Development and validation of the Cirrhotic Ascites Severity model—A patient-reported outcome-based model to predict 1-year mortality
Abstract
Ascites formation is a sign of decompensation of cirrhosis and heralds a poor prognosis. The widely used standard binary classification of ascites as diuretic-responsive or refractory does not cover the spectrum of ascites and has limited prognostic information. We developed the Cirrhotic Ascites Severity (CIRAS) model to predict 1-year mortality among 465 patients randomized to placebo in the satavaptan trials investigating treatment of cirrhotic ascites. We used multivariable logistic regression to derive the CIRAS model based on these variables: ascites discomfort score (≤50 or >50), plasma sodium (≥140, 133–139, 125–132, or <125 mmoL/L), and a composite of ascites accumulation and diuretic treatment. We validated the prediction model in 697 trial participants randomized to satavaptan treatment. The 1-year all-cause mortality was 19.6%. The area under the receiver operator curve was higher for the CIRAS model than for the standard ascites classification into refractory and diuretic-responsive in both the development cohort (0.68 [95% confidence interval (CI): 0.62–0.75] vs. 0.62 [0.57–0.68]), and the validation cohort (0.68 [0.64–0.72] vs. 0.55 [0.51–0.60]). The CIRAS model had similar discrimination to the Child-Pugh score and nearly as good as the Model for End-Stage Liver Disease (MELD), MELD-Na, and MELD 3.0. Conclusions: The CIRAS model based on simple ascites-relevant data is an easily applicable and patient-centered way to describe the severity and prognosis of all ascites grades. It carries more prognostic information than today's label of “refractory ascites” and forms the basis for earlier and better clinical decisions related to ascites management.
INTRODUCTION
Ascites formation is the most frequent manifestation of cirrhotic decompensation.[1] More than half of patients with cirrhosis develop ascites within 10 years,[2] and 10% of these patients progress to refractory ascites.[3, 4] The long-term survival is reduced in patients with cirrhosis with ascites compared to patients with ascites-free cirrhosis, and those developing refractory ascites have a particularly poor prognosis.[5] The diagnostic criteria for refractory ascites rely on a battery of assessments including the lack of effect of or intolerance to intensive diuretic therapy, adherence to a salt-restrictive diet, and evaluations of renal sodium excretion, development in body weight, and ascites recurrence rate.[3, 4, 6] (Table S1). Because the diagnostic criteria are complicated, the clinical definition of “refractory ascites” transfers poorly from one center to the next. This problem of ambiguity is compounded by the existence of the term “recidivant ascites” (Table S1), which is reserved for ascites that is severe but not quite refractory.[5] Furthermore, the binary descriptive classification does not yield clinical predictions pertaining to the whole spectrum of ascites, and in this way is less useful. It is therefore time to replace the clinical terms diuretic-responsive, recidivant, and refractory ascites with measures that apply to all degrees of ascites, are easily accessible and restricted to ascites-related characteristics, and hold broader prognostic information.
With the access to detailed data from three large multicenter randomized trials including 1198 patients with cirrhosis with ascites,[7] we aimed to develop and validate such a model that is both descriptive and predicts mortality, reported following the TRIPOD guideline.[8] The model relied on ascites-related variables to predict 1-year all-cause mortality in patients with any stage of cirrhotic ascites. We intended to compare the prognostic performance of this model with that of the binary classification into refractory or diuretic-responsive ascites.
METHODS
Patients
Three multicenter randomized placebo-controlled double-blind studies were performed between July 2006 and December 2008 to explore the safety and efficacy of long-term administration of the vasopressin V2 receptor antagonist, satavaptan, as treatment of ascites in patients with cirrhosis.[7, 9] Recruitment occurred from 33 countries across six continents. The total study population consisted of 1198 patients with cirrhosis with three different ascites profiles; patients with clinically evident ascites treated with sodium-restricted diet with or without diuretic treatments and without any large-volume paracentesis (LVP) performed during the past 6 months (n = 462, randomized 1:1 to satavaptan or placebo); patients with recurrent ascites treated with sodium-restricted diet in combination with diuretics with a history of LVP (n = 496, randomized 2:1 to satavaptan or placebo); and patients with recurrent ascites with a history of frequent LVP not being treated with diuretics (n = 240, randomized 2:1 to satavaptan or placebo). Note that, because of the different randomization schemes, patients on satavaptan had more severe ascites on average. Exclusion criteria were identical in the three trials: variceal bleeding or spontaneous bacterial peritonitis within the past 10 days, ongoing hepatic encephalopathy exceeding West-Haven grade 1, a functioning transjugular intrahepatic portosystemic shunt (TIPS), hepatocellular carcinoma exceeding the Milan criteria, use of potent modifiers of the cytochrome P450 3A pathway, use of drugs with an ability to prolong the QT interval, serum creatinine >150 μmol/L, serum potassium >5.5 mmol/L, plasma (P) sodium >142 mmol/L, serum bilirubin >150 μmol/L, international normalized ratio >3.0, platelets <30,000 /mm3, and neutrophils <1000 /mm3.
We created a three-way categorical variable combining ascites accumulation with diuretic treatment (Supporting Information S2 and S3): (1) ascites accumulation and no diuretics, (2) ascites accumulation and any diuretic treatment, and (3) no ascites accumulation. For this purpose, “ascites accumulation” was defined as at least one paracentesis during the past 3 months before inclusion into the study, or requiring a paracentesis at inclusion into the study. This ascites-diuretic variable does not correspond to the standard classification into diuretic-responsive, recidivant, and refractory ascites (Table S2).
All patients were followed up after 1 year to register survival or date of death. While participating in the trials, ascites was controlled with paracentesis and/or diuretic titration as required, and other cirrhosis-related complications were managed at the discretion of the treating hepatologist. The classification of patients with refractory, diuretic-responsive, or recidivant ascites was based on the managing clinician's judgment, with the trial protocols referring to consensus clinical guidelines.[4, 10] For the present work we excluded 36 patients with missing data on ascites accumulation, diuretic treatment, plasma sodium (P-sodium), or patient-reported ascites discomfort score. Consequently, 465 patients randomized to the placebo arms were included in the model development cohort, and 697 patients treated with satavaptan were used as a validation cohort.
Statistical analyses
The easily obtainable clinical ascites-related variables P-sodium, patient-reported ascites discomfort score on a 100-mm visual analogue scale with 0 reflecting no discomfort due to ascites and 100 reflecting maximum discomfort, and ascites-diuretic group, were selected for regression analyses (Supporting Information S3 and Table S3). P-sodium and ascites discomfort score were divided into categories to make the model easier to use. The outcome was 1-year all-cause mortality. We first performed univariable logistic regression analyses. All candidate variables achieved p-values below 0.10 and proceeded to a multivariable logistic regression model, in which p-values remained below the limit of 0.10 for inclusion or exclusion. We also tested the predictive performance of the ascites discomfort score in subgroups by continental region of recruitment to show that its predictive potential was ubiquitous (Supporting Information S4 and Table S4). Next, we turned all combinations of model coefficients into integers by multiplying them by two and then rounding to the nearest integer (Table S5).
We used the area under the receiver operator curve (AUROC) to examine the model's ability to discriminate between patients who were dead or alive after 1 year. This was examined in the development and validation cohorts and primarily compared with the discrimination ability of the binary classification into refractory or diuretic-responsive ascites, as we aimed to challenge this term. In addition, we compared with established predictive models/scores to demonstrate noninferiority of our model. To examine the model's ability to assign an accurate 1-year mortality risk to patients in the development and validation cohorts (i.e., its calibration ability), we computed the predicted 1-year mortality based on the final logistic regression model. Also, a Pearson goodness-of-fit test was applied. Next, we performed sensitivity analyses consisting of (1) a multivariable Fine and Gray regression analysis with liver transplantation treated as competing risk to death and (2) a multivariable logistic regression analysis with death or liver transplantation after 1 year as outcome. The candidate variables remained the same.
Finally, we divided patients in the development and validation cohorts into three arbitrary groups depending on their score (low, intermediate, and high grade). We used the Kaplan–Meier estimator to calculate the cumulative all-cause mortality in the three groups from randomization to the end of follow-up after 1 year.
RESULTS
Development cohort
The included 465 patients with cirrhosis were grouped according to their ascites classification: 175 (37.6%) with diuretic-responsive ascites, 91 (19.6%) with recidivant ascites, and 199 (42.8%) with refractory ascites. Compared to patients with recidivant ascites, patients with diuretic-responsive ascites had similar hepatic and renal biochemistry but fewer paracenteses. The diuretic-responsive and recidivant ascites groups as a whole had a better liver function and milder degree of ascites than patients with refractory ascites (Table 1). The 1-year mortality for diuretic-responsive (14.9%) and recidivant ascites (11.0%) were similar, but lower than refractory ascites (28.6%) (Figure S1). Thus, for the remainder of this work, we consider recidivant ascites as diuretic-responsive and proceed using only the binary standard classification of diuretic-responsive and refractory ascites.
| Diuretic-responsive ascites (n = 175) | Recidivant ascites (n = 91) | Diuretic-responsive and recidivant ascites combined (n = 266) | Refractory ascites (n = 199) | |
|---|---|---|---|---|
| Age (years) | 57 (51–63) | 56 (49–62) | 56 (50–63) | 56 (50–63) |
| Sex (male) | 132 (75.4%) | 59 (64.8%) | 191 (71.8%) | 133 (66.8%) |
| Ethnicity | ||||
| Black | 2 (1.1%) | 1 (1.1%) | 3 (1.1%) | 3 (1.5%) |
| Caucasian/White | 169 (96.6%) | 88 (96.7%) | 257 (96.6%) | 171 (85.9%) |
| Asian | 3 (1.7%) | 1 (1.1%) | 4 (1.5%) | 17 (8.5%) |
| Other | 1 (0.6%) | 1 (1.1%) | 2 (0.8%) | 8 (4.0%) |
| Etiology of cirrhosisa | ||||
| Alcohol | 119 (68.0%) | 63 (69.2%) | 182 (68.4%) | 131 (65.8%) |
| Hepatitis B virus infection | 12 (6.9%) | 13 (14.3%) | 25 (9.4%) | 14 (7.0%) |
| Hepatitis C virus infection | 35 (20.0%) | 17 (18.7%) | 52 (19.6%) | 49 (24.6%) |
| Nonalcoholic fatty liver disease | 7 (4.0%) | 2 (2.2%) | 9 (3.4%) | 4 (2.0%) |
| Cryptogenic | 10 (5.7%) | 3 (3.3%) | 13 (4.9%) | 14 (7.0%) |
| Autoimmune hepatic disease | 6 (3.4%) | 4 (4.4%) | 10 (3.8%) | 7 (3.5%) |
| Storage deficiency | 4 (2.3%) | 1 (1.1%) | 5 (1.9%) | 1 (0.5%) |
| Other | 2 (1.1%) | 4 (4.4%) | 6 (2.3%) | 4 (2.0%) |
| Biochemistry | ||||
| Albumin (g/L) | 34 (29–38) | 34 (29–37) | 34 (29–38) | 32 (28–35) |
| Bilirubin (μmol/L) | 28 (18–41) | 27 (17–50) | 27 (17–46) | 29 (19–50) |
| Creatinine (μmol/L) | 75 (65–93) | 74 (61–93) | 75 (64–93) | 79 (65–101) |
| INR | 1.4 (1.2–1.6) | 1.4 (1.3–1.7) | 1.4 (1.3–1.6) | 1.4 (1.2–1.7) |
| Sodium (mmol/L) | 138 (135–140) | 137 (134–140) | 138 (135–140) | 136 (132–138) |
| Platelets (x 109/L) | 115 (78–184) | 132 (94–180) | 124 (82–183) | 125 (88–190) |
| Urea (mmol/L) | 5.1 (4.1–6.8) | 5.8 (4.5–7.8) | 5.3 (4.2–7.0) | 6.1 (4.3–8.2) |
| MELD | 13 (10–15) | 13 (10–17) | 13 (10–16) | 13 (11–17) |
| MELD-Na | 15 (12–20) | 16 (13–21) | 15 (12–20) | 18 (14–22) |
| MELD 3.0 | 14 (11–18) | 15 (12–19) | 14 (11–18) | 16 (13–20) |
| Child-Pugh score | ||||
| Median score | 8 (7–9) | 8 (7–9) | 8 (7–9) | 9 (8–10) |
| A | 29 (16.6%) | 8 (8.8%) | 37 (13.9%) | 8 (4.0%) |
| B | 114 (65.1%) | 62 (68.1%) | 176 (66.2%) | 117 (58.8%) |
| C | 32 (18.3%) | 21 (23.1%) | 53 (19.9%) | 74 (37.2%) |
| Encephalopathy | ||||
| HE previously | 39 (22.3%) | 29 (31.9%) | 68 (25.6%) | 58 (29.2%) |
| HE at baselineb | 7 (4.0%) | 3 (3.3%) | 10 (3.8%) | 16 (8.0%) |
| Diuretics (mg) | ||||
| K-sparing drugs | 100 (50–100) | 100 (50–200) | 100 (50–100) | 50 (0–150) |
| Loop-diuretics | 40 (0–40) | 40 (0–80) | 40 (0–50) | 9 (0–50) |
| Last paracentesis (days) | 130 (30–398) | 23 (14–53) | 41 (19–180) | 19 (9–40) |
| <4 weeks | 17 (9.7%) | 42 (46.2%) | 59 (22.2%) | 115 (57.8%) |
| 4–12 weeks | 15 (8.6%) | 22 (24.2%) | 37 (13.9%) | 54 (27.1%) |
| >12 weeks | 44 (25.1%) | 12 (13.2%) | 56 (21.1%) | 12 (6.0%) |
| Never | 99 (56.6%) | 15 (16.5%) | 114 (42.9%) | 18 (9.1%) |
| Paracenteses the past year | 0 (0–1) | 3 (1–6) | 0 (0–2) | 4 (1–8) |
| 0 | 118 (67.4%) | 17 (18.7%) | 135 (50.8%) | 21 (10.7%) |
| 1–3 | 56 (32.0%) | 33 (36.3%) | 89 (33.5%) | 70 (35.7%) |
| 4–12 | 1 (0.6%) | 30 (33.0%) | 31 (11.7%) | 77 (39.3%) |
| >12 | 0 (0.0%) | 11 (12.1%) | 11 (4.1%) | 28 (14.3%) |
| Ascites accumulation | 35 (20.0%) | 66 (72.5%) | 101 (38.0%) | 171 (85.9%) |
| Ascites discomfort score (0–100) | 18 (5–46) | 39 (7–53) | 24 (5–48) | 41 (10–71) |
| Refractory type | ||||
| Diuretic-resistant | NA | NA | NA | 134 (67.3%) |
| Diuretic-intractable | NA | NA | NA | 61 (30.6%) |
| Both | NA | NA | NA | 1 (0.5%) |
| Unknown | NA | NA | NA | 3 (1.5%) |
| Time since ascites debut (months) | 13 (3–37) | 12 (5–32) | 12 (4–35) | 13 (6–31) |
| Ascites location | ||||
| Abdominal | 40 (100%) | 65 (98.5%) | 105 (99.1%) | 170 (98.8%) |
| Thoracic | 0 (0%) | 1 (1.5%) | 1 (0.9%) | 1 (0.6%) |
| Both | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.6%) |
| Ascites-diuretic groupc | ||||
| Ascites acc., no diu. treatment | 4 (2.3%) | 7 (7.7%) | 11 (4.1%) | 72 (36.2%) |
| Ascites acc., any diu. treatment | 31 (17.7%) | 59 (64.8%) | 90 (33.8%) | 99 (49.8%) |
| No ascites acc. | 140 (80.0%) | 25 (27.5%) | 165 (62.0%) | 28 (14.1%) |
| Treatments within trial period | ||||
| Liver transplantation | 7 (4.0%) | 7 (7.7%) | 14 (5.3%) | 12 (6.0%) |
| TIPS insertion | 1 (0.6%) | 3 (3.3%) | 4 (1.5%) | 7 (3.5%) |
| 1-year mortality | 24 (14.9%) | 10 (11.0%) | 34 (12.8%) | 57 (28.6%) |
- Note: The data in this table represent medians with interquartile ranges or numbers with proportions.
- Abbreviations: acc., accumulation; diu., diuretic; HE, hepatic encephalopathy; INR, international normalized ratio; K-sparring drugs, potassium-sparing drugs; NA, not applicable; TIPS, transjugular intrahepatic portosystemic shunt.
- a Autoimmune hepatic disease includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis. Storage deficiency includes hemochromatosis and Wilson disease. Other includes hepatitis D virus infection, alpha-1 antitrypsin deficiency, cardiogenic cirrhosis, and toxic hepatitis.
- b Grade 1 hepatic encephalopathy according to the West-Haven classification.
- c The three groups rely on the combination of diuretic therapy and whether the patients have ascites accumulation defined as paracentesis within the past 3 months and/or at study inclusion. The group without ascites accumulation includes patients on monotherapy with sodium restrictive diet as well as patients receiving any diuretic treatment regimen.
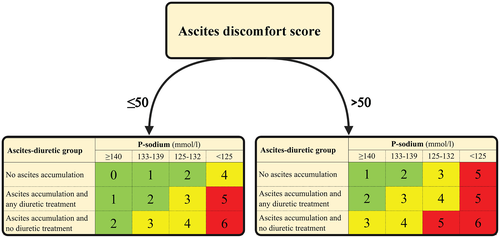
Cirrhotic Ascites Severity score
Table 2 provides the univariable and multivariable odds ratios for the candidate ascites-related variables. The prediction model included a triad of patient-reported, clinical, and biochemical variables ultimately creating the seven risk scores (scored 0–6) in the Cirrhotic Ascites Severity (CIRAS) model (Figure 1).
| Risk factors | Univariable analyses | Multivariable analyses | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | β | p-Value | OR (95% CI) | β | p-Value | |
| Ascites discomfort score (continuous score 0–100) | 1.01 (1.00–1.02) | 0.01 | 0.002 | — | — | — |
| Ascites discomfort score (>50 vs. ≤50) | 2.02 (1.25–3.25) | 0.7 | 0.004 | 1.54 (0.92–2.55) | 0.43 | 0.097 |
| Ascites-diuretic groups: | ||||||
| No ascites accumulation | 1.00 (reference) | — | — | 1.00 (reference) | — | — |
| Ascites accumulation and any diuretic treatment | 1.63 (0.94–2.84) | 0.49 | 0.081 | 1.41 (0.80–2.49) | 0.35 | 0.23 |
| Ascites accumulation and no diuretic treatment | 3.61 (1.95–6.67) | 1.28 | <0.001 | 2.59 (1.36–4.96) | 0.95 | 0.004 |
| P-sodium, mmol/L (continuous) | 0.90 (0.86–0.94) | −0.10 | <0.001 | — | — | — |
| P-sodium categories: | ||||||
| ≥140 mmoL/L | 1.00 (reference) | — | — | 1.00 (reference) | — | — |
| 133–139 mmoL/L | 1.70 (0.88–3.29) | 0.53 | 0.113 | 1.63 (0.84–3.17) | 0.49 | 0.149 |
| 125–132 mmoL/L | 3.81 (1.80–8.05) | 1.34 | <0.001 | 3.05 (1.41–6.57) | 1.12 | 0.004 |
| <125 mmoL/L | 11.1 (3.07–40.1) | 2.41 | <0.001 | 6.84 (1.81–25.9) | 1.92 | 0.005 |
- Note: All variables selected for the multivariable logistic regression model were independent predictors of 1-year mortality.
- Abbreviations: OR, odds ratio; β, regression coefficient.
Validation cohort
The validation cohort displayed a similar cirrhosis etiology distribution and baseline biochemistry profile as the development cohort, but the patients in the validation cohort were characterized by more severe ascites. The 1-year all-cause mortality was 25.8% (95% confidence interval [CI]: 22.6%–29.2%) in the validation cohort, which was higher than the development cohort with a 1-year all-cause mortality of 19.6% (95% CI: 16.1%–23.5%) (Table 3).
| Development cohort (n = 465) | Validation cohort (n = 697) | |
|---|---|---|
| Age (year) | 56 (50–63) | 58 (51–64) |
| Sex (male) | 322 (69%) | 486 (70%) |
| Ethnicity | ||
| Black | 6 (1.3%) | 6 (0.9%) |
| Caucasian/White | 428 (92.0%) | 645 (92.5%) |
| Asian | 21 (4.5%) | 20 (2.9%) |
| Other | 10 (2.2%) | 26 (3.7%) |
| Etiology of cirrhosisa | ||
| Alcohol | 313 (67.3%) | 485 (69.6%) |
| Hepatitis B virus infection | 39 (8.4%) | 57 (8.2%) |
| Hepatitis C virus infection | 101 (21.7%) | 144 (20.7%) |
| Nonalcoholic fatty liver disease | 13 (2.8%) | 17 (2.4%) |
| Cryptogenic | 27 (5.8%) | 45 (6.5%) |
| Autoimmune hepatic disease | 17 (3.7%) | 16 (2.3%) |
| Storage deficiency | 6 (1.3%) | 7 (1.0%) |
| Other | 10 (2.2%) | 14 (2.0%) |
| Biochemistry | ||
| Albumin (g/L) | 33 (29–36) | 33 (29–38) |
| Bilirubin (μmol/L) | 28 (18–46) | 27 (15–43) |
| Creatinine (μmol/L) | 78 (65–95) | 79 (64–97) |
| INR | 1.4 (1.3–1.6) | 1.4 (1.3–1.6) |
| Sodium (mmol/L) | 137 (134–139) | 136 (134–139) |
| Platelets (×109/L) | 125 (87–186) | 130 (92–191) |
| Urea (mmol/L) | 5.6 (4.3–7.7) | 6.1 (4.3–8.2) |
| MELD | 13 (11–16) | 13 (11–16) |
| MELD-Na | 16 (13–21) | 16 (13–21) |
| MELD 3.0 | 15 (12–19) | 15 (11–19) |
| Child-Pugh score | ||
| Median score | 8 (7–10) | 8 (7–9) |
| A | 45 (10%) | 53 (8%) |
| B | 293 (63%) | 471 (68%) |
| C | 127 (27%) | 173 (25%) |
| Encephalopathy | ||
| HE previously | 126 (27%) | 171 (25%) |
| HE at baselineb | 26 (6%) | 50 (7%) |
| Diuretics (mg) | ||
| K-sparing drugs | 100 (0–100) | 100 (0–100) |
| Furosemide | 34 (0–50) | 25 (0–40) |
| Ascites: | ||
| Last paracentesis (days) | 25 (13–63) | 20 (11–48) |
| Never drained | 132 (28%) | 124 (18%) |
| Paracenteses the past year | 1 (0–5) | 3 (1–6) |
| Ascites accumulation | 272 (58%) | 512 (73%) |
| Ascites discomfort score (0–100) | 32 (6–58) | 28 (6–56) |
| Ascites classification | ||
| Diuretic-responsive | 175 (38%) | 187 (27%) |
| Recidivant | 91 (20%) | 137 (20%) |
| Refractory | 199 (43%) | 373 (54%) |
| Time since ascites debut (months) | 13 (5–33) | 15 (5–36) |
| Abdominal ascites location | 275 (99%) | 508 (99%) |
| Ascites-diuretic groupc | ||
| Ascites accumulation and no diuretics | 83 (18%) | 168 (24%) |
| Ascites accumulation and any diuretic treatment | 189 (41%) | 344 (49%) |
| No ascites accumulation | 193 (42%) | 185 (27%) |
| Treatments within trial period | ||
| Liver transplantation | 26 (5.6%) | 37 (5.3%) |
| TIPS insertion | 11 (2.4%) | 8 (1.1%) |
| 1-year mortality | 91 (19.6%) | 180 (25.8%) |
- Note: The data in this table represent medians with interquartile ranges or numbers with proportions.
- a Autoimmune hepatic disease includes autoimmune hepatitis, primary sclerosing cholangitis, and primary biliary cholangitis. Storage deficiency includes hemochromatosis and Wilson disease. Other includes hepatitis D virus infection, alpha-1 antitrypsin deficiency, cardiogenic cirrhosis, and toxic hepatitis.
- b Grade 1 hepatic encephalopathy according to the West-Haven classification.
- c The three groups rely on the combination of diuretic therapy and whether the patients have ascites accumulation defined as paracentesis within the past 3 months and/or at study inclusion. The group without ascites accumulation includes patients on monotherapy with sodium restrictive diet as well as patients receiving any diuretic treatment regimen.
Discrimination and calibration
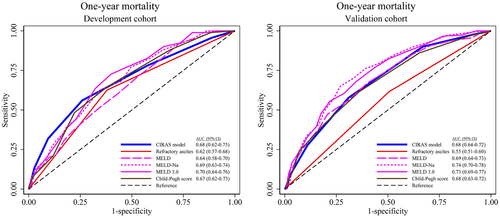
In the development cohort, the AUROC of the CIRAS model for 1-year all-cause mortality was 0.68 (95% CI: 0.62–0.75), which was better than the binary classification into refractory and diuretic-responsive ascites with an AUROC of 0.62 (95% CI: 0.57–0.68) (Figure 2A). Also in the validation cohort, the AUROC for 1-year all-cause mortality was 0.68 (95% CI: 0.64–0.72) for the CIRAS model and again was better than the binary ascites classification that gave an AUROC of 0.55 (95% CI: 0.51–0.60) (Figure 2B). The AUROC of the CIRAS model was similar to the Model for End-Stage Liver Disease (MELD), MELD-Na, MELD 3.0, and the Child-Pugh (CP) score in the development cohort, and in the validation cohort only MELD-Na and MELD 3.0 performed slightly better than the CIRAS model (Figure 2B). In the validation cohort we also explored the CIRAS model's performance within the groups with refractory and diuretic-responsive ascites. The AUROC for 1-year mortality was 0.73 (95% CI: 0.66–0.80) for patients with diuretic-responsive ascites and 0.62 (95% CI: 0.56–0.68) for patients with refractory ascites. As the median time from ascites onset was 13 months, we also performed a subgroup analysis exploring the predictive performance of the CIRAS model in patients with less than 6 months from ascites onset to study enrollment. The AUROC for 1-year mortality was 0.67 (95% CI: 0.53–0.81) in the development cohort (136 patients) and 0.72 (95% CI: 0.65–0.80) in the validation cohort (186 patients).
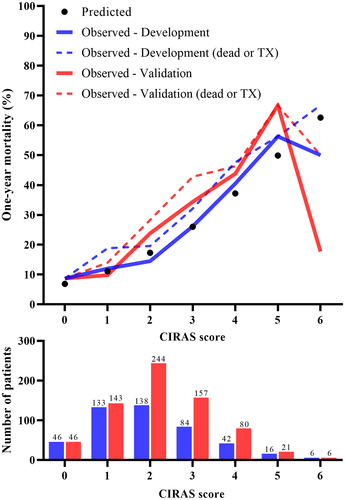
Next, we examined the calibration of the CIRAS model. The observed 1-year mortality risks in the development and validation cohorts agreed with the predicted risks, particularly for CIRAS model scores 0 to 5. Only few patients achieved a score of 6, of whom 25% underwent liver transplantation (Figure 3). Moreover, the CIRAS model performed well in calibration tests (Supplementary Information S6).
Sensitivity analyses
In a multivariable Fine and Gray regression analysis with liver transplantation treated as a competing risk to death, the β coefficients remained largely unchanged compared with the multivariable logistic regression analysis used previously. The β coefficients also remained unchanged when we assessed either liver transplantation or death after 1 year as outcome in a multivariable logistic regression analysis. In addition, discrimination was unaltered by the statistical approach used to derive the CIRAS model (Supporting Information S7 and Table S6).
Risk stratification with CIRAS score grades
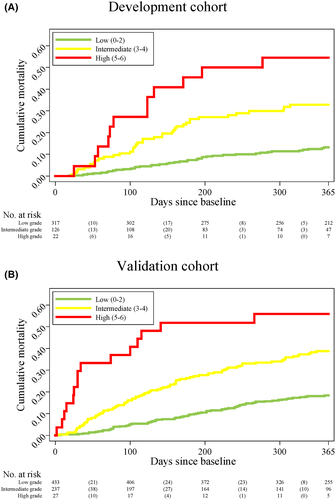
We introduced three CIRAS model grades. Model scores from 0–2, 3–4, and 5–6 were classified as low, intermediate, and high-grade ascites, respectively (Supporting Information S7 and Figure S2). In the development cohort, the 1-year mortality risk was 13% (95% CI: 9%–17%) for patients with low-grade ascites, 31% (95% CI: 23%–40%) for patients with intermediate-grade ascites, and 55% (95% CI: 32%–76%) for patients with high-grade ascites (Figure 4A). These risks largely persisted in the validation cohort, in which the corresponding 1-year mortality risks were 18% (95% CI: 14%–21%), 38% (95% CI: 31%–44%), and 56% (95% CI: 35%–75%), respectively (Figure 4B).
DISCUSSION
The main achievement of the present study is the development of the CIRAS model to predict 1-year all-cause mortality for patients with all degrees of cirrhotic ascites. The model is simple to use and provides patient-centered management of ascites by including only easily obtainable patient-reported, clinical, and biochemical data that relate directly to ascites: ascites discomfort score, ascites accumulation, diuretic treatment, and P-sodium. The model shows better survival discriminatory ability than the clinical standard classification into refractory or diuretic-responsive ascites in both the development and validation cohorts. Thus, the CIRAS model outperforms the standard binary refractory ascites classification and may replace the standard classification as the preferred assessment tool for clinicians in their management of patients with all stages of cirrhotic ascites.
The standard binary refractory ascites classification was developed to guide clinicians for timely evaluation of patient eligibility for invasive ascites treatments or liver transplantation, as well as to terminate ineffective and potentially harmful diuretic treatments and intensify the monitoring of renal and liver biochemistry, nutrition, and quality-of-life measures.[4] The classification was not set up for prognostic information, and particularly not so for all degrees of ascites. Furthermore, as ascites often worsens over time and has a long career before it reaches the refractory stage, there is a need for another classification system on which to base the clinical decisions earlier in the course of ascites. Therefore, a major strength of the CIRAS model is its ability to identify all patients with ascites by their 1-year mortality risk, enabling earlier pharmacological treatment optimization and improving the preparation time for invasive ascites treatments, including transplantation. This is further supported by the good discriminatory performance of the CIRAS model in the subgroup of diuretic-responsive patients. In addition to the clinical and biochemical variables, the CIRAS model includes ascites-related quality of life, which has also previously been demonstrated to be an independent predictor of mortality.[11] This paper disseminates the data on patient-reported ascites discomfort from the satavaptan trials.[7] As such, the CIRAS model brings a different perspective into the clinical assessment of patients with cirrhotic ascites.
All-cause mortality after 1 year was chosen as outcome. We believe that all-cause mortality is relevant and easier to communicate to patients than a composite endpoint of death or liver transplantation, which depends on access to liver transplantation. One year was considered a clinically relevant time frame, as cirrhosis decompensation markedly reduces survival. However, shorter follow-up time may be clinically relevant too. Therefore, we included cumulative incidence of mortality curves for the CIRAS model grades. These curves separate early, so we would probably have obtained similar results with an even shorter follow-up duration. This may illustrate the meaningfulness of the model. Additionally, in a logistic regression analysis with death or liver transplantation after 1 year as outcome, the β coefficients of the candidate variables and the discriminatory ability of the CIRAS model were unchanged. Similarly, the Fine and Gray regression analysis with liver transplantation treated as a competing risk to death did not change our results. This proves the value of a simple multivariable logistic regression analysis when complete outcome data are available and justifies its use in this work.
The limitations of the current study are that the inclusion in clinical trials usually requires the candidates to fulfill a list of prespecified criteria, limiting the generalizability of the findings. For example, candidates were ineligible if they had a recent variceal bleeding or spontaneous bacterial peritonitis, ongoing hepatic encephalopathy, an open functioning TIPS, or advanced hepatocellular carcinoma—all criteria that exclude patients with an a priori high mortality from the current work. This equates to a median time from onset of ascites of 13 months. However, subgroup analyses in patients with less than 6 months since ascites debut revealed similar discrimination ability for the CIRAS model. As a result, patients with refractory ascites in the development cohort showed a 1-year all-cause mortality of 29%, which is lower than reported in previous studies.[5, 12, 13] Second, renal sodium excretion is a mainstay in the assessment of patients with ascites, but baseline urine analyses were unavailable. Finally, the distribution of cirrhosis etiologies has changed, and antiviral treatments have improved since the satavaptan trials were conducted, which may affect the translatability of our results to today's spectrum of patients with cirrhosis.
For validation of the prediction model, we relied on the patients treated with satavaptan from the same trials whose placebo arms were used for the model development. However, the design of the trials dictated more patients with severe ascites be allocated to the active arms, so that the validation cohort had higher ascites severity and 1-year mortality (Table 3).[7] However, the CIRAS model showed the same performance in the validation cohort, in favor of the robustness of the model. Likewise, participants treated with TIPS insertion (1.8%) or liver transplantation (5.2%) during the trials contributed with 1-year follow-up data. Although patients are sicker in the validation cohort, we believe that data drift is not a significant problem. Because there is still close to a 50/50 distribution of refractory/diuretic-responsive patients in both cohorts, the discriminative ability of that information is not biased in the validation cohort (which it would have been if nearly all patients in the validation cohort had refractory ascites). Furthermore, with a small dataset for model development, the risk of overfitting was considered but mitigated by the small set of candidate variables and a similar performance in the validation cohort. Nevertheless, future validation in a truly external cohort is highly desired.
Our concept and goal were to develop an easy-to-use model that relies solely on simple ascites-related clinical, biochemical, and symptomatic variables, and we did not include biomarkers of liver or renal dysfunction, nor age, sex, ethnicity, or etiology of liver disease. Hence, such general data have already been evaluated in the development of the SAM (Severe Ascites Mortality) score, which was neither easy to use nor focused on ascites-related variables.[14] The CIRAS model, like the standard refractory ascites classification, applies exclusively to patients with cirrhotic ascites. However, the CIRAS model is superior to the refractory ascites term, as it is both descriptive and predictive. Well-established predictive scores such as the CP score and the MELD, MELD-Na, and MELD 3.0[15-17] are useful for all patients with cirrhosis, and supplementation of them with the CIRAS model is useful for the management of those who also have ascites. An AUROC of 0.68 implies that the CIRAS model alone may not be good enough to make decisions about, for example, liver transplantation. Hence, the CIRAS model is not intended as an alternative to the existing generic scores, but as a supplement that brings the patient in focus and demands the physician to evaluate diuretic therapy and quality of life. As such, the strengths of the CIRAS model are its ability to describe patients with all ascites severities and to predict 1-year mortality without clinically meaningful inferiority to MELD and its variants. We therefore advocate for implementation of the CIRAS model, as the CIRAS model score may change and motivate treatment optimization without changes in the generic scores. This approach is supported by recent reports of inconsistent predictive abilities of hyponatremia, the CP score, and the MELD score in patients with ascites.[14, 18] Thus, the CIRAS model fulfills the unmet need for a model developed with the core intention of both describing the whole spectrum of ascites and predicting the prognosis of patients with cirrhotic ascites.
CONCLUSIONS
We developed the CIRAS model combining P-sodium, ascites accumulation, diuretic treatment, and patient-reported ascites discomfort to describe the ascites severity and predict 1-year all-cause mortality for patients with all grades of cirrhotic ascites. The CIRAS model had better prognostic ability than today's labels of “refractory ascites” and “diuretic-responsive ascites” in both the development and validation cohort. This model may be a promising assessment tool to guide clinicians in their management of patients with cirrhotic ascites.
AUTHOR CONTRIBUTIONS
Study concept: Rasmus Hvidbjerg Gantzel, Niels Kristian Aagaard, Hendrik Vilstrup, Henning Grønbæk, Hugh Watson, and Peter Jepsen. Statistical analyses: Rasmus Hvidbjerg Gantzel and Peter Jepsen. Visualization: Rasmus Hvidbjerg Gantzel. Manuscript draft: Rasmus Hvidbjerg Gantzel. Writing, reviewing, and editing: Rasmus Hvidbjerg Gantzel, Niels Kristian Aagaard, Hendrik Vilstrup, Henning Grønbæk, Hugh Watson, and Peter Jepsen. Supervision: Henning Grønbæk and Peter Jepsen. All authors have approved the final manuscript.
FUNDING INFORMATION
Supported by a research grant from ADS AIPHIA Development Services AG (Switzerland).
CONFLICT OF INTEREST
H.W. owns shares in Sanofi. H.G. has received research grants from Intercept, AbbVie, ARLA Food for Health, and the NOVO Nordisk Foundation and consultant fees from Pfizer and Novo Nordisk Foundation.