Patterns and outcomes of subsequent therapy after immune checkpoint inhibitor discontinuation in HCC
Funding information
Wellcome Trust Strategic Fund (PS3416)
Abstract
The availability of immune checkpoint inhibitors (ICIs) for the management of advanced hepatocellular cancer (HCC) has changed the treatment paradigm. There are emerging questions regarding the efficacy of subsequent anticancer therapies. The primary aim of this retrospective, multicenter study was to examine the types of anticancer treatment received after ICIs and to assess the impact on post-ICI survival. We established an international consortium of 11 tertiary-care referral centers located in the USA (n = 249), Europe (n = 74), and Asia (n = 97), and described patterns of care following ICI therapy. The impact of subsequent therapy on overall survival (OS) was estimated using the Kaplan–Meier method and presented with a 95% confidence interval (CI). A total of 420 patients were treated with ICIs for advanced HCC after one line of systemic therapy (n = 371, 88.8%): 31 (8.8%) had died, 152 (36.2%) received best supportive care (BSC) following ICIs, and 163 patients (38.8%) received subsequent anticancer therapy. Tyrosine kinase inhibitors (TKIs, n = 132, 80.9%), in particular sorafenib (n = 49, 30.0%), were the most common post-ICI therapy followed by external beam radiotherapy (n = 28, 17.2%), further immunotherapy (n = 21, 12.9%), locoregional therapy (n = 23, 14.1%), chemotherapy (n = 9, 5.5%), and surgery (n = 6, 3.6%). Receipt of post-ICI therapy was associated with longer median OS compared with those who had received BSC (12.1 vs. 3.3 months; hazard ratio [HR]: 0.4 (95% CI: 2.7–5.0). No difference in OS was noted in those patients who received TKI before ICIs compared with those who received ICIs followed by TKI. Conclusion: Post-ICI therapy is associated with OS in excess of 12 months, suggesting a role for therapeutic sequencing. OS from TKI therapy was similar to that reported in registration studies, suggesting preserved efficacy following ICIs.
INTRODUCTION
Until recently, treatment for hepatocellular cancer (HCC) was dominated by tyrosine kinase inhibitors (TKIs), which provided an overall survival (OS) benefit of 2 months in the first-line setting compared with best supportive care (BSC).[1-3] However, the therapeutic landscape has dramatically changed with the introduction of immune checkpoint inhibitors (ICIs) both in the first-line and second-line setting.[4-6] Importantly, the recently published IMBrave 150 study reported a significant improvement in OS and progression-free survival (PFS) in patients treated with atezolizumab and bevacizumab compared to sorafenib, making this the new standard of care for newly diagnosed unresectable HCC.[7] This publication has heralded a number of combination ICI studies in HCC.[8] However, the response to combination ICIs varies from 20% to 30%, and invariably most patients will progress.[9] What is unclear is what treatments should be used following ICI exposure.
Regorafenib,[10] cabozantinib,[11] and ramucirumab[12] all have been shown to impart survival benefit following sorafenib failure but there is paucity of data regarding their activity following ICIs. The RESORCE study investigated the efficacy of regorafenib versus placebo following sorafenib.[10] Post hoc analysis of the RESORCE study illustrated that sequential treatment with regorafenib following sorafenib failure resulted in a median survival of 26 months compared with 19.2 months in those who were randomized to placebo.[13] Similar results were observed in a post hoc analysis of the CELESTIAL trial, a study that investigated cabozantinib or placebo in patients who had received prior systemic therapy.[11] The post hoc analysis illustrated that in patients who had received prior sorafenib, cabozantinib significantly improved OS, 24.5 months compared with 18.8 months, in those receiving placebo.[14] The REFLECT study was a noninferiority study of lenvantinib versus sorafenib for first-line treatment of unresectable HCC.[3] A post hoc analysis of the REFLECT data illustrates an OS benefit of second-line therapy compared with those who did not receive subsequent therapy, OS 20.8 versus 17.0 months (hazard ratio [HR] 0.87; 95% confidence interval [CI] 0.67–1.14).[15] The efficacy of subsequent anticancer therapy following ICI failure remains undefined. The primary objective of this retrospective, multi-institutional study was to explore types of therapies and survival following ICI therapy in HCC.
METHODS
Study population
Data were extracted from a large international database that has been described previously.[16, 17] Briefly, a consortium of 11 tertiary-care referral centers located in Europe, the USA, and Asia contributed to a prospectively maintained cohort of patients with HCC undergoing treatment with ICIs. To be included, patients had to have a diagnosis of HCC made by histopathology or imaging criteria according to international guidelines, not amenable to curative or loco-regional therapy following local multidisciplinary tumor board review and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.[18-20] At the censoring date of September 30, 2020, the multicenter database included 420 eligible patients. Patients were treated with ICIs either as a monotherapy or in combination with other agents between 2017 and 2020. Ethical approval to conduct this study was granted by the Imperial College Tissue Bank (Reference No. R16008), and the institutional review board in each participating institution also approved the study protocol. All study-related procedures and data collection were conducted in accordance with the Declaration of Helsinki and in accordance with Good Clinical Practice.
Study endpoints
The primary clinical endpoint of the study was OS, calculated from the date of permanent cessation of ICIs to the date of death and/or last follow-up. Those patients who died while receiving ICIs were excluded from the survival analysis of subsequent therapy. Best response to ICIs was defined according to RECIST v1.1 criteria.[20]
Statistical analysis
Continuous variables were presented as medians and interquartile ranges (IQRs), and categorical variables were summarized as proportions. Median OS and PFS and corresponding 95% CIs were estimated using the Kaplan-Meier method followed by log-rank test. Statistical analyses were performed using SPSS version 25.0 (IBM Inc., Chicago, IL, USA).
RESULTS
Baseline patient characteristics
A total of 420 patients with HCC were included in the study from the USA (n = 249), Europe (n = 74), and Asia (n = 97). The most common cause of underlying liver disease was HCV infection (n = 163, 38.8%) followed by hepatitis B virus (HBV) infection (n = 116, 27.6%), alcohol-associated liver disease (n = 76, 18.1%), and nonalcoholic fatty liver disease (n = 44, 10.5%). The mean age of the study population was 65.1 years (range 25–89 years). At the time of commencement of ICIs, most of the patients had Barcelona Clinic Liver Cancer C-stage disease (n = 299, 71.2%) and had preserved liver function (n = 319, 75.9%). Most patients (n = 371, 88.3%) had received at least one prior treatment for HCC before ICIs, including at least first line of systemic therapy (n = 289, 68.8%), with most having received sorafenib (n = 237, 56.4%). Demographic findings are provided in Table 1.
| Baseline characteristic | All patients (%), range n = 420 | Subsequent therapy (%) n = 165 |
|---|---|---|
| Center | ||
| USA | 249 (58.3) | |
| Europe | 73 (16.8) | |
| Asia | 45 (10.5) | |
| Age, years, median (IQR) | 65.1 (13) | 63.2 (10) |
| Sex | ||
| Male | 331 (78.8) | 126 (76.4) |
| Female | 89 (21.2) | 39 (23.6) |
| Etiology | ||
| Hepatitis C | 162 (38.6) | 57 (34.5) |
| Hepatitis B | 115 (27.3) | 60 (36.4) |
| Alcohol | 74 (17.6) | 27 (16.4) |
| Other | 59 (14.0) | 25 (15.1) |
| Child-Turcotte-Pugh Class | ||
| A | 319 (75.9) | 133 (80.6) |
| B | 97 (23.1) | 28 (17.0) |
| Barcelona Clinic Liver Cancer | ||
| A | 15 (3.6) | 9 (5.5) |
| B | 106 (25.2) | 38 (23.0) |
| C | 299 (71.2) | 118 (71.5) |
| Maximum tumor diameter | ||
| <7 cm | 180 (42.9) | 60 (36.4) |
| >7 cm | 101 (66.9) | 42 (25.5) |
| Portal vein thrombus | ||
| Absent | 220 (52.4) | 88 (53.3) |
| Present | 100 (23.8) | 37 (22.4) |
| AFP (ug/dL) | ||
| <400 | 244 (58.1) | 98 (59.4) |
| >400 | 158 (37.6) | 66 (40.00) |
| Cirrhosis | ||
| Absent | 117 (27.9) | 52 (31.5) |
| Present | 297 (70.7) | 133 (68.5) |
| Metastases | ||
| Absent | 166 (39.5) | 62 (37.6) |
| Present | 161 (38.3) | 64 (38.8) |
| Previous lines of treatment | ||
| 0 | 48 (11.4) | 15 (9.1) |
| 1 | 96 (22.9) | 32 (19.4) |
| 2 | 116 (27.6) | 43 (26.1) |
| >3 | 159 (37.9) | 74 (44.8) |
| Previous treatment | ||
| Resection | 132 (31.9) | 66 (40) |
| Radiofrequency/microwave ablation | 296 (5.9) | 43 (26.1) |
| Transarterial chemoembolization | 195 (47.1) | 83 (50.3) |
| Y90 | 93 (22.5) | 29 (17.6) |
| Radiotherapy | 49 (11.8) | 29 (17.6) |
| Sorafenib | 237 (57.2) | 94 (57.0) |
| Other | 102 (24.6) | 46 (35.9) |
- Abbreviations: AFP, α-fetoprotein; IQR, interquartile range.
Monotherapy (n = 358, 85.2%) with nivolumab (n = 310, 73.8%) was the most commonly administered ICIs followed by single-agent pembrolizumab (n = 22. 5.2%). In terms of combination therapy, 26 (6.2%) received combination programmed death ligand 1 (PD-L1)/cytotoxic T lymphocyte antigen 4 (CTLA-4) inhibitors, and 18 patients (4.3%) received combination ICIs with TKIs while 4 patients (1%) received PD-L1/CTLA-4/TKI. These combinations are outlined in Table S1. The best overall response to ICIs was assessed in 395 (94%) patients. Twenty-seven (6.8%) patients experienced a complete response (CR), 45 (11.4%) partial response (PR), 161 (38.3%) had stable disease (SD), and 162 (38.6%) patients experienced progressive disease (PD). The mean ICI duration was 6.5 months (SD ± 6.7). The median OS from commencement of ICI therapy was 15.4 months (95% CI 13.1–17.7 months) based on 216 events (49%), and median PFS was 3.6 months (95% CI 2.9–4.3 months) based on 237 events (56%). As there is emerging evidence suggesting viral etiology has an improved outcome to ICI therapy compared with nonviral liver disease,[21] survival was compared between these groups. No difference was observed between viral and nonviral disease etiology (16.1 months [95% CI 12.7–19.5] vs. 12.2 months [95% 7.7–16.7], p = 0.02).
After ICI therapy
At the time of analysis, 329 patients (78.3%) had discontinued ICIs, the main reason being disease progression (n = 219, 52.1%) (Figure 1). Thirty-one patients (7.4%) died while on ICIs and were excluded from further analysis. Following ICI therapy, 163 patients (49.5%) received a subsequent line of therapy, and 152 patients (46.5%) received BSC (Table 1). In terms of baseline characteristics, the subgroup that received subsequent therapy was more likely to be from the USA (62.6%) or Asia (29.4%) compared with Europe (8.0%) (p < 0.001), younger age at commencement of ICIs (median age 65.0 vs. 68.0 years, p = 0.03), and had higher rates of HBV (36.9 vs. 23.0%, p = 0.01) compared with those who received BSC. The subsequent therapy group was more likely to have preserved liver function at baseline (Child-Pugh A 82.4% compared with 69.1% of patients who received BSC; p = 0.008). Both groups were balanced in terms of tumor staging at the time of ICI initiation. At the time of ICI cessation, 86 (27.2%) of patients had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 131 (41.5%) had an ECOG PS of 1, 46 (14.6%) had an ECOG PS of 2, and 26 (5.0%) had an ECOG PS of 3. More patients who received BSC had an advanced PS compared with those who received subsequent therapy (p < 0.001). In terms of toxicity, those who received BSC were more likely to have experienced any grade of liver toxicity secondary to ICIs, compared with those who received subsequent therapy (20.7% vs. 11.7%, p = 0.01). No differences in any other toxicity were noted. Most patients receiving subsequent therapy had experienced primary resistance and PD to ICI therapy (n = 82, 50.3%), whereas 48 (29.4%) had initially experienced SD, and 11 (6.7%) achieved a PR and 6 (3.6%) achieved CR.
Most patients received only one further line of treatment (n = 115, 70.5%). TKIs were the most common next line of therapy (n = 109, 66.9%), in particular sorafenib (44.9%) and regorafenib (30.3%). Fifty-one patients (31.2%) received liver-directed therapies including radiofrequency ablation, transarterial chemoembolization, transarterial radioembolization, or external-beam radiotherapy, of whom 14 (8.5%) received liver-directed therapies in combination with other therapies (Table 2). Of note, 48 patients (29.4%) underwent multiple lines of further treatment, including 2 patients who underwent liver transplantation.
| Number of subsequent lines received | n (%) |
|---|---|
| 1 | 115 (67.9) |
| 2 | 32 (19.4) |
| >3 | 13 (7.9) |
| Treatments received | |
| TKI | 109 (66.1) |
| sorafenib | 49 (44.9) |
| lenvatinib | 31 (28.4) |
| regorafenib | 33 (30.3) |
| cabozantinib | 13 (11.9) |
| ramucirumab | 6 (5.5) |
| Radiotherapy | 28 (16.9) |
| Immunotherapy | 21 (12.7) |
| Transarterial chemoembolization/Y90 | 19 (11.5) |
| Chemotherapy | 9 (5.5) |
| Surgery | 6 (3.6) |
| Radiofrequency/microwave ablation | 4 (2.4) |
| Other | 23 (13.9) |
A significant benefit in median OS was observed for those patients who received therapy following ICIs (HR 0.4, 95% CI 0.3–0.5, p < 0.001), such that patients who received further treatment had a median OS following the cessation of ICIs of 12.2 months (95% CI 9.3–15.0) compared with 3.2 months (95% CI 1.8–4.5) in those who received BSC (log rank; p < 0.001) (Figure 2). Estimated survival rates from the end of ICIs were higher in those who received subsequent therapy from 6 months until 36 months (Table 3). We explored these parameters with other known predictors of survival from commencement of ICIs on multivariable analysis (Table 4). Receipt of subsequent therapy remained a significant predictor of OS on multivariable analysis (HR 0.4, 95% CI 0.3–0.6, p < 0.001). Patients who had received ICI first-line therapy had worse survival outcomes compared with those who had received ICIs as second-line or third-line therapy (median OS 10.9 months compared with 12.5 months; HR 1.6, 95% CI 1.01–2.5, p = 0.02). Responders to ICIs who received subsequent anticancer treatment (n = 16) had an improved OS compared with those who did not initially respond to ICIs (n = 142) (HR 0.3, 95% CI 0.1–0.9, p = 0.04).
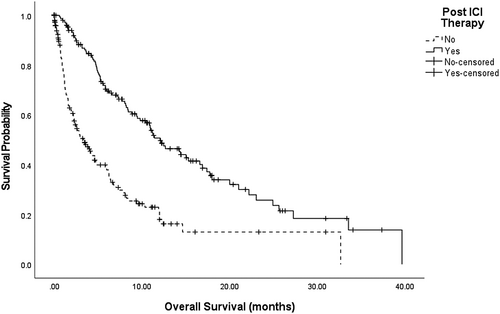
| Survival rate | Subsequent treatment (n = 162) | No subsequent treatment (n = 194) |
|---|---|---|
| 6 months | 69.8% | 36.9% |
| 12 months | 49.9% | 18.0% |
| 24 months | 23.7% | 13.0% |
| 36 months | 13.9% | — |
| Predictor | Univariable HR (95% CI) | p value | Multivariable HR (95% CI) | p value |
|---|---|---|---|---|
| Subsequent therapy | 0.4 (0.3–0.5) | <0.001 | 0.4 (0.3–0.6) | <0.001 |
| BCLC stage C/D vs. A/B | 1.5 (1.1–2.1) | 0.006 | ||
| ECOG PS (>1) | 1.2 (0.9–1.5) | 0.3 | ||
| CTP class (B/C vs. A) | 1.9 (1.4–1.5) | <0.001 | 1.4 (0.9–2.2) | 0.8 |
| Cirrhosis (presence vs. absence) | 1.1 (0.8–1.4) | 0.7 | ||
| PVT (presence vs. absence) | 2.1 (1.5–2.9 0 | <0.001 | 1.7 (1.2–2.5) | 0.006 |
| Geographic region | ||||
| USA vs. Europe | 0.9 (0.7–1.3) | 0.4 | ||
| USA vs. Asia | 1.3 (0.8–1.9) | |||
| Extrahepatic metastases (present) | 1.2 (0.9–1.7) | 0.2 | ||
| AFP > 400 (ug/dL) | 1.9 (1.4–2.6) | <0.001 | 1.7 (1.2–2.5) | 0.5 |
| Etiology (viral vs. nonviral) | 0.8 (0.6–1.1) | 0.1 |
- Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CTP, Child-Turcotte-Pugh; ECOG PS, Eastern Cooperative Oncology Group performance status; PVT, portal vein thrombosis.
We then considered whether sequencing of TKIs and ICIs had an impact on survival outcomes. No difference was observed between the survival of those patients who had previously received TKIs followed by ICIs compared with those who had ICIs followed by TKIs (15.5 months [95% CI 5.4–25.5] vs. 15.9 months [95% CI 13.7–18.2], p = 0.2). However, patients who received TKIs followed by ICIs and then TKIs had a significantly prolonged OS (22.3 months [95% CI 10.4–34.2], p = 0.04). Of interest, prior sorafenib therapy did not affect the subsequent efficacy of administered TKIs (HR 0.8, 95% CI 0.5–1.3, p = 0.5). We then considered the subgroup patients who had previously received sorafenib (n = 73) and were rechallenged with sorafenib following ICIs. Rechallenge with sorafenib resulted in a median OS of 12.5 months (95% CI 8.7–16.3). No statistical difference was observed in the efficacy of individual TKIs (Figure 3).

DISCUSSION
Since the initial approval of sorafenib for advanced HCC, subsequent development of active second-line therapies, improved patient selection, and treatment-stage migration[22] have pushed the median OS for this population.[13-15]
Immune-based therapies are reshaping the therapeutic armamentarium of HCC.[23] However, anti–programmed cell death-1 (PD-1) monotherapy[4, 5] and dual checkpoint inhibition with anti-CTLA-4[24] were approved by the FDA on the basis of response rates rather than evidence of convincing OS benefit. Although atezolizumab and bevacizumab prolong OS first-line, data on long-term survivorship and response to subsequent therapies are not yet available.[7] Most patients with advanced HCC will invariably progress, and there is very little evidence to guide recommendations for what treatments should be used after ICI therapy, the sequencing of these therapies, and the efficacy of subsequent lines of therapy.
Using a large, international, observational data set, we first attempted to evaluate patterns of anticancer therapy following ICI failure in patients with HCC. We found that most patients considered eligible for subsequent therapy received a TKI. Sorafenib was the most commonly prescribed TKI following ICIs, a point of interest given that most of our patients were sorafenib-experienced before ICIs. Notably, a significant proportion of patients who received second-line therapy, 31.2%, were considered for further ablative/locoregional or even radical therapy (including liver transplantation), suggesting that the disease-modulating effect of ICIs might have produced significant down-staging of the disease and treatment-stage migration, a concept that should be explored in future work. These results are particularly provocative in underscoring the need for multidisciplinary evaluation of tumor and patient status at the point of ICI progression, so that patients can be treated with the optimal therapeutic modality in an attempt to maximize OS.
Considering the clinical outcomes of our patient cohort, our results show that receipt of subsequent therapy of any type was associated with a significant improvement in OS compared with provision of BSC (HR 0.4 95% CI 2.7–5.0, p < 0.001). These results should be interpreted with caution. This is a registry study; therefore, selection bias must be considered when evaluating our results, particularly as we observed that patients receiving subsequent therapy were more likely to have preserved liver function and better PS at the completion of ICIs compared with those in the BSC cohort, which may account for their improved outcomes. The impact of PS and underlying liver function on survival outcomes with systemic therapy is well established and needs to be controlled for in future work.[25, 26] Moreover, we observed clear geographical differences, with patients from the USA and Asia more likely to receive subsequent therapies and those in Europe more likely to receive BSC, which may reflect the differing reimbursement patterns. Future studies would be strengthened by propensity score matching, which we were unable to perform due to the limited sample size. However, on multivariable analysis of the entire patient cohort, receipt of subsequent therapy remained an independent predictor of survival, suggesting that patient selection alone does not account for the improved survival in the subsequent therapy group—a finding that requires further evaluation.
Patients treated with TKIs following ICIs achieved median OS in about 12 months, in line with registration studies of TKIs in HCC.[1, 3] Particularly notable is the prolonged OS in patients who had received ICIs after TKIs compared to those treated with ICIs in the first-line setting. Multitargeted TKIs disrupt many pathways that promote a highly immunosuppressive tumor microenvironment, and TKIs have been shown to help deplete regulatory T cells and myeloid-derived suppressors from the tumor microenvironment[27]; indeed, early trials looking at the combination have demonstrated significant promise, and ongoing phase 3 trials will soon report the effects of TKI and ICI combinations, although these trials will not necessarily address potential benefits of sequencing therapies over concurrent administration.[28, 29] In addition, we speculate whether a proportion of patients treated with PD-1 inhibitors in first-line therapy might have experienced higher proportion of hyperprogression,[30] a paradoxical worsening of disease status that can affect up to 15% of patients with HCC.[31]
Evidence of efficacy of TKIs following ICIs in HCC is limited. A post hoc analysis of 14 patients in the CELESTIAL study who received cabozantinib third line following ICIs reported a median OS of 7.9 months (95% CI 5.1–NE), which was comparable to that of patients who had received two prior regimens (median OS 8.5 months [95% CI 7.4–9.7]).[32] Another abstract of 30 patients reported a median OS from commencement of TKIs following ICIs of 20 months (95% CI 4.0–NE).[33] Interestingly, our results mirror evidence produced in renal cell cancer (RCC), an oncological indication characterized by similar dependence on angiogenesis seen in HCC. In RCC, sequential TKI use following ICI therapy is associated with incremental OS benefit, leading to international guidelines recommending the use of any multitargeted TKI that has not been used in the first-line setting in combination with ICIs.[33-35] Notably, the half-lives of the checkpoints used in the treatment of HCC range from 2 to 4 weeks, so there may be a component of synergy also seen in patients who are receiving sequential treatment.
Most of the patients who had further treatment had experienced primary resistance to single-agent nivolumab, which was administered primarily as second-line therapy following sorafenib failure, and the median OS reported in this study is consistent with that observed in Keynote-240.[36] Following ICIs, most patients subsequently received TKIs, although any differences in efficacy between individual TKIs cannot be resolved due to the small size of the data set. Data were not collected on patterns of disease failure with each line of therapy, which would be of interest particularly with regard to emerging work investigating the immune infiltrate in metastases compared with the primary site giving insight into patterns of immune escape.[37] It should be noted that most patients had received sorafenib in the first-line setting; this did not affect the survival benefit from subsequent TKIs and supports the findings of the post hoc analyses of the RESORCE, CELESTIAL, and REFLECT studies that illustrate additive survival benefit with sequential TKIs owing to their nonredundant molecular targets.[13-15]
There is a complex interplay between angiogenesis and the immune response, and much research has addressed the role of anti-angiogenics in enhancing the response to immunotherapy. However, emerging evidence suggests that immunotherapy also affects the tumor vasculature and may improve the efficacy of anti-angiogenics.[38, 39] In an elegant paper using an in vivo model of breast cancer, Tian et al. demonstrated that immunotherapy resulted in vessel normalization secondary to infiltration of CD4+ T cells and secretion of interferon-γ.[40] It is conceivable, therefore, that the vascular normalization induced by ICIs resulted in enhanced delivery and effect of any subsequent therapy, a concept that needs to be further investigated in the preclinical setting.[38]
There are a number of limitations of this analysis, including the retrospective nature of the data, the lack of response and toxicity data that is of particular importance in the palliative setting, and the diversity of TKIs in the post-ICI space. Selection bias in terms of patients receiving subsequent lines of treatment also needs to be considered. Time to tumor progression data with TKIs would also be of key interest. Despite the introduction of atezoluzimab and bevacizumab for the first-line treatment of HCC, these data are of interest given the results regarding subsequent treatments following ICIs.[7] International guidelines have approved the use of TKIs following immunotherapy with a distinct lack of evidence, and this work supports the continued sequencing of these agents in the management of HCC.(41, 42) Validation of these findings in future clinical trials is warranted in order to delineate the second and subsequent line therapeutic space in HCC.
ACKNOWLEDGMENT
The authors acknowledge the infrastructure support provided by the Imperial Experimental Cancer Medicine Center, Cancer Research UK Imperial Center, and the Imperial College Healthcare NHS Trust Tissue Bank. They also thank Musharraf Navaid, Bo Yu, and Neil Nimkar for their contribution to the data.
CONFLICT OF INTEREST
A.P. advises Eisai, Exelixis, AztraZeneca, Genentech, and Replimune (Data and Safety Monitoring Board). She is on the speakers’ bureau of Simply Speaking Hepatitis. N.P. consults for and received grants from Amgen and Servier. She consults for Merck Serono and received grants from Basilea. T.P. consults for and received grants from Bayer. She also received grants from Lilly and Roche. D.P. consults for and is on the speakers’ bureau of Roche. He consults for EISAI, AZ, H3B, and Mina Therapeutics. He is on the speakers’ bureau of and received grants from BMS and MSD. He is also on the speakers’ bureau of Ipsen. A.K. consults for, advises, and received grants from BMS, Merck, Eisai, Exelixis, Roche, and Bayer. He consults for and advises AstraZeneca. A.S. advises and received grants from Bristol Myers Squibb. He advises, is on the speakers’ bureau of, and received grants from AstraZeneca. He advises and is on the speakers’ bureau of Daiichi Sankyo. He advises Merck and Pfizer and received grants from Exelixis. L.R. consults for ArQule, Basilea, BMS, Celgene, Genenta, Hengrui, IQVIA, and Servier. She is on the speakers’ bureau of AbbVie, Gilead, and Merck Serono. She received grants from Agios, ARMO BioSciences, BeiGene, and Fibrogen. She also consults for and is on the speakers’ bureau of Amgen, Bayer, and Sanofi. She consults for and received grants from AstraZeneca, MSD, Exelixis, Nerviano Medical Sciences, and Zymeworks. She consults for, received grants from, and is on the speakers’ bureau of Eisai, Ipsen, Lilly, Roche, and Incyte. D.B. consults for Bayer, Shionogi, and Boston Scientific and is on the speakers’ bureau of Falk Foundation. T.M. advises and received grants from Regeneron and received grants from Boehringer Ingelheim and BMS.
AUTHOR CONTRIBUTIONS
Study concept: Rohini Sharma. Data analysis: Rohini Sharma and David James Pinato. Data interpretation: Rohini Sharma, Thomas Urban Marron, David Szafron, Abdul Rafeh Naqash, Lorenza Rimassa, and David James Pinato. Manuscript preparation: Rohini Sharma and David James Pinato. Manuscript submission: Rohini Sharma. Data acquisition and manuscript review: Anjana Pillai, Thomas Urban Marron, Petros Fessas, Anwaar Saeed, Tomi Jun, Sirish Dharmapuri, David Szafron, Abdul Rafeh Naqash, Anuhya Gampa, Yinghong Wang, Uqba Khan, Mahvish Muzaffar, Chieh-Ju Lee, Pei-Chang Lee, Anushi Bulumulle, BY, Sonal Paul, NN, Dominic Bettinger, Hannah Hildebrand, Mohammed Yehia, Tiziana Pressiani, Ahmed Kaseb, Yi-Hsiang Huang, Celina Ang, Masatoshi Kudo, Naoshi Nishida, Nicola Personeni, Lorenza Rimassa, and David James Pinato.
STATEMENT OF ETHICS
Ethical approval to conduct this study was granted by the Imperial College Tissue Bank (Reference No. R16008), and the institutional review board in each participating institution approved the study protocol. All study-related procedures and data collection were conducted in accordance with the Declaration of Helsinki and in accordance with Good Clinical Practice.




