Diagnostic Performance of Transient Elastography in Biliary Atresia Among Infants With Cholestasis
Supported by the Taiwan Children Liver Foundation (TCLF 2020) and National Taiwan University Hospital (NTUH 110-T05).
Potential conflict of interest: Nothing to report.
Abstract
Biliary atresia (BA) is a challenging liver disease in infancy. Early diagnosis of BA is important for timely hepatoportoenterostomy. We evaluated the age-specific diagnostic performance of transient elastography (TE) with a liver stiffness measurement (LSM) greater than 7.7 kPa in BA among infants with cholestasis. A total of 61 infants with cholestasis (5-121 days of age) were enrolled in this prospective follow-up study; 15 infants were BA. Four age groups were defined (≤30, 31-60, 61-90, and 91-180 days). Picrosirius red staining was performed to quantify the percentage of collagen fibers in liver specimens. The utility of an LSM greater than 7.7 kPa for diagnosis of BA among infants with cholestasis was compared among age groups. In all four groups, TE showed high diagnostic power for BA using the criterion of an LSM greater than 7.7 kPa. Positive predictive values were 100%, 100%, and 100% in the groups aged 30 days or younger, 31 to 60 days, and 61 to 90 days, respectively. Respective negative predictive values were 90.9%, 94.7%, and 100%, and respective diagnostic accuracies were 92.9%, 95.2%, and 100%. The positive predictive value, negative predictive value, and diagnostic accuracy were 100%, 100%, and 100%, respectively, for LSM greater than 8.8 kPa in the group aged 91 to 180 days. The LSM was positively correlated with the percentage of collagen fibers stained by picrosirius red (P = 0.03). Conclusion: In this prospective follow-up study, TE had good diagnostic accuracy for differentiation of BA from non-BA cholestasis in infants with cholestasis who were 90 days of age or younger. The LSM was significantly positive correlated with the liver fibrosis status stained by picrosirius red in infants with cholestasis.
Abbreviations
-
- BA
-
- biliary atresia
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- HPE
-
- hepatoportoenterostomy
-
- IOC
-
- intraoperative cholangiography
-
- LSM
-
- liver stiffness measurement
-
- MRCP
-
- magnetic resonance cholangiopancreatography
-
- NPV
-
- negative predictive value
-
- PPV
-
- positive predictive value
-
- TE
-
- transient elastography
Biliary atresia (BA) is a destructive and inflammatory cholangiopathy of unclear etiology that affects the intrahepatic and extrahepatic bile ducts of neonates, contributing to fibrotic obliteration of the biliary tract and cholestatic liver injury.(1, 2) Early diagnosis of BA is essential, because early hepatoportoenterostomy (HPE) before 60 days of age may reestablish bile flow, thereby prolonging native liver survival.(3) Despite surgical treatment, BA remains the leading cause of pediatric liver transplantation worldwide.(4-6)
Early identification of BA is hampered by the overlapping clinical presentations of BA and other causes of neonatal cholestasis.(7, 8) Ultrasonography, hepatobiliary scintigraphy, and magnetic resonance cholangiopancreatography (MRCP) are helpful, but their diagnostic accuracies are insufficient. The ultrasonographic appearance of a “triangular cord sign” is suggestive of BA, with sensitivities of 49% to 73%.(9) Hepatobiliary scintigraphy has high sensitivity for BA (98.7%), but its specificity is relatively low (33%-80%).(10) MRCP for BA has a sensitivity of 90% to 99%, and specificity of 36% to 77%.(11, 12) Invasive tests such as liver biopsy and intraoperative cholangiography (IOC) remain necessary to assist in the diagnosis of BA.(13)
A noninvasive and accurate diagnostic tool for BA is required for timely diagnosis of BA; it is also necessary for the reduction of unnecessary IOCs in infants with non-BA cholestasis. Using the data available at initial assessment, Shneider et al. built a predictive model for BA using three significant factors (gamma-glutamyl transpeptidase [GGT] level [cutoff ≥ 204 IU/L], acholic stool, and weight z-score [cutoff ≤ −1.28]); this model achieved good accuracy (area under the curve = 0.83).(14)
Transient elastography (TE) is a noninvasive and reliable diagnostic tool for liver fibrosis. Because liver fibrosis progresses rapidly in patients with BA before HPE, TE is performed to assess liver stiffness in infants with cholestasis and to facilitate the differential diagnosis of BA.(15, 16) Liver stiffness measurement (LSM) by TE can be used for diagnosis of BA. An LSM cutoff of 7.7 kPa was proposed for diagnosis of BA in infants with cholestasis younger than 90 days of age; it demonstrated diagnostic accuracy of 85.3%, sensitivity of 80%, and specificity of 97%.(17) However, variations in the accuracy of BA diagnosis by TE among infants with cholestasis in different age groups are unclear.
In this prospective follow-up study, we investigated the age-specific diagnostic accuracy and predictive value of TE with an LSM greater than 7.7 kPa for BA in infants with cholestasis.
Patients and Methods
Patients and Clinical Data
In this prospective cohort study, we recruited 61 infants with cholestasis (43 boys and 18 girls; 5-121 days of age) from the Department of Pediatrics of National Taiwan University Hospital (NTUH) from January 2018 to August 2019. Patients who presented with cholestasis (serum direct bilirubin level > 1 mg/dL) were divided into two groups: BA (n = 15) and non-BA (n = 46). The diagnosis of BA was confirmed by IOC in 13 patients; it was confirmed in 2 additional patients at the time of liver transplantation. Patients in the non-BA group (n = 46) had received cholangiography to exclude BA or had a specific etiology confirmed by genetic testing or liver biopsy for the diagnosis of cholestatic diseases. These etiologies were neonatal hepatitis in 18 patients, total parenteral nutrition-related cholestasis in 6 patients, choledochal cyst in 2 patients, neonatal intrahepatic cholestasis caused by citrin deficiency in 1 patient, inborn error of bile acid metabolism in 1 patient, Zellweger syndrome in 1 patient, and hepatic hemangioma in 1 patient. Of the patients, 16 infants without a confirmed etiology did not receive liver biopsy; however, they had cholestasis that resolved spontaneously during follow-up and were therefore presumed to have idiopathic cholestasis. All patients with cholestasis underwent the routine workup for cholestasis, including blood, urine, and metabolic tests and abdominal ultrasonography. Liver biopsy was performed in 31 of 61 (50.8%) infants with cholestasis for diagnostic purposes. All patients underwent regular follow-up after workup and clinical management in our institution. Tests for blood biochemical parameters (total and direct bilirubin levels; alanine aminotransferase and GGT levels) and abdominal ultrasonography were performed regularly during follow-up. The study protocol was approved by the institutional review board of NTUH.
Liver Fibrosis Measurement by TE
From January 2018 to August 2019, TE (FibroScan 502 Touch; Echosens, Paris, France) was performed to assess the LSM during cholestatic workup before the final diagnosis. The S1 probe (5 MHz) was used for LSM with 10 shots within 3 to 5 minutes in each infant. Measurements were carried out until 10 validated results had been obtained. An interquartile range/median LSM less than 0.3 was defined as valid. The median value of 10 validated scores was considered the elastic modulus of the liver and was expressed in kilopascals.
Picrosirius Red Staining for Liver Fibrosis
Picrosirius red staining is used for histological visualization of collagen fibers.(18, 19) Liver specimens from 31 patients were obtained by needle biopsy during cholestatic workup or by wedge biopsy during surgery; 2 of these patients had inadequate specimens for further picrosirius red analysis. Picrosirius red (Abcam, Cambridge, MA) was used to quantify the percentage of collagen fibers in these 29 available liver specimens. Biopsy samples were formalin-fixed, paraffin-embedded, cut into sections, and stained with picrosirius red. The sections were scanned using a light microscope (Axioplan2; Carl Zeiss, Hallbergmoos, Germany). The picrosirius red–positive area was quantified in 10 randomly selected high-power fields per section using ImageJ software (National Institutes of Health, Bethesda, MD). Data are expressed as the proportion of the total tissue area stained with picrosirius red.
Statistical Analysis
Stata (version 14; StataCorp, College Station, TX) and MedCalc (version 19.4; MedCalc Software, Ostend, Belgium) were used for statistical analysis. For continuous variables, Student t test or the nonparametric Mann-Whitney U test was performed to assess differences among groups. The age-specific positive predictive values (PPVs), negative predictive values (NPVs), and diagnostic accuracies of LSM cutoffs greater than 7.7 kPa and GGT greater than or equal to 204 IU/L were assessed.(14, 17) Analysis of variance was performed to evaluate the statistical significance of differences among multiple groups. Linear regression analysis was also performed. A P value < 0.05 was considered statistically significant.
Results
General Patient Characteristics
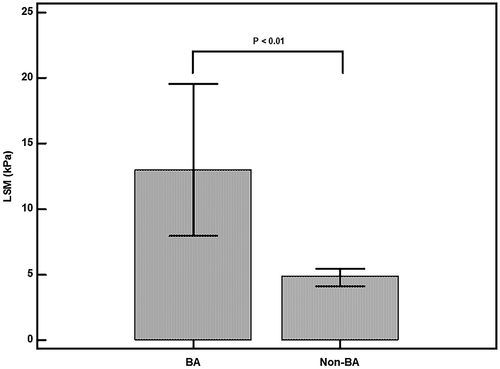
A total of 61 infants with cholestasis (43 boys and 18 girls) were enrolled in this prospective cohort study; 15 (24.6%) were diagnosed with BA, and 46 (75.4%) were diagnosed with non-BA cholestasis. The 15 infants with BA had a higher direct bilirubin level, GGT level, and LSM value than infants with non-BA cholestasis at a similar age (P < 0.01; Table 1 and Fig. 1). The median (interquartile range) age of the patients with BA who underwent TE was 30 days (range, 22-63 days). Among the patients with BA, 14 underwent MRCP for evaluation of cholestasis before IOC; the findings indicated BA in 11 patients (78.6%) (Table 2). The remaining 3 patients (LSM = 25.5, 8.3, and 17.9 kPa) were diagnosed with choledochal cysts initially by MRCP and then diagnosed with cystic-type BA during IOC. The median LSM values of the patients with BA were 8.4, 10.0, 19.4, and 40.8 kPa in the groups aged 30 days or younger, 31 to 60 days, 61 to 90 days, and 91 to 180 days, respectively (Table 3).
| Infants With Non-BA Cholestasis (n = 46) | BA (n = 15) | P Value | |
|---|---|---|---|
| Male sex, n (%) | 34 (73.9%) | 9 (60%) | 0.35 |
| Age, median (IQR), days | 35.5 (24-51.3) | 30 (22-63) | 0.64 |
| T-bil, median (IQR), mg/dL | 7.7 (5.6-11.7) | 8.5 (6.9-10.9) | 0.91 |
| D-bil, median (IQR), mg/dL | 2.7 (1.7-4.8) | 4.9 (4.1-5.9) | <0.01 |
| GGT, median (IQR),U/L | 134.5 (90-229) | 399 (184-563) | <0.01 |
| AST, median (IQR), U/L | 54 (32.5-129) | 115 (47-218) | 0.33 |
| ALT, median (IQR), U/L | 33 (13.5-78) | 79 (27-110) | 0.53 |
| LSM, median (IQR), kPa | 4.9 (3.6-5.7) | 13 (7.8-19.7) | <0.01 |
| Collagen in liver specimens by picrosirius red, median (IQR), % | 8.5 (6.5-12.2) (n = 14)* | 19.9 (13.8-27.6) (n = 15) | 0.02 |
- * A total of 31 patients underwent liver biopsy; 29 of these patients (non-BA, 14; BA, 15) had adequate specimens for picrosirius red staining.
- Abbreviations: D-bil, direct bilirubin; IQR, interquartile range; T-bil, total bilirubin.
| Sex | LSM Workup Age (Days) | LSM (kPa) | GGT (IU/L) | Total/Direct Bilirubin Ratio | Gallbladder Size (cm) | MRCP Favors BA | Cystic BA* |
|---|---|---|---|---|---|---|---|
| Female | 13 | 6.5 | 706 | 0.37 | 1.4 | Yes | No |
| Male | 14 | 25.5 | 709 | 0.5 | 2.1 | No | Yes |
| Female | 21 | 8.3 | 563 | 0.4 | 2 | No | Yes |
| Female | 22 | 13.5 | 184 | 0.58 | 1.8 | Yes | No |
| Female | 24 | 7.8 | 484 | 0.65 | 1.7 | Yes | No |
| Male | 27 | 3.3 | 114 | 0.56 | 1.9 | Yes | No |
| Male | 30 | 8.4 | 411 | 0.56 | 0 | Yes | No |
| Female | 30 | 17.9 | 169 | 0.43 | 2.2 | No | Yes |
| Male | 32 | 5.5 | 89 | 0.63 | 1.4 | N/A | No |
| Female | 39 | 10 | 1019 | 0.59 | 0.9 | Yes | No |
| Male | 50 | 13 | 399 | 0.63 | 0 | Yes | No |
| Male | 63 | 19.7 | 303 | 0.58 | 2.4 | Yes | No |
| Male | 82 | 19.1 | 377 | 0.63 | 0 | Yes | No |
| Male | 105 | 55.5 | 458 | 0.62 | 0 | Yes | No |
| Male | 121 | 26 | 285 | 0.58 | 1.6 | Yes | No |
- * Type of BA confirmed by intraoperative cholangiography.
- Abbreviation: NA, not applicable.
| Age Group (Days) | LSM of BA, Median (IQR), kPa | LSM of Non-BA, Median (IQR), kPa |
|---|---|---|
| ≦30 | 8.4 (6.8-16.8) (n = 8) | 4.2 (3.3-5.4) (n = 20) |
| 31-60 | 10 (5.5-13) (n = 3) | 5.4 (3.8-6.2) (n = 18) |
| 61-90 | 19.4 (19.1-19.7) (n = 2) | 5.5 (4.5-6.1) (n = 5) |
| 91-180 | 40.8 (26-55.5) (n = 2) | 3.8 (3.2-8.8) (n = 3) |
- Abbreviation: IQR, interquartile range.
Correlation Between LSM and Liver Fibrosis on Liver Histology
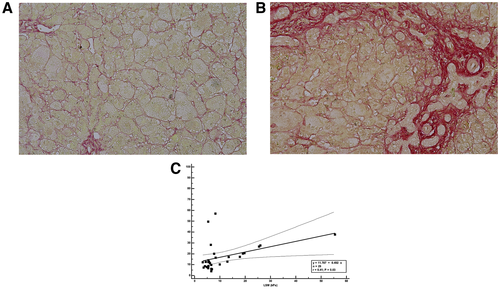
The quantity of collagen fibers in the liver was significantly higher in patients with BA than in patients with non-BA cholestasis of similar age at cholestatic workup (19.9% vs. 8.5%; P = 0.02; Table 1 and Fig. 2A, B). A significant positive correlation was detected between the LSM and the percentage of collagen fibers (P = 0.03; Fig. 2C).
Age-Specific Diagnostic Role of an LSM Greater Than 7.7 kPa for BA
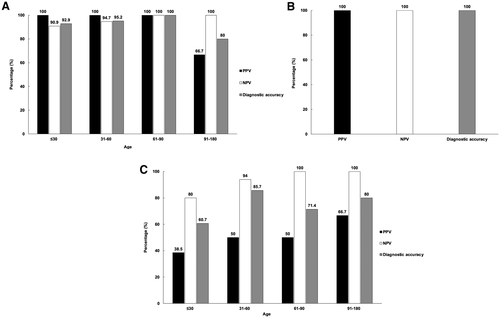
In the overall cohort, the PPV and NPV of an LSM cutoff greater than 7.7 kPa for BA among all infants with cholestasis were 92.3% and 93.8%, respectively; a diagnostic accuracy of 93.4% was achieved.
The patients with cholestasis were divided into four age groups (≤30, 31-60, 61-90, and 91-180 days) according to the age at which they underwent TE for cholestatic workup. The diagnostic performance of TE for BA is shown in Fig. 3A. In the group aged 30 days or younger (n = 28), an LSM threshold greater than 7.7 kPa yielded a PPV, NPV, and diagnostic accuracy of 100%, 90.9%, and 92.9%, respectively. In the group aged 31 to 60 days (n = 21), the criterion achieved a PPV of 100%, an NPV of 94.7%, and a diagnostic accuracy of 95.2%. In the group aged 61 to 90 days (n = 7), the PPV, NPV, and diagnostic accuracy were all 100%. In the group aged 91 to 180 days (n = 5), the criterion achieved a PPV of 66.7%, an NPV of 100%, and a diagnostic accuracy of 80%. For the group aged 91 to 180 days, a higher LSM cutoff greater than 8.8 kPa was analyzed by receiver operating characteristic curve to predict BA measured by TE (sensitivity = 100%; specificity = 100%; area under the curve [AUC] = 100%; P < 0.001). The PPV, NPV, and diagnostic accuracy were 100%, 100%, and 100%, respectively, for LSM greater than 8.8 kPa in the group aged 91 to 180 days (Fig. 3B). This group included a patient with non-BA cholestasis whose LSM was 8.8 kPa at 107 days of age. This patient exhibited cholestasis at 30 days of age; BA had been excluded by IOC. The final diagnosis of this patient was inborn errors of bile acid metabolism, caused by cytochrome 7B1 (CYP7B1) mutations, which were confirmed by genetic analysis.
Diagnostic Role of a Serum GGT Level of Greater Than or Equal to 204 IU/L for BA
A GGT level of greater than or equal to 204 IU/L was used to distinguish BA from non-BA.(14) The criterion was applied to assess the age-specific diagnostic performance for BA of a GGT of greater than or equal to 204 IU/L (Fig. 3C). In the group aged 30 days or younger (n = 28), the PPV was 38.5%, NPV was 80%, and diagnostic accuracy was 60.7%. In the group aged 31 to 60 days (n = 21), the criterion yielded a PPV of 50%, an NPV of 94%, and a diagnostic accuracy of 85.7%. In the group aged 61 to 90 days (n = 7), the criterion achieved a PPV, NPV, and diagnostic accuracy of 50%, 100%, and 71.4%, respectively. In the group aged 91 to 180 days (n = 5), a GGT greater than or equal to 204 IU/L yielded similar PPV, NPV, and diagnostic accuracy (66.7%, 100%, and 80%, respectively) compared with an LSM greater than 7.7 kPa (66.7%, 100%, and 80%, respectively).
Discussion
The infant stool-color card is used to screen for infants with cholestasis.(14, 20) Harpavat et al. implemented newborn screening for BA with direct or conjugated bilirubin measurements.(21) The age of patients at the time of cholestatic workup for BA was significantly younger after these screening programs.(21) However, the time needed for the workup of BA among these young infants with cholestasis was unchanged.(21) Furthermore, clinicians may encounter younger infants with cholestasis after these screening programs. Hence, a noninvasive, feasible, and accurate diagnostic modality should be developed; age-specific criteria for these diagnostic modalities are needed.
TE could be used to assess liver stiffness as a surrogate marker for liver fibrosis; moreover, liver stiffness is strongly correlated with histological fibrosis stage.(15-17, 22-24) TE can rapidly and reliably measure liver stiffness in adults and children with various liver disorders. Wu et al. demonstrated that the optimal cut-off LSM for diagnosis of BA in infants with cholestasis was 7.7 kPa.(17)
We performed a follow-up study to assess the utility of TE with an LSM cutoff of 7.7 kPa in the diagnosis of BA among groups of infants with cholestasis at different ages. The results demonstrated that noninvasive scoring for liver fibrosis with TE was effective among infants with cholestasis. Using an LSM cutoff of greater than 7.7 kPa defined in our previous study,(17) this criterion was applied to our four age groups, and its diagnostic value for significant fibrosis was assessed. The diagnostic accuracy of LSM greater than 7.7 kPa for differentiation between BA and non-BA was high in patients younger than 90 days of age. The NPV of LSM greater than 7.7 kPa for the diagnosis of BA increased from 90.9% in the group aged 30 days or younger to 94.7% in the group aged 31 to 60 days; it was 100% in the groups aged 61 to 90 days and 91 to 180 days. The increasing NPV with age indicates that all infants with BA developed significant liver fibrosis after 61 days of age, indicating that HPE in infants older than 60 days of age has reduced clinical benefit. Infants with cholestasis who exhibit an initial LSM less than 7.7 kPa may need further serial TE monitoring to alleviate cholestasis.(24)
Although the PPV of an LSM greater than 7.7 kPa for BA was 100% in infants younger than 90 days of age, it decreased to 66.7% in the group aged 91 to 180 days. These results indicate that although the progression of liver fibrosis caused by other cholestatic liver diseases in infancy is slower than in infants with BA, it can progress to chronic fibrotic liver disease over time, resulting in considerable liver stiffness. This explains the reduction of PPV in infants older than 91 days of age. In the group aged 91 to 180 days, a patient diagnosed with inborn errors of bile acid metabolism had an LSM value of 8.8 kPa at 107 days of age. The liver may develop significant fibrosis in other non-BA cholestatic diseases in infants older than 91 days of age.
Cystic-type BA is a variant of BA. It can be difficult to differentiate cystic BA from choledochal cysts because they look very similar.(25, 26) In this study, 3 patients had an initial MRCP interpretation of choledochal cysts but were later diagnosed with cystic-type BA by IOC. Their LSM values were greater than 7.7 kPa (25.5, 8.3, and 17.9 kPa), providing early signs of the development of liver fibrosis. Surgical intervention was arranged, and the patients underwent HPE. Therefore, the use of LSM and MRCP during cholestatic evaluation could enable early diagnosis of BA.
Shen et al. explored the diagnostic accuracy for BA of parallel testing of GGT and LSM by the SuperSonic system among patients with cholestasis separated into three age groups (≤60 days, n = 124; 61-90 days, n = 124; and 91-120 days, n = 34) in a retrospective study.(27) Infants younger than 30 days of age (9 patients) were not grouped separately because of the small number. The optimal cutoff LSM value in the group aged 60 days or younger was 7.5 kPa (AUC of 0.761, sensitivity of 87.8%, specificity of 58.0%). After the initiation of cholestatic screening programs, particularly infant stool-color card and direct bilirubin, a greater number of infants with cholestasis aged 30 days or younger may be encountered.(14, 21) In our prospective study, an LSM greater than 7.7 kPa yielded a high PPV, NPV, and diagnostic accuracy (100%, 90.9%, and 92.9%, respectively) in infants with cholestasis aged 30 days or younger.
The predicted probability model for BA of Shneider et al. (GGT ≥ 204 IU/L, acholic stools, and weight z-score ≤ −1.28) enabled accurate diagnosis of BA in 290 of 357 patients (81%) with a predictive probability of greater than 0.8.(14) We applied the criterion of GGT greater than or equal to 204 IU/L to our study groups and compared its diagnostic value with that of LSM for BA. LSM had higher diagnostic accuracy in infants with cholestasis younger than 90 days of age.
Histopathologic assessment of liver fibrosis on liver biopsy remains the gold standard for determining the severity of fibrosis; the METAVIR scoring system is widely used.(28) Several studies used TE to determine LSM in children with various liver diseases, using different cutoff points for advanced fibrosis. Shen et al. reported that an LSM cutoff of 15.15 kPa was optimal for identification of stage F4 in patients with BA in the METAVIR scoring system.(15) A prospective study at Boston Children’s Hospital revealed that LSM cutoffs of 8.6 and 11.5 kPa were predictive of METAVIR F3-F4 and F4 fibrosis in unselected children and young adults.(29) Our study demonstrates the correlation between quantification of percentage collagen stained by picrosirius red staining with LSM in cholestatic infants. A higher abundance of collagen fibers was found in the liver specimens of patients with BA. Infants with BA had a greater severity of liver fibrosis than infants with cholestasis who did not exhibit BA (P = 0.02). We also demonstrated that the LSM value was significantly correlated with the percentage of collagen fibers stained by picrosirius red (P = 0.03). For infants with cholestasis in whom liver biopsy poses a considerable health risk, LSM may represent a noninvasive alternative method for assessment of liver fibrosis status.
The LSM of patients with BA increased significantly with age before HPE.(17) This trend was also observed in the present study; LSM and liver fibrosis quantified using picrosirius red correlated with increasing age in both patients with BA and patients with non-BA cholestasis. This finding indicates that in the presence of increasing severity or longer duration of biliary fibrosis, liver stiffness may increase over time.
A notable limitation of this study was that, although the overall cohort is the largest among published articles thus far, it included relatively few patients in each age group. In the group of 91-180 days of age, only 1 patient in the non-BA group (n = 3) had an LSM of 8.8 kPa. On the other hand, high LSM in the BA group (n = 2) with more than 90 days of age may be caused by portal hypertension with time. For this group, we identified a higher LSM cutoff greater than 8.8 kPa to predict BA measured by TE, but our case number is relatively small in this group compared with others. Under the regular screening for cholestasis using the infant stool-color card, most of the patients could be identified earlier, and the rate of patients who diagnosed over the age of 90 days was significantly decreased in Taiwan. (20) A different cutoff should be provided for this group, to accurately differentiate BA from non-BA cholestatic diseases in the future, when more case numbers are included. Furthermore, patients with some rare cholestatic liver diseases were not included in the study; a greater number of patients with different diagnoses is therefore needed to validate our results.
In conclusion, LSM determined by TE is a noninvasive liver fibrosis marker for infants with cholestasis. Infants with BA had significantly greater liver fibrosis severity according to picrosirius red staining and LSM values, compared to other infants with cholestasis. A cutoff LSM of greater than 7.7 kPa had high diagnostic accuracy for BA in a variety of age groups, particularly infants aged 30 days or younger, 31 to 60 days, and 61 to 90 days. TE is a safe and well-tolerated tool for prediction of liver fibrosis in patients with BA and may serve as a surrogate predictor of BA among infants with cholestasis. With increasing data regarding the validity of LSM in pediatric patients, TE may enable assessment of liver fibrosis status in patients with BA as well as patients with other cholestatic liver disorders of early infancy.
Acknowledgment
The authors thank Ms. Hui-Chuan Lee from the Department of Pediatrics of National Taiwan University Hospital for the technical support in TE examination, and Prof. Chia-Hsiang Yang from National Taiwan University Hospital for the assistance with data analysis.