| New onset HTN at 1 year | 0.42 (0.12, 1.46) p = 0.17 | 0.41 (0.12, 1.42) p = 0.16 | ||
| New onset obesity after transplant (BMI > 30) | 1.31 (0.42, 4.11) p = 0.64 | 1.48 (0.46, 4.70) p = 0.51 | ||
| Renal failure (after SLKT) | 1.73 (0.80, 3.74) p = 0.16 |
- Note: CVE defined as a hospitalization for ICD-9 and ICD-10 codes for acute coronary syndrome, arrhythmias, heart failure, stroke, peripheral vascular disease, valvular heart disease, hypertensive urgency, or pulmonary embolism. Model 1: adjusted for age, sex, race. Model 2: Model 1 + adjusted for HTN, DM, obesity, CAD, cardioprotective medication use at time of listing, and NASH cirrhosis. Model 3: Model 1 + adjusted for new onset HTN, DM, new onset obesity. Model 4: Model 3 + adjusted for after SLKT renal failure.
- Abbreviations: Afib, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CVE, cardiovascular event; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HTN, hypertension; ICD, International Classification of Diseases; NASH, nonalcoholic steatohepatitis; OR, odds ratio; SLKT, simultaneous liver–kidney transplant.
- a Cardioprotective medications include aspirin, statin, or selective beta blocker use.
DISCUSSION
This study provides the first estimate of CVEs after SLKT in a large contemporary cohort of SLKT recipients, which is estimated as high as 14.2%. This estimate is somewhat lower than what has been reported for early (1-year) CVEs in the liver-alone liver transplant recipient (15.2%–30.0%) but is higher than what is reported early after kidney transplantation (3.5%–5.0%).[3, 4, 9] The most common CVEs observed in SLKT recipients were arrhythmias (particularly AF) and CHF. This is consistent with findings in liver transplantation that have found that the most common cause of an early CVE is arrhythmias, in particular AF.[5, 6] Arrhythmias have also been found to be a common complication after kidney transplant, with one study demonstrating that ventricular arrhythmias occurred in up to 30% of patients after kidney transplant.[10] Another found that AF occurred in over 7% of kidney transplant recipients within 3 years of kidney transplantation.[11] A study of 54,697 liver transplant recipients found that stroke was responsible for 12.5% of early CVD mortality.[3] Another study showed that stroke accounted for 9% of hospital readmissions within 90 days of liver transplantation.[5] Among kidney transplant recipients, cerebrovascular events occurred in 7% of recipients at 3 years posttransplant.[11] Notably, we observed an 11.3% 1-year rate of stroke among SLKT recipients, predominantly due to early hemorrhagic stroke. ACS was relatively less common in our SLKT cohort at both 6 months (8.3%) and 12 months (11.3%). This is consistent with prior studies in liver transplantation that have shown that early CVEs are primarily noncoronary events but contradicts findings of studies in kidney transplantation that ischemic heart disease is the most common etiology of early CVEs after kidney transplant.[4, 5]
We found that increasing age was a risk factor for the development of 1-year CVEs independent of other cardiac risk factors. As increasing age is a risk factor for developing CVD, older age at time of transplant may represent a surrogate marker for subclinical and clinical CVD. Prior studies have established that increasing age is a risk factor for CVD as well as a risk factor for CVEs in the perioperative and postoperative setting.[3, 5, 9, 12] Age is included in some surgical risk assessment models, including the American College of Surgeons National Surgical Quality Improvement Program, which demonstrates good discrimination for cardiac complications in noncardiac surgeries with a C statistic of 0.895, although it has not been specifically studied in liver or kidney transplant recipients.[13] However, older age has been identified as a risk factor for CVEs, 30-day CVD mortality, and 1-year CVD mortality after liver transplant and is a key component of the cardiovascular risk in the orthotopic liver transplantation score, the cardiac arrest risk index score, and the CAD in liver transplantation score, all of which were specifically developed in liver transplant candidates to predict various cardiac outcomes.[3, 12, 14-16] The Patient Outcomes in Renal Transplant is a risk assessment calculator specifically designed for kidney transplant patients and identifies age as a risk factor for major adverse cardiac events at 30 days and 1 year.[17, 18] With the adoption of MELD and “Share 35” resulting in an older and sicker population qualifying for SLKT, this group is at higher risk for adverse cardiac events at 1 year after SLKT.[2]
The presence of CAD before SLKT was also significantly associated with CVE within 1 year of SLKT. Pre-liver transplant CAD is a significant risk factor for 30-day postoperative myocardial infarction and death.[7, 19, 20] Of note, the prevalence of CAD may actually be underestimated in the SLKT population. Keeling et al.[21] found that asymptomatic CAD is prevalent in the end-stage liver disease (ESLD) population, with only 9.2% having normal coronary arteries and 33.8% having greater than 50% stenosis of the coronary arteries. The high incidence of CAD among patients with ESRD also presents a significant challenge for pretransplant cardiovascular screening.[22] The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-CKD trial found that stable coronary disease and moderate or severe ischemia—an initial invasive strategy of coronary angiography and revascularization—did not have a lower risk of death or nonfatal myocardial infarction among patients with advanced kidney disease compared to initial conservative management with medical therapy.[23] This suggests that while preoperative cardiac evaluation and detection of CAD is important for appropriate risk stratification, revascularization may not prevent postoperative cardiovascular complications, at least in the kidney transplant-alone population.[22, 24] In the current study, only 4.1% of patients underwent pre-SLKT revascularization; no patients proceeded to transplant with significant CAD without revascularization. Whether or not revascularization actually alters outcomes in SLKT candidates with significant CAD requires further prospective study.
We also found that the prevalence of 1-year CVEs increased 4-fold from transplant era as defined by the adoption of MELD in 2002 to OPTN version 1 in 2009 and OPTN version 2 in 2012. Our current system of SLKT allocation is based on a “sickest first” policy. The adoption of OPTN version 1 and version 2 sought to standardize listing criteria and judiciously allocate scarce organs to both ESLD and ESRD transplant candidates. However, kidney graft survival among SLKT recipients remains inferior to kidney transplant-alone recipients.[1] In addition, with the adoption of Share 35 in June 2013, this has further prioritized liver grafts to a sicker population and has contributed to both an increase in the absolute number of SLKT and an increase in the proportion of SLKT making up liver transplants.[2] This population is more likely to be older, critically ill, and thus more likely to have clinical and subclinical CVD.
Our study has several limitations, including the retrospective design. CVEs were defined broadly in this cohort, and thus the rates reported herein do not reflect traditional definitions of major adverse CVEs. This was intentional as the goal of the current study was to characterize the patterns and prevalence of the spectrum of CVEs that occur in SLKT recipients. CVEs were defined using ICD 9/10 codes, and as such, certain events may be underestimated (e.g., CHF) relative to others (e.g., ACS, stroke).[25-27] We also only looked at outcomes at two time points of 6 months and 12 months after transplant because dates of hospitalizations were not captured in this study. Thus, early peri-operative and postoperative CVEs (e.g., <30 days) and related mortality after SLKT could not be investigated. Information on results of pretransplant cardiac testing was also not available in the SLKT database. However, the goals of the current study were to estimate prevalence and types of CVEs in SLKT recipients, which has not been previously described. Finally, we did not have information on use of cardioprotective medications after SLKT, which may be important risk modifiers.
In conclusion, this study found that there was a 4-fold increase in the prevalence of 1-year CVEs over time in a large contemporary cohort of SLKT recipients. Increasing age, prevalent CAD, and AF were the main potential explanatory factors for this trend, independent of other cardiac risk factors. The combination of an older patient with prior CAD and AF plus need for SLKT identifies a high-risk group for adverse cardiac outcomes within 1 year of transplant. This has implications for appropriate pretransplant cardiovascular evaluation, risk stratification, and posttransplant management of this high-risk population.
FUNDING INFORMATION
Michigan Medicine, Educational Committee and Intramural MCUBE 3.0 grant; American Society of Transplantation Liver and Intestinal Community of Practice; National Heart, Lung and Blood Institute, Grant Number: K23 HL136891; Asociación Española para el Estudio del Hígado, Joan Rodés Grant 2021.
CONFLICT OF INTEREST
Lisa VanWagner consults for Gerson Lerhmann Group and Noble Insights; she received grants from W.L. Gore & Associates. Yuval Patel consults for Intercept. The other authors have nothing to report.