Association Between Transient Elastography and Controlled Attenuated Parameter and Liver Ultrasound in Children With Cystic Fibrosis
Supported by the Cystic Fibrosis Foundation (NARKEW17AB0 to M.N.) and the National Institutes of Health NIDDK U01 DK062453 to M.N. and NIDDK U24 DK062456 to J.M.).
Potential conflict of interest: Dr. Molleston received grants from Mirum, Gilead, AbbVie, and Albireo. Dr. Ling received grants from AbbVie and Gilead. Dr. Murray consults for Gilead and Albireo. Dr. Narkewicz consults for Vertez. He received grants from Gilead and AbbVie. Dr. Schwarzenberg consults for UpToDate. She received grants from Gilead. Dr. Towbin consults for Applied Radiology. He received grants from Guerbet. He has intellectual property rights in Elsevier. Dr. Karnsakul consults for Albireo and Gilead.
Abstract
Methods to identify children with cystic fibrosis (CF) at risk for development of advanced liver disease are lacking. We aim to determine the association between liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) with research ultrasound (US) patterns and conventional hepatic markers as a potential means to follow liver disease progression in children with CF. ELASTIC (Longitudinal Assessment of Transient Elastography in CF) is a nested cohort of 141 patients, ages 7-21, enrolled in the Prediction by US of Risk of Hepatic Cirrhosis in CF (PUSH) Study. We studied the association between LSM with research-grade US patterns (normal [NL], heterogeneous [HTG], homogeneous [HMG], or nodular [NOD]) and conventional hepatic markers. In a subgroup (n = 79), the association between controlled attenuation parameter (CAP) and US pattern was explored. Among 133 subjects undergoing VCTE, NOD participants (n = 26) had a significantly higher median (interquartile range) LSM of 9.1 kPa (6.3, 15.8) versus NL (n = 72, 5.1 kPa [4.2, 7.0]; P < 0.0001), HMG (n = 17, 5.9 kPa [5.2, 7.8]; P = 0.0013), and HTG (n = 18, 6.1 kPa [4.7, 7.0]; P = 0.0008) participants. HMG participants (n = 14) had a significantly higher mean CAP (SD) (270.5 dB/m [61.1]) compared with NL (n = 40, 218.8 dB/m [46.5]; P = 0.0027), HTG (n = 10, 218.1 dB/m [60.7]; P = 0.044), and NOD (n = 15, 222.7 dB/m [56.4]; P = 0.041) participants. LSM had a negative correlation with platelet count (rs =  0.28, P = 0.0071) and positive correlation with aspartate aminotransferase–to-platelet ratio index (rs = 0.38, P = 0.0002), Fibrosis-4 index (rs = 0.36, P = 0.0007), gamma-glutamyltransferase (GGT; rs = 0.35, P = 0.0017), GGT-to-platelet ratio (rs = 0.35, P = 0.003), and US spleen size z-score (rs = 0.27, P = 0.0073). Conclusion: VCTE is associated with US patterns and conventional markers in patients with liver disease with CF.
0.28, P = 0.0071) and positive correlation with aspartate aminotransferase–to-platelet ratio index (rs = 0.38, P = 0.0002), Fibrosis-4 index (rs = 0.36, P = 0.0007), gamma-glutamyltransferase (GGT; rs = 0.35, P = 0.0017), GGT-to-platelet ratio (rs = 0.35, P = 0.003), and US spleen size z-score (rs = 0.27, P = 0.0073). Conclusion: VCTE is associated with US patterns and conventional markers in patients with liver disease with CF.
Abbreviations
-
- APRI
-
- AST to platelet ratio index
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CAP
-
- controlled attenuation parameter
-
- CF
-
- cystic fibrosis
-
- CFLD
-
- cystic fibrosis–associated liver disease
-
- CFLD-NET
-
- CFLD Network
-
- CI
-
- confidence interval
-
- ELASTIC
-
- Longitudinal Assessment of Transient Elastography in Cystic Fibrosis
-
- FIB-4
-
- Fibrosis-4 index
-
- GGT
-
- gamma-glutamyltransferase
-
- GPR
-
- GGT-to-platelet ratio
-
- HMG
-
- homogenous
-
- HTG
-
- heterogenous
-
- ICC
-
- intraclass correlation coefficient
-
- IQR
-
- interquartile range
-
- LSM
-
- liver stiffness measurement
-
- NL
-
- normal
-
- NOD
-
- nodular
-
- PUSH
-
- Prediction by US of Risk of Hepatic Cirrhosis in CF
-
- SSAZ
-
- spleen-size-for-age z-score
-
- VCTE
-
- vibration-controlled transient elastography
-
- US
-
- ultrasound
Advanced cystic fibrosis–associated liver disease (CFLD) is evidenced by cirrhosis with or without portal hypertension, or nodular regenerative hyperplasia with portal hypertension, and occurs in approximately 7%-10% of patients with cystic fibrosis (CF).(1) Cirrhosis is seen primarily in childhood,(1, 2) whereas noncirrhotic portal hypertension affects all ages.(3) More subtle liver involvement including hepatomegaly, persistently elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT) or gamma-glutamyltransferase (GGT), and/or ultrasound (US) image-pattern abnormalities may occur in 40%-90% of patients with CF by 18 years of age.(4) However, noninvasive methods to detect and monitor patients who progress to advanced CFLD are limited.(5)
Vibration-controlled transient elastography (VCTE) is a noninvasive alternative to liver biopsy that uses shear wave velocity to measure liver stiffness (LSM). VCTE has been validated in a number of chronic liver diseases (e.g., hepatitis B and C, primary biliary cirrhosis, and nonalcoholic fatty liver disease [NAFLD])(6) and has been shown to reflect progression of fibrosis over time.(7) In addition to LSM, VCTE by FibroScan (Echosens, Paris, France) can provide controlled attenuation parameter (CAP) measurements to provide an estimate of hepatic steatosis, which has been reported in up to 60% of patients with CF.(8) VCTE has been studied in patients with CF, but its interpretation is confounded by nonuniform definitions of CFLD as well as a wide spectrum of proposed cutoff values.(9-15)
ELASTIC (Longitudinal Assessment of Transient Elastography in Cystic Fibrosis; clinicaltrials.gov: NCT03001388) is a nested study within the previously described Prediction by Ultrasound of the Risk of Hepatic Cirrhosis in Cystic Fibrosis (PUSH; clinicaltrials.gov: NCT01144507) study conducted by the CFLD Network (CFLD-NET).(16) Ultimately, the ELASTIC study will determine whether VCTE, when combined with US pattern characterization, can improve the identification of children with, and who will progress to, advanced CFLD. The aims of the current analysis are to determine the association between VCTE (FibroScan)–generated LSM and CAP and research-defined US patterns, and explore the relationship between LSM and conventional laboratory markers in a well-characterized, multicenter pediatric cohort with CF.
Materials and Methods
Study Population
CFLD-NET is investigating predictors of the development of a nodular US pattern in CF in a prospective study, PUSH, whose detailed methodology has been previously reported.(16) Briefly, between January 12, 2010, and October 17, 2013, 725 participants underwent screening US at the beginning of the PUSH study. Children 3-12 years of age were eligible for the study. Liver US studies were performed after participants had fasted for at least 4 hours and were timed to avoid coinciding with an acute respiratory exacerbation. US findings were classified by liver parenchymal patterns as normal (NL), heterogeneous (HTG), homogeneously hyperechoic (HMG), or nodular (NOD). Classification was based on consensus of three specially trained study radiologists blinded to the readings by the other radiologists, VCTE measurements, clinical information, and demographic data. Among the 725 screened participants, 245 eligible patients entered the 9-year prospective longitudinal study. A recent interim analysis that concluded the need for continuation of the study also noted the benefit of including VCTE in the study’s goals.(17)
Between March 8, 2017, and December 31, 2018, the ELASTIC study enrolled participants from the PUSH cohort at eight of the 11 CFLD-NET centers where FibroScan was available. All participants who fulfilled inclusion criteria for PUSH at the eight participating centers were eligible for enrollment. Local internal review board approval was obtained at each participating center.
FibroScan Examination
FibroScan was completed at the ELASTIC baseline visit, with two additional annual examinations to be completed at future visits. Comprehensive clinical data and biosamples were collected annually, and research gray-scale US was performed every 2 years according to the PUSH protocol. FibroScan measurements were completed using a standardized protocol at each of these visits by a designated investigator(s) who had undergone appropriate training by Echosens to ensure consistent and standardized acquisition of complete data. The thoracic perimeter of the patient was measured and recorded to determine the size of the probe to be used per standard protocol as follows: >75-cm M-probe, 45-cm to ≤75-cm S2 probe, and ≤45-cm S1 probe.
The results of LSM (in kilopascals) were reported as the median of 10 valid measurements and were considered valid if the ratio of the interquartile range (IQR) and median was ≤0.3. If no valid LSM measurements were obtained at the first visit, an additional visit was scheduled to allow an additional attempt. To account for the described patchy nature of CFLD,(8) a secondary site at least one intercostal space away was identified, and the same protocol repeated. If no valid LSM measurements were obtained at the secondary site, this was recorded but a second visit was not scheduled. For participants in whom the M-probe was used, CAP (in decibel-milliwatts [dB/m]) was also recorded using the same 10 valid measurements used for LSM. CAP can only be determined with the M probe, so only those participants for whom the M probe was appropriate had determination of CAP. Four CAP measurements below the detection limit (100 dB/m) were excluded, as this was felt to be a poor study with inconclusive findings, as recommended by the manufacturer. In addition to LSM, CAP and IQR, the number of invalid measurements and success rate to obtain a valid scan were recorded.
ELASTIC Baseline Data
At enrollment into ELASTIC, demographics were collected in addition to most-recent US grade and Pseudomonas status. Conventional biomarkers of liver disease including platelet count, AST, ALT, and GGT within 1 year of the FibroScan were recorded. AST-to–platelet ratio index (APRI; AST/upper limit of normal of AST/platelet count), Fibrosis-4 index (FIB-4; age × AST)/(platelet count × √ALT), and GGT-to-platelet ratio (GPR) were calculated. Additionally, spleen size was measured on the participant’s most-recent US as the longest craniocaudal dimension and expressed as a spleen-size-for-age z-score (SSAZ) using age-specific normal ranges.(18) Reference ranges are given in Supporting Table S1.
Statistical Analysis
To evaluate the feasibility of VCTE in children and young adults with CF, we calculated the proportion of participants with valid LSM measurements at the primary liver location on first attempt or with multiple attempts among all participants for whom FibroScan was attempted with 95% confidence intervals (CIs).
To examine the variability of LSM and CAP measurements taken at two different liver locations in the same patient, we calculated the intraclass correlation coefficient (ICC) to assess similarity between these two measurements. Because LSM is severely skewed, to avoid the influence of extreme leverage points and inflated ICC values, LSM was log-transformed before calculation. ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively. A recent meta-analysis of LSM by VCTE suggested a cutoff of 5.95 kPa to prompt an investigation for liver disease among patients with CF.9 Using this threshold, we examined the consistency of LSM from the two liver locations using a 2 × 2 table with McNemar test and kappa statistics.
To assess whether LSM and CAP are associated with US pattern, we used the analysis of variance test to compare the log-transformed LSM and CAP values at the primary liver location among all US groups. Because US was performed on a different day than the day of FibroScan, the US grade closest in time to the baseline FibroScan visit was used. Post hoc tests were conducted to compare pair-wise group difference.
We then assessed whether LSM was associated with conventional biomarkers of liver disease–severity (ALT, GGT, GPR, APRI, FIB-4, platelet count, and SSAZ) graphically penalized splines. Using the LSM values from the primary liver location, we calculated Spearman correlation coefficients and their CI between LSMs with each of these conventional biomarkers. Statistical significance was defined as P < 0.05.
Results
Study Population and Baseline Characteristics
A total of 141 PUSH participants were enrolled into ELASTIC between March 2017 and December 2019 (Fig. 1). Baseline VCTE was attempted in 137 participants, of whom 5 were less than 8 years of age. The demographic and laboratory information for 133 participants with valid LSM measurements is given in Table 1. Within a year before VCTE, 30.8% participants had a Pseudomonas-positive sputum culture. At the US visit closest (mean: 7.8 months before VCTE scan; range: 31.6 months before to 9.6 months after) to the ELASTIC baseline visit (before or after), most of the participants (54.1%) had a NL US pattern, and 13.5%, 12.8%, and 19.5% had HTG, HMG, and NOD patterns, respectively. The median (IQR) platelet count, GPR, and FIB-4 of ELASTIC participants was 281.5 103/mm3 (232.0, 347.0), 0.06 (0.04, 0.13), and 0.25 (0.19, 0.33), respectively, and within normal limits. The median values for GGT (17 U/L [IQR: 13.0, 40.0]), APRI (0.4 [IQR: 0.27, 0.71]), and US SSAZ (0.9 [IQR: -0.11, 2.33]) were within normal ranges, but mean values for GGT (30.8 U/L), APRI (0.6), and US SSAZ (1.2) trended toward abnormally high values, suggesting skewedness of the data.
| Characteristic | n (%) | |
|---|---|---|
| Total | 133 | |
| Age (years) | Mean (SD) | 14.3 (3.4) |
| Median (IQR) | 14.7 (11.9, 17) | |
| Min, Max | 7, 21 | |
| Male gender | n (%) | 70 (52.6%) |
| Ethnicity | Hispanic | 10 (7.5%) |
| Non-Hispanic | 123 (92.5%) | |
| Race | Asian | 1 (0.8%) |
| Black or African American | 2 (1.5%) | |
| Multiracial | 1 (0.8%) | |
| White | 129 (97%) | |
| Pseudomonas-positive at enrollment | n (%) | 41 (30.8%) |
| US grade | NL | 72 (54.1%) |
| HTG | 18 (13.5%) | |
| HMG | 17 (12.8%) | |
| NOD | 26 (19.5%) | |
| Platelets (103/mm3) | Mean (SD) | 288.8 (95.6) |
| Median (IQR) | 281.5 (232, 347) | |
| Min, Max | 51, 628 | |
| <150 | 5 (5.3%) | |
| <100 | 3 (3.2%) | |
| APRI | Mean (SD) | 0.6 (0.8) |
| Median (IQR) | 0.4 (0.3, 0.7) | |
| Min, Max | 0.1, 5.2 | |
| >1.0 | 13 (14.9%) | |
| >1.5 | 6 (6.9%) | |
| FIB-4 | Mean (SD) | 0.3 (0.3) |
| Median (IQR) | 0.2 (0.2, 0.3) | |
| Min, Max | 0.1, 1.6 | |
| GGT (U/L) | Mean (SD) | 30.8 (37.1) |
| Median (IQR) | 17 (13, 40) | |
| Min, Max | 4, 274 | |
| GGT and GPR | Mean (SD) | 0.2 (0.4) |
| Median (IQR) | 0.1 (0, 0.1) | |
| Min, Max | 0, 2.5 | |
| Spleen size (cm) | Mean (SD) | 11.1 (2) |
| Median (IQR) | 10.8 (9.8, 12.3) | |
| Min, Max | 7.1, 18 | |
| Spleen size z-score | Mean (SD) | 1.2 (2.1) |
| Median (IQR) | 0.9 (−0.1, 2.3) | |
| Min, Max | −3.8, 8 |
Feasibility of FibroScan and Reproducibility at Two Liver Locations
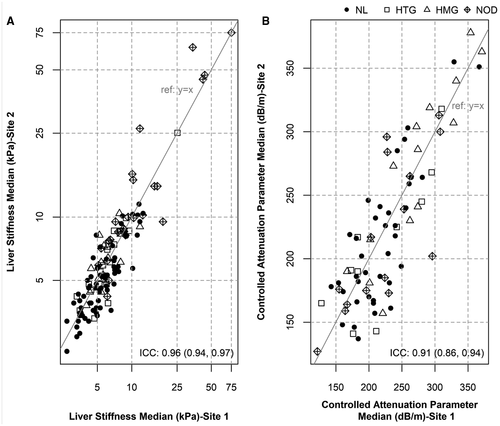
Among the 137 participants for whom FibroScan was attempted, 120 (87.6%; 95% CI: 80.9, 92.6) had valid scans at the first probe location on first attempt. The rate of a valid scan on the first attempt was 80% (95% CI: 28.4%, 99.5%) for participants less than 8 years of age (n = 5) and 87.9% (95% CI: 81.1%, 92.9%) for 8 years old and older (n = 132). Among these 120 cases, all but one also had a valid scan at the second probe location. Among the 17 participants who failed the initial scan, 14 underwent repeat scan(s) with 12 successful studies at the second attempt; 1 participant required a third scan to obtain a valid measurement, while the final subject did not have a successful measurement even with an additional attempt. The scan success rate at the first probe location was 97.1% (95% CI: 92.7%, 99.2%) overall. The final success rate at both probe sites was 96.4%.
In total there were 19 failed scans in 17 patients (Supporting Table S2). Incorrect probe size (as determined by central review by the study’s data coordinating center) was the most commonly reported reason (n = 10), followed by calibration issues (n = 5), uncooperative (n = 2), and IQR/median ratio >0.3 (11%). Each participant with a failed scan was invited to return to their local center and have their scan repeated with the appropriate probe size. Only the scan with the correct probe size was included in the data set for analysis. Given the relatively small sample size of participants younger than 8 years of age (n = 5), we did not perform any statistical test on the difference in feasibility between younger and older participants with CF in this cohort.
The median (IQR) LSM at the first probe location was 5.9 kPa (4.6, 8.0), and ranged from 2.7 to 75 kPa. There were 83 participants for whom the M probe was appropriate and thus were eligible to have CAP determined. Four (3 HTG and 1 NL) were excluded due to CAP below detection limit (100 dB/m), as recommended by the manufacturer. The 79 available CAP measurements demonstrated a mean (SD) of 228.6 dB/m (55.5) and median (IQR) of 225 dB/m (183, 262), and ranged from 122 to 371. The only difference between those with and without CAP determination was that those with CAP determination were older compared to those without CAP (Supporting Table S3).
The ICC for LSM (log-transformed) and CAP between the first and second probe locations was excellent: 0.955 (95% CI: 0.937, 0.968) and 0.912 (95% CI: 0.862, 0.944), respectively (Fig. 1). However, further review showed that 39.4% of participants had a > 1 kPa difference between LSM measurements at the two locations. This percentage was 61.5% among the 26 subjects categorized as having a NOD pattern. Overall, 8 (6%) of subjects had a > 3 kPa difference between the two probe locations (Table 2). Among NOD subjects, 6 (23%) had a difference > 3 kPa. Using the 5.95-kPa threshold that has been suggested as a cutoff to prompt evaluation for liver disease in CF,(9) 16.7% of participants would have had discordant LSM values (1 value ≥ 5.95 and 1 < 5.95 kPa) between the two probe locations. The marginal distribution of this dichotomized version was not significantly different between the two locations (P = 0.09), and the corresponding kappa statistic was 0.67 (0.54, 0.79) (Supporting Table S4). The clinical impact is that performance of LSM at a single location would have led to evaluation for liver disease in 48% versus 55% of participants if two probe locations were used, if using the proposed cutoff value of 5.95 kPa (Supporting Table S4).
| All | NL | HTG | HMG | NOD | |
|---|---|---|---|---|---|
| N | 132 | 71 | 18 | 17 | 26 |
| Mean (SD) | 1.5 (3.0) | 1.0 (0.9) | 0.7 (0.6) | 1.1 (1.0) | 3.5 (6.2) |
| Median (Q1, Q3) | 0.8 (0.4, 1.6) | 0.8 (0.4, 1.4) | 0.7 (0.3, 1.0) | 1.0 (0.2, 1.6) | 1.6 (0.6, 2.8) |
| Min, Max | 0.0, 29.6 | 0.0, 4.5 | 0.1, 2.3 | 0.0, 3.0 | 0.0, 29.6 |
| Difference > 1, n (%) | 52 (39.4%) | 24 (33.8%) | 4 (22.2%) | 8 (47.1%) | 16 (61.5%) |
| Difference > 2, n (%) | 22 (16.7%) | 8 (11.3%) | 1 (5.6%) | 3 (17.6%) | 10 (38.5%) |
| Difference > 3, n (%) | 8 (6.1%) | 2 (2.8%) | 0 (0%) | 0 (0%) | 6 (23.1%) |
| Difference > 4, n (%) | 6 (4.5%) | 1 (1.4%) | 0 (0%) | 0 (0%) | 5 (19.2%) |
| Difference > 5, n (%) | 4 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (15.4%) |
Association Between LSM and US Consensus Grade
LSMs differed significantly among US groups (Fig. 2A). The median (IQR) LSM was 5.1 kPa (4.2, 7.0), 6.1 kPa (4.7, 7.0), and 9.1 kPa (6.3, 15.8) for NL, HTG, and NOD participants, respectively. NOD participants had a significantly higher median LSM level than both NL (P < 0.0001) and HTG (P = 0.0013) participants. Among subjects with HMG US patterns, the median (IQR) LSM was 5.9 kPa (5.2, 7.8), which was also significantly lower than the NOD group (P = 0.0008). The highest LSM of 75 kPa (upper limit of FibroScan LSM) was observed in a NOD participant. There was not a significant difference in the data if only the participants with an US within 1 year of the VCTE are used (versus all) (Supporting Table S4).
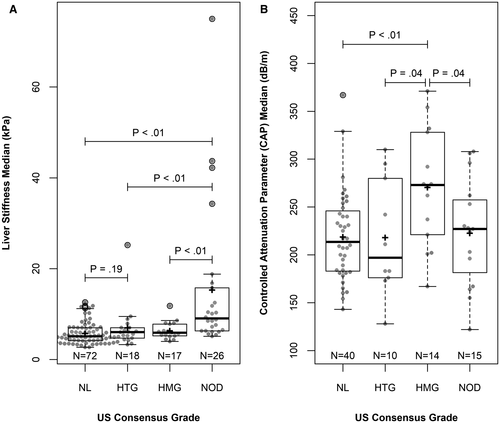
Association Between CAP and US Grade
Among the 79 patients with valid CAP measurements, CAP level differed significantly among US groups (Fig. 2B). The HMG US group had the highest mean (SD) CAP of 271 dB/m (61), which was significantly higher than all the other three groups; the mean (SD) CAP was 219 dB/m (47) for NL (P = 0.0027), 218 dB/m (60.7) for HTG (P = 0.044), and 223 dB/m (56) for NOD (P = 0.041). Of note, there was a weak and insignificant association between CAP and body mass index z-score (rs = 0.18; P = 0.1545). Similar to LSM, there was not a significant difference between values obtained at the two locations (P = 0.41), and the corresponding kappa statistic was 0.66 (0.49, 0.83), as shown in Supporting Table S5.
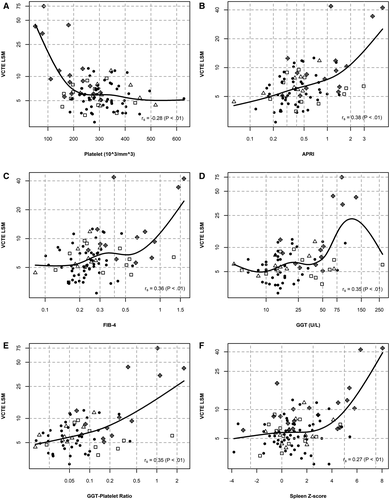
Association Between LSM and Lab Values/US Findings
LSM was significantly associated with conventional laboratory biomarkers of liver disease (platelet count, APRI, FIB-4, GGT, and GPR) and US spleen size z-score (Fig. 3). Increased LSM was associated with lower platelet count (Spearman correlation coefficient rs =  0.28; P = 0.0071) and a higher APRI (rs = 0.38; P = 0.0002), FIB-4 (rs = 0.36; P = 0.0007), GGT (rs = 0.35; P = 0.0017), GPR (rs = 0.35; P = 0.003), and US spleen size z-score (rs = 0.27; P = 0.0073). Laboratory biomarkers among subjects with a NOD US pattern accounted for most of the values seen at the extremes of the respective graphs and matches the association between these markers and US grade as previously reported by our group.(19) We have included details of LSM and those participants with clinically detected portal hypertension in Supporting Table S6.
0.28; P = 0.0071) and a higher APRI (rs = 0.38; P = 0.0002), FIB-4 (rs = 0.36; P = 0.0007), GGT (rs = 0.35; P = 0.0017), GPR (rs = 0.35; P = 0.003), and US spleen size z-score (rs = 0.27; P = 0.0073). Laboratory biomarkers among subjects with a NOD US pattern accounted for most of the values seen at the extremes of the respective graphs and matches the association between these markers and US grade as previously reported by our group.(19) We have included details of LSM and those participants with clinically detected portal hypertension in Supporting Table S6.
Discussion
The CFLD-NET PUSH study represents the largest, well-characterized, prospective cohort of pediatric patients with CF dedicated to determining the natural history of CFLD.(17) The use of LSM and CAP in this cohort has demonstrated excellent feasibility, reproducibility between two anatomical sites, and strong associations with biochemical and nodular US findings of liver disease. The ELASTIC cohort had 54.1% NL, and 13.5%, 12.8%, and 19.5% HTG, HMG, and NOD US patterns, respectively, representing a cohort more enriched in non-NL than previously reported.(16, 17)
In this study, obtaining valid FibroScan studies at two separate liver sites was highly successful, confirming its feasibility in the clinical setting. Patchy involvement of CFLD has been well described,(20, 21) but there was minimal discordance between LSM and CAP values at the two locations, as evident by ICCs greater than 91% for both. However, the patients with the largest differences in LSM between the two sites were those with a NOD pattern, suggesting that patchy liver fibrosis in advanced CFLD remains characteristic.
The initial LSM and CAP values of the ELASTIC study are similar to those previously reported in other studies evaluating FibroScan in CFLD.(9, 11, 22) Gominon et al., in a single-center experience, reported higher LSM among patients characterized as having CFLD compared with no CFLD (4.7 kPa vs. 3.6 kPa) based on clinical criteria. Clinical US was obtained as part of the study but the association between LSM and US was not evaluated.(11) Similarly, in a single-center trial, Lewindon et al. reported higher LSM (10.7 kPa vs. 4.6 kPa) in CFLD compared to CF without liver diseases, determined by liver biopsy, which is not routinely obtained in the clinical setting.(12) Additionally, a metanalysis suggested that patients with CF whose LSM is ≥ 5.95 kPa and APRI ≥ 0.329 should be evaluated for CFLD. Although these studies focus on the ability to differentiate CFLD from no liver disease, our study is unique in its aim to detect changes throughout the spectrum of liver involvement in CF over time. Although the current analysis provides cross-sectional baseline data, the ultimate goal is to use information gained form ELASTIC and PUSH, to predict those patients at greatest risk of developing advanced disease in a well-characterized, multicenter, longitudinal research cohort.
In our population, patients with a NOD US pattern had a significantly higher median LSM compared with NL, HTG, and HMG patterns. Interestingly, there appeared to be a step-wise increase in LSM from NL to HTG to NOD. Although several studies have evaluated the use of LSM by FibroScan in CF,(2, 28) our study aimed to determine the association between LSM and US patterns in a multicenter and well-characterized pediatric CF cohort. Furthermore, our study further expands on the previously noted association between increased LSM and decreased platelet count among patients with CF.(22) We showed that LSM was associated with an increase in US SSAZ and decreased platelet count, suggesting that an elevated LSM may be used as a surrogate for portal hypertension.
Together, our data suggest that VCTE combined with routine screening biomarkers could be used to identify those individuals with CF most likely to have a NOD US pattern, but its use in clinical practice requires further exploration through longitudinal assessment. VCTE specifically has the advantage of being performed in a clinic quickly and at low cost, compared with conventional US. The Spearman correlation coefficients between biomarkers and LSM are weak but significant. This is largely driven by the relationship between increased LSM with conventional biomarkers outside of or at the end of their reference ranges, and influenced largely by patients with a NOD US pattern (Fig. 4). These findings are consistent with previous reports of conventional laboratory markers and biomarker indices (APRI,(2, 9, 12, 15, 22, 25) FIB-4,(2, 25) and ALT(2, 11)) having similarly been shown to be associated with LSM. Our group has reported similar relationships between US grade and conventional biomarkers as well,(19) suggesting that US, VCTE, and conventional markers of liver disease may all have some utility, independently or in combination, in the evaluation of advanced CFLD.
In our cohort, we found that CAP was significantly associated with US patterns, consistent with previous reports. Median CAP in the HMG group was significantly higher and above the previously reported pediatric steatosis cutoff.(29) In a single-center study of 129 adolescents and adults with CF who underwent a FibroScan examination, 33% were found to have steatosis, as determined by a CAP measurement > 230 dB/m (median CAP = 210 dB/m). The presence of steatosis was significantly higher among participants categorized as CFLD without portal hypertension (48%), as compared to those described as CFLD with portal hypertension (20%) and CF without liver disease (27%).(23) We found a higher mean CAP in our cohort, which may have been influenced by our decision to exclude 4 patients with a measurement of ≤100 dB/m. HMG US pattern is a common finding in steatosis but may be influenced by inflammation and fibrosis.(29) Our data suggest that a HMG US pattern may be a reasonable indicator of steatosis in CF, although overlap between US patterns was observed with mean CAP measurements among NOD subjects in particular nearing the previous described cutoff of 230 dB/m for CF steatosis.(23) This is an important consideration, as the association between increased steatosis and CAP measurements may be associated with increased LSM, as noted in some studies of adults with NAFLD,(30, 31) although others have shown no association.(32) Given the lack of knowledge concerning potential progression of steatosis to advanced liver disease in CF, and the overlap of CAP measurements between NOD and HMG groups, both LSM and CAP should be obtained and considered, along with conventional hepatic biomarkers, when conducting a FibroScan study with a M or XL probe, to most accurately examine the liver.
Our study is limited by the lack of liver biopsy data due to ethical considerations regarding the safety and utility of repeat biopsies in this population. As such, the precise etiology of the NOD pattern cannot be definitely attributed to cirrhosis, as compared with other causes of a NOD US pattern such as obliterative portal venopathy or nodular regenerative hyperplasia, which have been well described in CFLD.(33) There is a large time interval between some VCTE and US studies that was unavoidable, due to a small percentage of patients missing ideal study-window visits or study windows between PUSH and ELASTIC being offset. Given the primary aims of both studies, these windows could not be altered. Given the anticipated slow changes in both LSM and CAP, it was decided to include all data captured in the initial analysis, as this is reflective of clinical practice. Similarly, blood work to evaluate for cholestasis and/or increase in ALT (both of which have been shown to affect LSM) was not obtained at study visit. However, given that scans were completed while clinical stable, and we have previously reported rare cholestasis or significant increase in ALT in our cohort,(13) we do not feel that this affected our results. Additionally, among some US subgroups, numbers remain relatively small, which may influence the impact of our analysis. Finally, IQR ranges were broad, with overlap apparent between US classifications, likely representing the spectrum and potential progression of liver involvement in CF. Importantly, these data serve as our cohorts’ baseline, as the ELASTIC study continues to follow patients longitudinally and will look to determine whether changes in LSM and CAP are associated with changes in US pattern, conventional biomarkers of liver disease, and clinical outcomes over time.
As highly effective modulator therapies become available for most persons with CF, there is a need for reliable, noninvasive methods that detect phenotype as well as incremental changes in the severity of CFLD over time. Earlier identification of those patients at greatest risk for progression to advanced CFLD are potentially those that may benefit the greatest from earlier therapy. Here we have shown that LSM by VCTE is a noninvasive, clinically feasible tool associated with research US patterns and conventional biomarkers of liver disease. Longitudinal follow-up will allow us to examine whether changes in LSM and CAP are associated with changes in US pattern, laboratory markers, and clinical outcomes as markers of disease progression over time.