Patterns of kidney dysfunction in acute-on-chronic liver failure: Relationship with kidney and patients’ outcome
Funding information
Supported by PFIS (FI17-00115), granted by Instituto de Salud Carlos III; Contratos Río Hortega (CM19/00044), granted by Instituto de Salud Carlos III; “Emili Letang” award granted by Hospital Clínic de Barcelona; “Ajut per a la Iniciació a la Recerca” award granted by Societat Catalana de Digestologia; Plan Nacional Investigación Cientifíca, Desarrollo e Innovación Tecnológica (I + D + I) and cofunded by Instituto Carlos III–Subdirección General de Evaluación and the European Regional Development Fund (PI18/00727); FIS PI20/00579 funded by Instituto de Salus Carlos III and co-financed by the European Union.
Abstract
Impairment of kidney function is common in acute-on-chronic liver failure (ACLF). Patterns of kidney dysfunction and their impact on kidney and patient outcomes are ill-defined. Aims of the current study were to investigate patterns of kidney dysfunction and their impact on kidney and patient outcomes in patients with acute decompensation (AD) of cirrhosis, with or without ACLF. This prospective study includes 639 admissions for AD (232 with ACLF; 407 without) in 518 patients. Data were collected at admission and during hospitalization, and patients were followed up for 3 months. Urine samples were analyzed for kidney biomarkers. Most patients with ACLF (92%) had associated acute kidney injury (AKI), in most cases without previous chronic kidney disease (CKD), whereas some had AKI-on-CKD (70% and 22%, respectively). Prevalence of AKI in patients without ACLF was 35% (p < 0.001 vs. ACLF). Frequency of CKD alone was low and similar in both groups (4% and 3%, respectively); only a few patients with ACLF (4%) had no kidney dysfunction. AKI in ACLF was associated with poor kidney and patient outcomes compared with no ACLF (AKI resolution: 54% vs. 89%; 3-month survival: 51% vs. 86%, respectively; p < 0.001 for both). Independent predictive factors of 3-month survival were Model for End-Stage Liver Disease–Sodium score, ACLF status, and urine neutrophil gelatinase–associated lipocalin (NGAL). AKI is almost universal in patients with ACLF, sometimes associated with CKD, whereas CKD alone is uncommon. Prognosis of AKI depends on ACLF status. AKI without ACLF has good prognosis. Best predictors of 3-month survival are MELD-Na, ACLF status, and urine NGAL.
INTRODUCTION
Impairment in kidney function is very common in cirrhosis, particularly in patients with decompensated cirrhosis, with ascites, gastrointestinal bleeding, hepatic encephalopathy, and/or bacterial infections.[1, 2] A large body of evidence indicates that impairment of kidney function, whichever criteria are used in its definition, is associated with poor prognosis.[2, 3] In fact, kidney impairment is one of the worst prognostic indicators of cirrhosis.[4-6]
Investigation on pathogenesis and clinical relevance of kidney function in cirrhosis has been spurred by the recent introduction of the term acute kidney injury (AKI) to better define the acute impairment of kidney function. AKI definition and diagnostic criteria were first established for intensive care unit (ICU) patients, particularly after cardiac surgery, but has been validated for use in patients with cirrhosis.[2] Its main innovation compared with previous definitions consists of taking into account small acute increases in serum creatinine concentration (of only 0.3 mg/dl) as indicative of acute impairment in kidney function, thereby allowing detection of kidney function impairment in very early stages compared with previous definitions. The use of AKI in clinical practice in patients with cirrhosis is now supported by clinical practice guidelines of main international societies of hepatology.[7-11]
This terminology and classification of AKI coexists now with the recent description of a syndrome that may develop in patients with cirrhosis, known as acute-on-chronic liver failure (ACLF), which is characterized by failure of the liver and/or extrahepatic organs, including the kidneys.[12, 13] Interestingly, the criteria used for definition of kidney dysfunction or failure in ACLF is based on fixed values of serum creatinine (either 1.5 or 2 mg/dl, respectively) rather than on the more dynamic definition of AKI. Moreover, recent studies indicate that chronic kidney disease (CKD), characterized by a chronic impairment of kidney function of variable severity, is common in patients with decompensated cirrhosis and is associated with poor outcome.[10, 11] Although kidney failure is one of the most common organ failures in ACLF, information about the patterns of kidney disfunction and its outcome in patients with ACLF is very limited.
Therefore, there is need for better understanding of the interrelationship between AKI and CKD and ACLF in patients with cirrhosis. Thus, the current study aimed to investigate the patterns of kidney dysfunction in a large prospective series of patients with cirrhosis admitted to hospital with acute decompensation (AD) of the disease with and without ACLF. The primary endpoints of the study were kidney and patient’s outcome as well as the role of kidney biomarkers in outcome prediction.
METHODS
Patient population and study design
The current study was performed in a cohort of 639 patients with cirrhosis admitted to the Liver Unit of the Hospital Clínic of Barcelona for management of an AD of the disease during a 32-month period. The number of individual patients was 518, because some patients were admitted more than once during the study period. All patients were included in a prospective database of all consecutive patients with cirrhosis admitted to hospital for treatment of an AD of the disease with biobank collection. The diagnosis of cirrhosis was based on liver biopsy or combination of clinical, biochemical, ultrasonography, and endoscopy findings.
Exclusion criteria were age < 18 or > 85 years, previous kidney/liver transplantation, hepatocellular carcinoma outside the Milan criteria or any other advanced malignancy, severe comorbidities (e.g., congestive heart failure NYHA (New York Heart Association) > 2, chronic obstructive pulmonary disease GOLD (Global Initiative for Chronic Obstructive Lung Disease) > 2, chronic kidney disease requiring dialysis), human immunodeficiency virus infection, and lack of written inform consent. Patients admitted to hospital for scheduled therapeutic or diagnostic procedures were not included. Disposition of patients is shown in Figure S1.
The study was approved by the institutional review board of our center (HCB/2018/0402), and all patients or relatives, regardless of whether patients had hepatic encephalopathy, signed a written informed consent to participate in the study. The creation of the biobank collection was presented and approved by the Ethics Committee (registration numbers HCB/2015/0653 and HCB/2016/0390). All samples were stored at the biobank as required by Spanish legislation. Sample storage and use was done following current national and institutional guidelines.
Study objectives
The study aimed to investigate the patterns of kidney failure in ACLF, specifically (1) to assess the prevalence and characteristics of AKI in patients with ACLF, compared with those of patients without ACLF; (2) to evaluate the prevalence and characteristics of CKD, either alone or with associated AKI, in patients with and without ACLF; (3) to evaluate the dynamics of kidney biomarkers in these patients; and (4) to investigate kidney and patient outcomes according to the pattern of kidney failure in patients with ACLF and compare with those of patients without ACLF.
Study protocol and patient assessment
Demographic, clinical, and analytical data were collected prospectively at hospital admission and at regular intervals during hospitalization. Blood work was done at least twice a week in patients admitted in the regular ward and more frequently in those admitted in the ICU. AKI and ACLF were assessed concomitantly when analytical data were available. Patients were followed up for at least 3 months after discharge. Urine samples were collected in patients with AKI at the time of inclusion in the study and at days 3, 7, and 14 (if patients were still in the hospital), to measure urine biomarkers, including albumin, interleukin-18 (IL-18), β-2 microglobulin, and neutrophil gelatinase–associated lipocalin (NGAL).
Definitions
AKI
AKI was defined according to internationally accepted criteria for patients with cirrhosis as an increase in serum creatinine ≥ 0.3 mg/dl with respect to baseline.[1, 2] The presence of AKI was assessed at hospital admission and throughout hospitalization. For the diagnosis of AKI at admission, the baseline serum creatinine used was the most recent stable value available in the previous 3 months before admission, as reported previously.[2]
AKI was categorized in four stages (1A, 1B, 2, or 3) according to modified European Association for the Study of the Liver clinical guidelines, which divide patients with stage 1 into two categories based on serum creatinine value at diagnosis of AKI (1A: < 1.5 mg/dl and 1B: ≥ 1.5 mg/dl).[4-8]
AKI was also classified into four types according to the cause of kidney injury: acute tubular necrosis (ATN), Hepatorenal syndrome AKI (HRS-AKI), hypovolemia-induced AKI, or miscellaneous. The type of AKI was adjudicated by the investigator’s team at the time of diagnosis of AKI on the basis of pre-established definitions (see subsequently).
Resolution of AKI
Resolution of AKI was defined as the decrease of serum creatinine to a value not greater than 0.3 mg/dl of baseline value during hospital stay, which means that the definition of AKI was no longer met.[2]
Progression of AKI
Progression of AKI was defined as an increase of at least one AKI stage during hospitalization: from stage 1A to stage 1B or greater; from stage 1B to stage 2 or 3; or from stage 2 to stage 3 during the hospitalization. Patients with AKI stage 3 who subsequently required renal replacement therapy (RRT) were not considered as having progression of AKI.[4]
Type of AKI
AKI was classified into four types: (1) hypovolemia-induced AKI, when there was history of fluid losses (e.g., overdiuresis, diarrhea) or bleeding (e.g., gastrointestinal bleeding) within the days before development of AKI; (2) HRS-AKI, defined according to the ICA (International Club of Ascites) criteria[2]; (3) ATN, defined according to a combination of clinical and laboratory data, as reported previously[4]; and (4) miscellaneous conditions.
CKD
CKD was defined according to the Kidney Disease Improving Global Outcomes guidelines as an estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2 during at least 3 months.[10] All eGFR values were calculated using the abbreviated modified diet in renal disease (MDRD-4) equation: eGFR (ml/min/1.73 m2) = 186 × creatinine−1.154 × age−0.203 × (0.742 if female) × (1.212 if black).[11]
ACLF
ACLF was defined and graded according to the CANONIC definition as follows: (1) ACLF grade 1: patients with single kidney failure (serum creatinine > 2 mg/dl), patients with single failure of the liver, coagulation, circulation, or respiration with serum creatinine from 1.5 to 1.9 mg/dl and/or mild to moderate hepatic encephalopathy, and patients with single cerebral failure with serum creatinine from 1.5 and 1.9 mg/dl; (2) ACLF grade 2: patients with two organ failures; and (3) ACLF grade 3: patients with three organ failures or more.[12, 13]
Analytical methods
Urine samples were centrifuged at 3000 rpm for 10 min within the first 4 h after collection, and the supernatant was stored at −80°. Urine NGAL, IL-18, albumin, and β-2 microglobulin were measured as previously reported.[14]
Statistical analysis
Comparisons of normally distributed continuous variables were made with Student t-test or analysis of variance. Comparisons of nonnormally distributed continuous variables were reported with Mann–Whitney U or Kruskal-Wallis tests. Results for continuous variables are expressed as median and interquartile range.
Categorical variables were reported as number and percentage and compared with chi-squared test with continuity correction or Fisher exact test, if appropriate. The 3-month survival was estimated by the Kaplan–Meier method and compared by means of log-rank test. For survival analysis, in patients who had more than one admission, only the first hospital admission was considered. Patients transplanted during follow-up were considered censored at time of transplantation. Multivariate Cox regression was performed to identify the independent predictors of 3-month survival, and the hazard ratios and their 95% confidence intervals were calculated. There was no specific calculation of the sample size. However, based on previous experience of studies in patients with cirrhosis, it was considered that more than 100 patients had to be included in the study to achieve a significant number of outcomes in terms of AKI resolution and 3-month mortality. Nonnormally distributed continuous variables were log-transformed to be included in multivariate models. When scores of liver disease were included in multivariate analyses, their components were excluded to avoid collinearity. The significance for all statistical tests was set at 0.05 two-tailed. The statistical analysis was performed using SPSS statistical package, version 23.0.
RESULTS
Baseline characteristics of patient population
The characteristics of patients at inclusion in the study are given in Table 1. As anticipated, most patients had advanced cirrhosis and severe liver failure, with high Child-Pugh and Model for End-Stage Liver Disease (MELD) scores. Diagnostic criteria of ACLF were met in 232 of the 639 admissions (36%). Of these 232 patients, 130 (56%) had grade I ACLF, 63 (27%) had grade II, and 39 (17%) had grade III. Frequency and type of organ failure in patients with ACLF are provided in Table S1.
| Variables | |
|---|---|
| Age (years) | 61 (54–68) |
| Gender (male) | 437 (68) |
| Etiology of cirrhosis | |
| Alcohol | 289 (45) |
| HCV | 152 (24) |
| NASH | 54 (9) |
| Othera | 144 (22) |
| Diabetes mellitus | 222 (35) |
| Chronic kidney diseaseb | 88 (14) |
| Ascites at admission | 424 (66) |
| Hepatic encephalopathy at admission | 178 (28) |
| Bacterial infection at admission | 339 (53) |
| Shock at admission | 73 (11) |
| Serum creatinine (mg/dl) | 1.2 (0.8–1.8) |
| Serum bilirubin (mg/dl) | 2.3 (1.1–4.9) |
| INR | 1.5 (1.3–1.8) |
| Serum sodium (mEq/L) | 136 (132–139) |
| Serum albumin (g/L) | 29 (25–33) |
| Platelets (cells × 103/μl) | 84 (53–132) |
| Leukocytes (cells × 103/μl) | 5.4 (3.8–8.2) |
| Blood polymophonuclears (cells × 103/μl) | 3.9 (2.4–6.3) |
| C-reactive protein (mg/dl) | 2 (0.8–4.4) |
| Mean arterial pressure (mm Hg) | 80 (72–89) |
| ACLF | 232 (36) |
| MELD score | 17 (13–22) |
| Child-Pughc | |
| Score | 8 (7–10) |
| Class A | 126 (20) |
| Class B | 290 (45) |
| Class C | 209 (33) |
- Note: Data are median (interquartile range) or number (percentage).
- a Other: Alcohol associated with HCV or HBV infection 52 (8%); cryptogenic cirrhosis 29 (5%), HBV infection 13 (2%), primary biliary cholangitis 18 (3%), autoimmune hepatitis 16 (2%), and other causes 16 (2%).
- b As defined by glomerular filtration rate < 60 ml/min/1.73 m2 for ≥ 3 months.
- c Available in 625 cases included in the study.
- Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; NASH, nonalcoholic steatohepatitis.
Frequency and characteristics of AKI and CKD and relationship with ACLF
Table 2 indicates the prevalence of the different patterns of kidney dysfunction, either AKI, AKI on CKD, or CKD, in all patients included in the study. Most patients with ACLF had associated AKI (214 of 232 patients, 92%). Of these, 163 had AKI without CKD, whereas 51 had AKI on top of CKD (acute-on-chronic kidney disease) (70% and 22% of patients with AKI, respectively). In contrast, few patients had ACLF with CKD alone or ACLF without either AKI or CKD (4% for both). However, the frequency of the different patterns of kidney dysfunction in patients without ACLF was markedly different. Prevalence of AKI in patients without ACLF was only 35% (vs. 92% of patients with ACLF; p < 0.001), with only 5% of no ACLF cases having AKI on top of CKD. Prevalence of isolated CKD in patients without ACLF was similar to that of patients with ACLF. Finally, almost two thirds of patients without ACLF had neither AKI nor CKD. A comparison of characteristics of patients with AKI with and without associated ACLF is found in Table S2.
| No ACLF (n = 407) | ACLF (n = 232) | p-Value | |
|---|---|---|---|
| AKI (all cases) | 142 (35%) | 214 (92%) | <0.001 |
| Not on CKD | 123 (30%) | 163 (70%) | 0.01 |
| On CKD | 19 (5%) | 51 (22%) | 0.01 |
| CKD | 11 (3%) | 9 (4%) | <0.001 |
| No AKI/no CKD | 254 (62%) | 9 (4%) | <0.001 |
- Abbreviation: CKD, chronic kidney disease.
There was a strong relationship between severity of AKI, as estimated by AKI stage, and association ACLF. In fact, stage 1A was more frequent in patients without ACLF compared to those with ACLF (53% vs. 12%, respectively), whereas stages 2 and 3 were more common in patients with ACLF than in those without (42% vs. 7%, respectively) (Table 3). As for the type of AKI, hypovolemia-induced AKI and HRS-AKI were slightly more common in patients without ACLF than in those with ACLF; remarkably, no case of ATN occurred in patients without ACLF versus 13% in patients with ACLF (Table 3).
| AKI without ACLF N = 142 | AKI with ACLF, N = 214 | p-value | |
|---|---|---|---|
| AKI stage | |||
| AKI 1 | 132 (93%) | 125 (59%) | <0.001 |
| AKI 1A/AKI 1B | 75 (53%)/57 (40%) | 25 (12%)/100 (47%) | |
| AKI 2 | 8 (6%) | 55 (26%) | |
| AKI 3 | 2 (1%) | 34 (16%) | |
| AKI type | |||
| Hypovolemia-induced | 84 (59%) | 85 (40%) | <0.001 |
| ATN | 0 | 28 (13%) | |
| HRS-AKI | 45 (32%) | 57 (25%) | |
| Other | 13 (9%) | 44 (21%) |
- a Patients with AKI on top of CKD were excluded from this analysis.
- Abbreviations: ATN, acute tubular necrosis; HRS, hepatorenal syndrome.
Kidney outcomes
Outcome of kidney function was much better in patients with AKI without ACLF than in those with associated ACLF. Notably, resolution of AKI occurred in most patients without ACLF, whereas progression of AKI was very uncommon (89% and 4%, respectively). In contrast, patients with AKI and associated ACLF had a much lower rate of resolution and higher rate of progression compared to those in patients without ACLF (54% and 28%, respectively) (Figure S2). There as an inverse relationship between kidney outcome and severity of ACLF, so that patients with ACLF grade III had a poorer kidney outcome compared with those of grade II, and patients with ACLF grade II had poorer kidney outcome compared to those with grade I (Table S3). Interestingly, resolution of AKI was more frequent in patients with ACLF with renal failure alone compared to patients with ACLF and renal failure associated with “extrarenal” organ failures (64% vs. 45%, respectively; p < 0.001). Resolution of AKI in patients with ACLF was unrelated to the presence of an underlying CKD (78% vs. 62% in patients with and without CKD, respectively; p = 0.15). In contrast, progression of AKI was lower in patients with AKI on CKD compared with AKI alone (16% vs. 32%, respectively; p = 0.04). Finally, outcome of kidney function was independent of whether AKI or ACLF was present at admission or developed during hospitalization, yet there was a trend for a lower resolution rate in patients who developed AKI during hospitalization than in those with AKI at admission (63% vs. 71%, respectively; p = 0.06).
Relationship of kidney biomarkers and AKI and ACLF
Kidney biomarker levels in patients with AKI categorized according to presence or absence of ACLF are provided in Table S4. The urinary levels of NGAL and albumin at time of AKI diagnosis and after 48 h were markedly different between the two groups of patients. In contrast, IL-18 and β2-microglobulin levels were statistically significant only after 48 h from the AKI diagnosis.
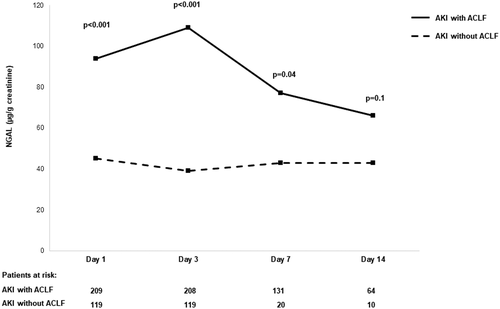
The time course of urinary NGAL (uNGAL) levels in patients with AKI during a 14-day period are shown in Figure 1. uNGAL levels at diagnosis of AKI were significantly higher in patients with AKI with associated ACLF compared to those of patients with AKI without associated ACLF and were statistically significant from day 1 to day 7 but not at day 14. IL-18 levels were significantly higher at day 3, and urinary albumin levels were significantly higher from day 1 to day 7 (Figure S3).
Patient survival
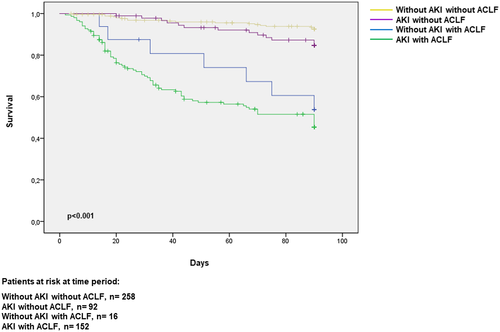
We next sought to determine the impact of different patterns of kidney dysfunction and their association with ACLF on patient’s survival. Overall, of the 518 patients included, 113 patients had died, 22 had been transplanted, 19 had been lost to follow-up, and 364 were alive at the end of the 3-month follow-up period. The corresponding figures in patients with AKI were 88, 15, 9, and 132, respectively. Patients with AKI at admission had better 3-month survival compared to those with AKI during hospitalization (60% vs. 42%, respectively; p < 0.0019). In patients with ACLF, mortality correlated with ACLF severity, but there were no differences between mortality when ACLF was present at admission versus ACLF that developed during hospitalization (data not shown). Patients with ACLF associated with AKI on CKD had a better survival compared to those with AKI without CKD (68% vs. 31%, respectively; p ≤ 0.001; Table S5).
Three-month survival probability curves of patients classified in four groups are shown in Figure 2. The highest probability of 3-month survival corresponded to patients without AKI with no associated ACLF, followed by patients with AKI but without ACLF (93% and 86%, respectively; p = 0.04), whereas the worst survival was that of patients with both AKI and ACLF (51%; p < 0.001). Patients with ACLF without AKI had an intermediate survival. Interestingly, in patients with ACLF, AKI stage was not associated with prognosis. In fact, 3-month survival probability of patients with ACLF was quite similar regardless of AKI stage (Figure 3). Only patients with AKI stage 1A had a slightly better probability of survival than those with stage 3 AKI.
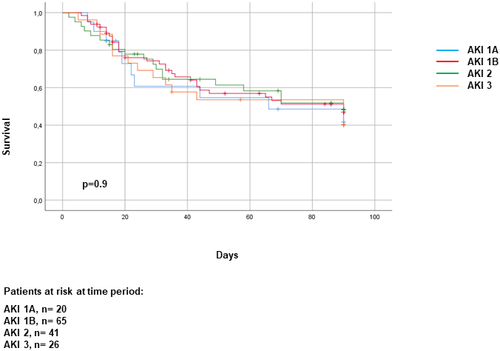
Because renal failure is included in the definition of ACLF, teasing out the relevance of AKI severity and that of ACLF in patient’s outcome is problematic. In a multivariate analysis, the presence of ACLF but not AKI stages was an independent predictive factor of 3-month survival. Moreover, patients with ACLF with renal and extrarenal organ failures had markedly worse survival compared to patients with ACLF and renal failure only (27% vs. 67%, respectively; number of patients 105 vs. 47, respectively; p < 0.001), whereas patients with ACLF with only extrarenal organ failures had an intermediate prognosis (44%, 16 patients), but the small number of the latter cases did not allow for representative comparisons between groups.
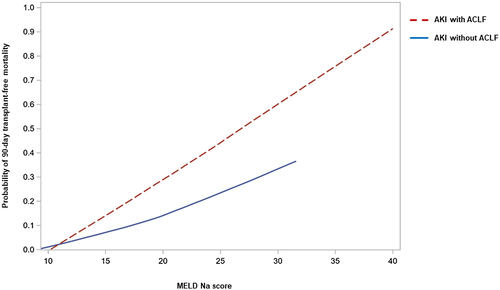
We then investigated factors predictive of survival in patients with AKI, with and without associated ACLF. Univariate analysis of factors predictive of survival at 3 months is found in Table S6. In the multivariate analysis, independent predictive factors of 3-month survival were MELD-Na score, presence of ACLF, and uNGAL measured at day 3 of AKI diagnosis (Table 4). Figure 4 shows a plot of the relationship between 90-day transplant-free mortality and MELD-Na score in patients with AKI categorized according to presence or absence of ACLF. For any given value of MELD-Na score, the probability of death was higher in patients with AKI associated with ACLF compared to that in patients with AKI without ACLF. Neither urine albumin nor IL-18 were independent predictors of survival. Three-month probability of survival of patients with AKI and ACLF categorized into two groups according to uNGAL values is shown in Figure S4.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Model 1 | |||
| Urine-NGAL at day 3 | 1.17 | (1.0–1.36) | 0.04 |
| MELD Na | 1.11 | (1.06–1.15) | <0.001 |
| ACLF | 2.22 | (1.02–4.85) | 0.05 |
| Model 2 (without MELD-Na) | |||
| Urine-NGAL at day 3 | 1.26 | (1.02–1.54) | 0.03 |
| Bilirubin | 1.06 | (1.03–1.09) | <0.001 |
| INR | 1.2 | (1.0–1.37) | 0.05 |
| ACLF | 2.78 | (1.30–5.93) | 0.009 |
- Note: Variables included in the equation: NGAL day 1, NGAL day 3, urinary albumin day 1, interleukin-18 day 1, interleukin-18 day 3, MELD Na, ACLF.
- Abbreviations: CI, confidence interval; HR, hazard ratio.
DISCUSSION
The results of the current study provide valuable clinical information on characteristics and patterns of kidney dysfunction in patients with decompensated cirrhosis with and without ACLF. The main findings of the study are as follows: (1) Most patients with ACLF have AKI, in some patients associated with CKD, whereas only a minority of patients have CKD alone; (2) severity of AKI is markedly greater in ACLF versus no ACLF; consistent with this, kidney outcome is markedly worse in patients with ACLF than in those without; (3) presence of AKI is associated with markedly impaired survival in ACLF, whereas survival is only slightly reduced in patients without ACLF; (4) ACLF status should be considered when using MELD-Na score in the assessment of prognosis in decompensated cirrhosis, because for the same MELD-Na value prognosis is worst if ACLF is present; and (5) urine NGAL is a very useful biomarker of prognosis in patients with cirrhosis and AKI.
One of the main findings of this study is that most (92%) patients with ACLF have acute impairment of kidney function, according to the definition of AKI. The 92% prevalence of AKI found in the current study is in contrast to the much lower prevalence of kidney failure reported in the CANONIC or NACSELD studies, which was 56% and 15%, respectively.[13-15] This discrepancy is due to the more severe criteria for definition of kidney failure used in both studies (serum creatinine > 2 mg/dl or need for RRT, respectively) compared with the definition of AKI, which is the standard definition of acute impairment of kidney function currently used worldwide.[1-3] Therefore, it is important to emphasize the message that kidney dysfunction is almost a universal feature of the ACLF syndrome. This finding is of major interest from pathogenic and therapeutic perspectives in ACLF. Other studies have reported a lower frequency of AKI in patients with ACLF; this was probably related to the use of different diagnostic criteria for ACLF other than those of the CANONIC study, or that baseline serum creatinine used for AKI diagnosis was that of admission and not from before hospitalization.[13, 15] It is well known that in a high proportion of patients, AKI is already present at hospital admission.[16-18] It is important to note that in the current cohort, one fourth of patients with ACLF developed AKI on top of CKD. Notably, AKI on CKD was associated with better kidney and patient outcomes compared with those of AKI alone, with a lower rate of progression of AKI and higher survival rate. The reason for these paradoxical findings is unknown and deserves investigation.[19, 20] Interestingly, the frequency of CKD alone in patients with ACLF was very low (4%), suggesting that, in practice, increased values of serum creatinine in a patient with ACLF should be considered most likely due to AKI and not to CKD. Finally, patients with AKI developing during hospitalization had a poor outcome compared to those with AKI present at admission, with lower (yet not significant) AKI resolution and poor survival.
AKI occurring in the setting of ACLF was more severe than that in patients without ACLF (AKI stage 2 and 3 occurred in 42% of patients with ACLF vs. only 7% of patients without). This could be due either to the fact that patients with more severe kidney impairment met the definition of ACLF, as mentioned previously, and/or to a higher intensity of the factor(s) driving kidney impairment in ACLF versus no ACLF. Consistent with this greater severity, kidney outcome was markedly worse in ACLF than in AD without ACLF, resolution of AKI being considerably lower and progression higher in patients with ACLF versus those without. Moreover, kidney outcome correlated inversely with ACLF grade.
It is well established that AKI is associated with poor prognosis in cirrhosis, and that presence of kidney failure in patients with ACLF portends a very poor outcome.[1, 3, 21-23] The current study increases the current understanding on AKI by identifying a specific group of patients with a particularly good prognosis (i.e., those patients with AD of cirrhosis with AKI but without ACLF). Three-month survival probability in these patients was greater than 90% and only slightly lower than that of their AD counterparts without AKI. In contrast, 3-month probability of survival of patients with AKI and associated ACLF was markedly lower (only 51%) and correlated inversely with ACLF grade. Most importantly, our study shows that the prognostic value of MELD-Na score in patients with AD of cirrhosis and AKI should be adjusted for the presence of ACLF. This is due to the fact that for any given value of MELD-Na score, 3-month transplant-free mortality is markedly higher in patients with ACLF compared to those without, the difference being more marked in patients with higher MELD-Na scores than those in greater need of transplant. These findings support the observation made by recent studies that patients with ACLF are underserved with the current MELD-Na system for organ allocation in liver transplantation and that a system should be designed to increase the transplant rate of patients with ACLF and ultimately their survival rate.[24]
The diagnosis of ACLF with the CANONIC criteria used in this study is based on presence of organ failures.[12, 13] In this regard, renal failure is defined when serum creatinine is above 2 mg/dl. Therefore, it is obvious that severity of AKI in patients with ACLF should be greater than that in patients without ACLF, as shown in the current study, which may be responsible, at least in part, for their poor outcome. On the other hand, patients with ACLF may have a number of organ failures other than renal failure, which may also account for their poor prognosis. Addressing the potential contribution of the kidney as well as that of “extrarenal” organs to patient’s outcome is therefore challenging. In a multivariate analysis, ACLF but not AKI stage was an independent predictive factor of survival, suggesting the relevance of extrarenal organs in determining prognosis. Moreover, as expected, patients with renal and “extra-renal” organ failures had worst outcome compared with those who had renal failure alone. Prognosis of patients with extrarenal organ failures but without renal failure could not be compared with that of renal failure alone due to low sample size of the former group. Finally, kidney outcome was worst in patients with renal failure associated with other extrarenal organ failures compared with that in patients with renal failure alone, which emphasizes the important contribution of extrarenal organ failures to patient’s and kidney’s outcome.
The relevance of urinary biomarkers, particularly uNGAL, in the AKI patient population warrants a specific comment. In the current study, there were two interesting observations related to uNGAL. First, uNGAL levels were higher in patients with AKI and ACLF compared to those without, not only at diagnosis of AKI but also throughout the observation period. Second, uNGAL levels measured after 2 days of diagnosis of AKI had independent predictive value of 3-month survival. These results are in keeping with a previous report in an independent series of patients from the CANONIC study showing that uNGAL, but not plasma NGAL, is an independent predictor of short-term survival in patients with ACLF.[25] With respect to the origin of NGAL in urine, we cannot be certain whether it arises from the kidney or from extrarenal organs or both. Previous studies have shown that patients with hypovolemia-induced AKI have low levels of uNGAL compared with other etiologies of AKI.[14] This could explain at least in part the lower levels seen in patients without ACLF, because hypovolemia-induced AKI was the most frequent cause of AKI in this group of patients, whereas it was less common in patients with ACLF. However, because NGAL has low molecular weight, NGAL present in plasma is filtered by the glomeruli, and therefore an extrarenal origin of the increased uNGAL cannot be excluded. A previous study from our group demonstrated increased expression of the lipocalin gene (the gene responsible for NGAL synthesis) in the liver of patients with ACLF.[25] Whichever the cause of the increased uNGAL levels is, our study clearly shows that uNGAL levels are increased in the high-risk group of patients with AKI and ACLF and correlate with prognosis, thus suggesting that NGAL may be of value in addition to MELD-Na score in prognosis assessment in these patients.
The current study has some strengths as well as limitations. Main strengths are the large sample size, the prospective design, as well as measurement of kidney biomarkers. The main limitation is that the study was performed in a single center. Therefore, findings should ideally be reproduced in a multicenter study. Another limitation derives from the definition of ACLF, which is a determinant of the high prevalence of AKI.[13]
In conclusion, the results of this large prospective series of patients hospitalized for AD of cirrhosis demonstrate that AKI is an almost universal finding in patients with ACLF and may occur either in patients with previous normal kidney function or on top of CKD, whereas CKD alone is extremely uncommon. Of clinical interest, AKI has markedly greater severity in patients with ACLF compared to those without and is associated with poorer kidney and patient outcomes. AKI without ACLF has good 3-month survival just slightly lower than that of patients without AKI. Independent predictors of 3-month survival in patients with AD of cirrhosis are MELD-Na score, ACLF status, and uNGAL. These factors could be useful to guide treatment decisions in patients with AD of cirrhosis.
ACKNOWLEDGMENT
The authors thank Nicki Van Berckel, Roser Poblet, and Beatriz Marquez for the administrative support. They also thank the faculty and nurses of the Liver Unit, and the patients who participated in the study and their families. They are indebted to the HCB-IDIBAPS Biobank, integrated in the Spanish National Biobanks Network, for the technical processing of biological human samples.
CONFLICT OF INTEREST
P.G. received research funding from Mallinckrodt, Grifols, and Gilead. He participated on advisory boards for Novartis, Gilead, and Martin Pharmaceuticals. The other authors have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
All authors contributed to this manuscript and approved the final submission. Study concept, design, data acquisition, and manuscript draft: Laura Napoleone, Cristina Solé, Elsa Solà, and Pere Ginès. Data analysis and interpretation: Laura Napoleone, Cristina Solé, Elsa Solà, Pere Ginès, Adrià Juanola, Ann T. Ma, Marta Carol, Martina Pérez-Guasch, Ana-Belén Rubio, Marta Cervera, Emma Avitabile, Octavi Bassegoda, Jordi Gratacós-Ginès, Manuel Morales-Ruiz, Núria Fabrellas, Isabel Graupera, Elisa Pose, and Gonzalo Crespo. Data generation and collection, data assembly, analysis of the results, and critical revision of the manuscript for important intellectual content: Adrià Juanola, Ann T. Ma, Marta Carol, Martina Pérez-Guasch, Ana-Belén Rubio, Marta Cervera, Emma Avitabile, Octavi Bassegoda, Jordi Gratacós-Ginès, Manuel Morales-Ruiz, Núria Fabrellas, Isabel Graupera, Elisa Pose, and Gonzalo Crespo.