Telomerase reverse transcriptase mutations in plasma DNA in patients with hepatocellular carcinoma or cirrhosis: Prevalence and risk factors
Potential conflict of interest: Nothing to report.
Supported by the National Institutes of Health/National Cancer Institute (award number P30CA016672, for the use of the Sequencing and Microarray Facility, to E. D). UTHealth Innovation for Cancer Prevention Research Training Program Postdoctoral Fellowship (grant RP160015 to J.J.), National Cancer Institute Cancer Prevention Fellowship (grant R25E CA056452 to G.W.), and Trans-Texas Hepatocellular Carcinoma Study and a start-up fund from the University of Texas MD Anderson Cancer Center, and National Institutes of Health/National Cancer Institute R01 award 1R01CA195524 (to L.B.).
Abstract
Telomerase reverse transcriptase (TERT) mutation is the most frequent genetic alteration in hepatocellular carcinoma (HCC). Our aims were to investigate whether TERT mutations can be detected in circulating cell-free DNA (cfDNA) of patients with HCC and/or cirrhosis and characterize clinical parameters associated with these mutations. We retrieved data on TERT C228T and C250T promoter mutations in 196 HCCs from The Cancer Genome Atlas. We measured these TERT mutations in plasma cfDNA in 218 patients with HCC and 81 patients with cirrhosis without imaging evidence of HCC. The prevalence of TERT mutations in The Cancer Genome Atlas HCC specimens was 44.4%. TERT mutations were detected with similar prevalence (47.7%) in plasma cfDNAs from 218 patients with HCC. TERT mutations, either within the HCC or in cfDNA, were associated with male sex, hepatitis C virus (HCV), alcoholic cirrhosis, family history of cancer, and poor prognosis. The high prevalence of TERT mutations in HCCs in male patients with cirrhosis caused by HCV and/or alcohol was confirmed in an independent set of HCCs (86.6%). Finally, TERT mutations were detected in cfDNA of 7 out of 81 (8.6%) patients with cirrhosis without imaging evidence of HCC, including 5 male patients with cirrhosis due to HCV and/or alcohol. Genes involved in xenobiotic and alcohol metabolism were enriched in HCCs with TERT mutations, and vitamin K2 was identified as an upstream regulator. Conclusion: TERT mutations are detectable in plasma cfDNA. Long-term imaging surveillance of patients with cirrhosis with cfDNA TERT mutations without evidence of HCC is required to assess their potential as early biomarkers of HCC. (Hepatology Communications 2018;2:718-731)
Abbreviations
-
- ABCB
-
- adenosine triphosphate binding cassette subfamily B
-
- AOR
-
- adjusted odds ratio
-
- cfDNA
-
- cell-free DNA
-
- CI
-
- confidence interval
-
- ddPCR
-
- droplet digital polymerase chain reaction
-
- FDR
-
- false discovery rate
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- IPA
-
- ingenuity pathway analysis
-
- mRNA
-
- messenger RNA
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- OR
-
- odds ratio
-
- PCR
-
- polymerase chain reaction
-
- SNP
-
- single-nucleotide polymorphism
-
- TCGA
-
- The Cancer Genome Atlas
-
- TERT
-
- telomerase reverse transcriptase
-
- UGT
-
- uridine diphosphoglucuronate-glucuronosyltransferase
-
- wt
-
- wild-type
Hepatocellular carcinoma (HCC) is the predominant form of liver cancer and the second leading cause of cancer mortality worldwide.1 In the United States, the incidence of HCC is increasing at the second fastest rate behind thyroid cancer. Furthermore, HCC mortality is increasing at the highest rate of all cancers despite a 25% decline in overall cancer mortality from 1991 to 2014.2 Cirrhosis of the liver, regardless of etiology, is the primary risk for HCC development. Other comorbid risk factors for HCC include chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infections, alcohol abuse, nonalcoholic fatty liver disease (NAFLD), and aflatoxin exposure. Currently, prognosis for patients with HCC is dismal, with a 5-year survival rate of 17%. HCC can be cured when detected at an early stage when it is amenable to resection or orthotopic liver transplantation. However, only 30% of patients with HCC are candidates for surgical intervention at the time of diagnosis.3 Thus, there is an urgent unmet need for biomarkers to identify individuals at high risk for HCC and to detect HCC at an early stage.
Recent genomic studies have identified telomerase reverse transcriptase (TERT), tumor protein p53 (TP53), and catenin beta 1 (CTNNB1) as the most frequently mutated genes in HCC.4-6 TERT promoter mutation is the most frequent genetic alteration in HCC6, 7 and a central and ancestry-independent node of hepatocarcinogenesis.4 Most importantly, TERT promoter mutations occur also in cirrhotic preneoplastic macronodules (25%) and in hepatocellular adenomas with malignant transformation to HCC (44%), therefore representing early recurrent genetic events in HCC in the cirrhotic and noncirrhotic liver.7, 8 In cirrhosis, TERT promoter mutations are highly related to stepwise hepatocarcinogenesis, with mutations identified in 6% of low-grade dysplastic nodules, 19% of high-grade dysplastic nodules, and 61% of early HCCs.9
Considering the high frequency of TERT promoter mutations in HCC and their occurrence in premalignant lesions, we investigated whether TERT promoter mutations could be detected in plasma cell-free DNA (cfDNA) in patients with HCC and in patients with cirrhosis. We also characterized the demographic or clinical parameters associated with the presence of these mutations in these patient groups.
Materials and Methods
PATIENTS, DATA SOURCES, AND BIOSPECIMENS
This study was approved by the institutional review boards of all collaborating institutions. The Cancer Genome Atlas (TCGA) HCC data were extracted from a recent study,6 the cBioPortal online platform, and Firebrowse.10 Clinical and demographic parameters from 196 HCCs with available TERT promoter mutations data in TCGA are summarized in Supporting Table S1. Plasma samples from 218 histologically confirmed patients with HCC were collected between 2002 and 2010, prior to treatment at MD Anderson Cancer Center. The demographic and clinical parameters of these 218 patients with HCC are described in Supporting Table S2. Formalin-fixed paraffin-embedded HCCs of 15 patients with HCC were collected at the University of Texas Medical Branch. The demographic and clinical parameters of these patients with HCC are described in Supporting Table S3. Plasma samples from 81 patients with cirrhosis but no HCC were collected at Baylor-St Luke's Hospital. The demographic and clinical parameters of these 81 patients with cirrhosis are described in Supporting Table S4. cfDNA was extracted from plasma samples (250-500 μL) using the QiAamp circulating nucleic acid kit (Qiagen). cfDNA peaks at a size range of 75-225 base pairs were visualized on a fragment analyzer (Advanced Analytical Technologies) and their heights used to estimate cfDNA amount. DNA was extracted from tumor, adjacent liver, and distant nontumoral liver areas, using the QIAamp DNA formalin-fixed paraffin-embedded tissue kit (Qiagen). DNA samples were then quantified using the Qubit fluorometer and the double-stranded DNA high-sensitivity assay kit (Thermo Fisher Scientific). DNA quality was assessed using a fragment analyzer and high sensitivity genomic DNA analysis kit (Advanced Analytical Technologies).
TCGA HCC MESSENGER RNA EXPRESSION ANALYSIS
Tumoral messenger RNA (mRNA) expression data and TERT promoter mutation data were available for 193 HCCs in TCGA. We compared gene expression in tumors with and without TERT promoter mutations, using feature-by-feature t tests and fit the resulting P values to the beta-uniform mixture model to allow for estimation of the false discovery rate (FDR) and to determine P value cutoffs for specified FDR values. Linear gene expression fold changes were then calculated for each gene between the two tumor groups. We considered genes to be differentially expressed if the linear fold change had an absolute value greater than 1.5 and the P value was less than the modeled cutoff at an FDR of 5% (corresponding P value of 0.0038). Gene expression analysis was conducted in R version 3.3 using the ClassComparison and ClassDiscovery packages.
DROPLET DIGITAL POLYMERASE CHAIN REACTION
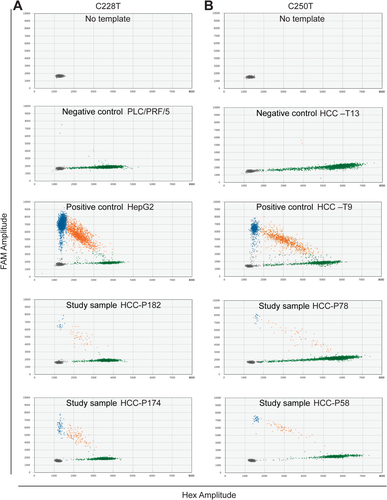
TERT C228T and C250T promoter mutations were detected by polymerase chain reaction (PCR) using the QX200 droplet digital PCR (ddPCR) system (Bio-Rad Laboratories, Inc.) and the dHsaEXD72405942 assay for TERT C228T and dHsaEXD46675715 assay for TERT C250T. Mutant and wild-type (wt) TERT alleles were differentiated by the fluorophores attached to the probes, with HEX fluorescence for wt TERT alleles and FAM fluorescence for mutant TERT alleles. A total of 4 μL of DNA per 20 μL ddPCR reaction was used in each reaction. Thermocycling conditions were 95°C for 10 minutes followed by 50 cycles of 96°C for 30 seconds and 62°C for 1 minute followed by 98°C for 10 minutes. The sealed plates were then placed in the droplet reader for detection of complete ddPCR reactions in individual droplets. The data were analyzed using QuantaSoft software (Bio-Rad Laboratories, Inc.). Samples with a mutant TERT allele fraction ≥0.1% and with at least two visible mutant signals were considered positive for the mutation, as described.11, 12 Because the two TERT promoter mutations are mutually exclusive,6, 7 we measured TERT C250T mutation in samples that were negative for TERT C228T mutation.
SANGER SEQUENCING
TERT promoter PCR was performed in 20-μL reactions and using a hotstart DNA polymerase (KAPA Biosystems) activation (95°C, 15 minutes), 40 denaturation cycles (94°C, 30 seconds), primer annealing (62°C, 30 seconds), and extension (72°C, 30 seconds) followed by a final extension (72°C, 5 minutes). PCR products were purified using the QIAquick Gel Extraction kit (Qiagen). Purified PCR products were sent to the MD Anderson Sequencing and Microarray Facility for Sanger sequencing. The primers used for PCR amplification and sequencing were forward CAGCGCTGCCTGAAACTC, reverse GTCCTGCCCCTTCACCTT. The sequences were visualized and analyzed using FinchTV software (Geospiza).
STATISTICAL ANALYSIS
Two-tailed χ2 tests and Fisher exact tests were used to test independence of patient characteristics and TERT promoter mutations in all data sets. Univariable logistic regression was used to generate odds ratios (ORs) and 95% confidence intervals (CIs) for the association of each patient characteristic with TERT promoter mutations. In addition, we calculated the adjusted OR (AOR) and 95% CI for each clinical parameter after adjusting for age and sex. To ensure that our results were not confounded by the amount of cfDNA extracted from plasma, we repeated the logistic regression analysis controlling for cfDNA concentration. We assessed the difference in overall survival in patients with and without TERT promoter mutations by visualizing Kaplan-Meier survival curves and testing the difference of the survival curves using the log-rank test. Analyses were conducted in R version 3.3.
Results
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PATIENTS WITH HCC WITH TERT MUTATIONS IN TUMORS
Two TERT promoter mutations, chr5 1,295,228 G>A (C228T) and 1,295,250 G>A (C250T), were measured by Sanger sequencing in 196 patients with HCC in TCGA and recently reported.6 The clinical and demographic characterization of these 196 patients are summarized in Supporting Table S1. The majority of these patients were male (65.8%) and non-Hispanic Caucasian individuals (57.8%), and the prevalence of obesity was 22.9%. These HCCs were associated with HCV (17.9%), HBV (22.4%), or NAFLD (4.3%), and for 27.4% of them, no known risk factor was identified. Only 25.3% of these patients had underlying cirrhosis. The majority of these HCCs were early stage (41.3% stage I and 23.4% stage II). The overall prevalence of TERT promoter mutations was 44.4%, and the two mutations C228T and C250T were mutually exclusive. We further characterized the patients with HCC with TERT promoter mutations (Table 1). We found that the presence of TERT promoter mutations was associated with male sex (OR, 2.5; 95% CI, 1.3-4.7; P = 0.005), older age (>65 years) (OR, 2.3; 95% CI, 1.3-4.3; P = 0.003), and HCV (OR, 2.5; 95% CI, 1.2-5.4; P = 0.002) and negatively associated with HBV (OR, 0.4; 95% CI, 0.2-0.8; P = 0.011). We also found that TERT promoter mutations were associated with family history of cancer (OR, 2.1; 95% CI, 1.1-4.1; P = 0.018), the presence of cirrhosis (OR, 2.0; 95% CI, 1.0-3.9; P = 0.044), and alcoholic etiology (OR, 2.3; 95% CI, 1.2-4.3; P = 0.005). Indeed, TERT promoter mutations were found in most patients with alcoholic etiology (58.5%), alcoholic cirrhosis in particular (66.7%). After adjusting for age and sex, there were no changes in significance or direction of association with male sex, older age, and HBV; significance became stronger for family history of cancer (AOR, 2.7; 95% CI, 1.3-5.9; P = 0.007), and association with cirrhosis and alcoholic etiology became nonsignificant. Finally, we did not find any association with obesity, NAFLD, tumor stage, or any laboratory test (alpha-fetoprotein, platelet count, albumin, and bilirubin).
| Mutated | Nonmutated | OR (95% CI) | P value | AOR* (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Overall (n = 196) | 87 (44.4%) | 109 (55.6%) | - | - | - | - |
| Sex | 0.005 | 0.002 | ||||
| Female | 20 (29.9%) | 47 (70.1%) | REF | REF | ||
| Male | 67 (51.9%) | 62 (48.1%) | 2.5 (1.3-4.7) | 2.8 (1.5 -5.5) | ||
| Age at diagnosis† | 66 (20-90) | 60 (20-84) | 1.5 (1.2-1.9) | <0.001 | 1.5 (1.2-2.0) | <0.001 |
| Age at diagnosis | 0.003 | 0.003 | ||||
| ≤65 years | 41 (35.7%) | 74 (64.3%) | REF | REF | ||
| >65 years | 46 (56.8%) | 35 (43.2%) | 2.3 (1.3-4.3) | 2.6 (1.4-5.1) | ||
| Smoking‡ | 0.336 | 0.282 | ||||
| No | 80 (44.0%) | 102 (56.0%) | REF | REF | ||
| Yes | 7 (58.3%) | 5 (41.7%) | 1.8 (0.6-6.3) | 2.0 (0.6-7.3) | ||
| Family history of cancer | 0.018 | 0.007 | ||||
| No | 31 (33.7%) | 61 (66.3%) | REF | REF | ||
| Yes | 37 (52.1%) | 34 (47.9%) | 2.1 (1.1-4.1) | 2.7 (1.3-5.9) | ||
| BMI | 26.0 (14.5-44.3) | 23.8 (16.3-56.1) | 1.0 (1.0-1.0) | 0.254 | 1.0 (1.0-1.1) | 0.546 |
| Obese (BMI ≥30) | 0.925 | 0.743 | ||||
| No | 55 (43.0%) | 73 (57.0%) | REF | REF | ||
| Yes | 17 (43.6%) | 22 (56.4%) | 1.0 (0.5-2.0) | 0.9 (0.4-1.9) | ||
| Cirrhosis | 0.044 | 0.145 | ||||
| No | 56 (39.4%) | 86 (60.6%) | REF | REF | ||
| Yes | 27 (56.3%) | 21 (43.8%) | 2.0 (1.0-3.9) | 1.7 (0.8-3.4) | ||
| Cirrhosis/alcohol | 0.067 | 0.284 | ||||
| No | 56 (39.4%) | 86 (60.6%) | REF | REF | ||
| Yes (nonalcoholic) | 15 (50%) | 15 (50%) | 1.5 (0.7-3.3) | 1.5 (0.6-3.4) | ||
| Yes (alcoholic) | 12 (66.7%) | 6 (33.3%) | 3.0 (1.1-9.0) | 2.2 (0.8-7.0) | ||
| Alcoholic etiology | 0.005 | 0.176 | ||||
| No | 46 (38.0%) | 75 (62.0%) | REF | REF | ||
| Yes | 38 (58.5%) | 27 (41.5%) | 2.3 (1.2-4.3) | 1.5 (0.8-3.0) | ||
| HCV | 0.002 | 0.04 | ||||
| Negative | 65 (40.4%) | 96 (59.6%) | REF | REF | ||
| Positive | 22 (62.9%) | 13 (37.1%) | 2.5 (1.2-5.4) | 2.3 (1.1-5.2) | ||
| HBV | 0.011 | 0.018 | ||||
| Negative | 75 (49.3%) | 77 (50.7%) | REF | REF | ||
| Positive | 12 (27.3%) | 32 (72.7%) | 0.4 (0.2-0.8) | 0.4 (0.2-0.8) | ||
| NAFLD | 0.105 | 0.177 | ||||
| No | 78 (43.8%) | 100(56.2%) | REF | REF | ||
| Yes | 6 (75%) | 2 (25%) | 3.8 (0.9-26.7) | 3.3 (0.7-25.3) | ||
| No known risk factor | 0.097 | 0.463 | ||||
| No | 67 (48.9%) | 70 (51.1%) | REF | |||
| Yes | 18 (35.3%) | 33 (64.7%) | 0.6(0.3-1.1) | 0.7(0.3-1.7) | ||
| Stage | 0.338 | 0.835 | ||||
| I | 37 (48.7%) | 39 (51.3%) | REF | REF | ||
| II | 17 (39.5%) | 26 (60.5%) | 0.7 (0.3-1.5) | 0.9 (0.4-2.1) | ||
| III/IV§ | 24 (36.9%) | 41 (63.1%) | 0.6 (0.3-1.2) | 0.8 (0.4-1.7) | ||
| AFP ‖ | 11 (1-308,836) | 24 (1-2,035,400) | 0.7 (0.5-1.0) | 0.255 | 1.0 (0.9-1.1) | 0.644 |
| Platelet count‖ | 200 (88-608) | 243 (102-602) | 1.0 (1.0-1.0) | 0.553 | 1.0 (1.0-1.0) | 0.512 |
| Serum albumin‖ | 3.8 (0.4-5.2) | 4 (0.2-6.9) | 1.1 (0.8-1.5) | 0.615 | 1.2 (0.9-1.6) | 0.277 |
| Bilirubin‖ | 0.7 (0.3-2.3) | 0.7 (0.1-13) | 1.0 (0.6-1.7) | 0.981 | 0.8 (0.4-1.5) | 0.462 |
- Data are presented as frequency (%) or as median (range). *Adjusting for age and sex; †ORs calculated for 10-year age intervals; ‡smoking noted as “other risk factor” in patient record; §collapsed stage III/IV due to small number of stage IV cases; ‖ORs for log-transformed values.
- Abbreviations: AFP, alpha-fetoprotein; BMI, body mass index; REF, reference value.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PATIENTS WITH HCC WITH TERT PROMOTER MUTATIONS IN PLASMA cfDNA
We then measured TERT C228T and C250T promoter mutations in cfDNA extracted from plasma collected from 218 patients with HCC. The demographic and clinical parameters of these 218 patients are summarized in Supporting Table S2. The ethnicity/race distribution among these 218 patients with HCC was non-Hispanic Caucasian (54.6%), Hispanic (16.1%), Asian (4.6%), Black (7.8%), and unknown (17%). The majority of these patients were male individuals (72%), and 37.9% were obese, 40.8% had diabetes, 32.5% were positive for HCV, 20.9% were positive for HBV, and 10.9% had NAFLD. Underlying cirrhosis was present in 54.5% of the patients, with 19.1% alcoholic cirrhosis and 35.4% nonalcoholic cirrhosis. No known risk factor could be identified in 9.5% of the patients. The distribution of well-, moderately, and poorly differentiated tumors was 39.6%, 34.3%, and 26.1%, respectively. We measured TERT C228T and C250T mutations in plasma cfDNA using ddPCR. Droplets positive for mutant alleles, positive for wt alleles, and double positive for both mutant and wt alleles were clearly separated, as shown in Fig. 1. Black, blue, green, and orange dots represent empty droplets, mutant positive droplets, wt-positive droplets, and double-positive wt and mutant droplets, respectively. PLC/PRF-5 and HepG2 cell lines, not harboring and harboring TERT C228T mutation, respectively, were used as controls (Fig. 1A). HCC tumor samples HCC-T13 and HCC-T9, not harboring and harboring TERT C250T mutation, respectively, as determined by Sanger sequencing (Supporting Fig. S1), were also used as controls (Fig. 1B). TERT C228T or C250T mutation was detected in 104 out of the 218 HCCs (47.7%), with mutant allele fractions ranging from 0.1% to 50.2%. As observed in the tumors, the distribution of TERT promoter mutations differed according to demographic and clinical characteristics (Table 2). As for TCGA HCCs, we found that the presence of TERT promoter mutations was associated with male sex (OR, 3.1; 95% CI, 1.6-6.3; P = 0.0003) and the presence of cirrhosis (OR, 1.7; 95% CI, 1.0-3.1; P = 0.041) with alcoholic cirrhosis in particular (OR, 2.2; 95% CI, 1.1-4.8). TERT promoter mutations were again found in most patients with alcoholic cirrhosis (62.5%). Associations with HCV (OR, 1.5; 95% CI, 0.8-2.6; P = 0.192) and with family history of cancer (OR, 1.7; 95% CI, 0.9-3.0; P = 0.080) were also found but did not reach significance. As for TCGA HCCs, the prevalence of TERT promoter mutations was low in patients with NAFLD. We further found an inverse association between TERT promoter mutations and obesity (OR, 0.6; 95% CI, 0.3-0.8; P = 0.039) or history of diabetes (OR, 0.5; 95% CI, 0.3-0.8; P = 0.010). In contrast to TCGA HCCs, no associations with older age or inverse association with HBV were found. After adjusting for age and sex, there were no changes in significance or direction of association with male sex, the presence of cirrhosis, HCV, and family history of cancer; the inverse association became stronger with diabetes (AOR, 0.4; 95% CI, 0.2-0.7; P = 0.003), while association with alcoholic cirrhosis and inverse association with obesity became not significant. Additional associations were identified, such as place of birth in the United States or Canada (AOR, 2.7; 95% CI, 1.4-5.6; P = 0.047) and multicentric tumor (AOR, 2.2; 95% CI, 1.2-4.2; P = 0.012). In addition, the odds of having a TERT promoter mutation increased with tumor stage (overall P value 0.0003). However, tumor stage was also associated with male sex (P = 0.0171), with over 75% of stage III/IV tumors diagnosed in male patients (data not shown). Therefore, male sex partially explains the association between stage and TERT promoter mutations.
| Mutated | Nonmutated | OR (95% CI) | P value | AOR* (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Overall (n = 218) | 104 (47.7%) | 114 (52.3%) | - | - | - | - |
| Sex | 0.0003 | 0.0008 | ||||
| Female | 16 (29.1%) | 39 (70.9%) | REF | REF | ||
| Male | 79 (56.4%) | 61 (43.6%) | 3.1 (1.6-6.3) | 3.2 (1.6-6.3) | ||
| Age at diagnosis† | 64 (41-88) | 67 (30-85) | 1.0 (0.7-1.2) | 0.726 | 1.0 (0.7-1.2) | 0.775 |
| Age at diagnosis | 0.278 | 0.399 | ||||
| ≤ 65 years | 53 (52.5%) | 48 (47.5%) | REF | REF | ||
| >65 years | 42 (44.7%) | 52 (55.3%) | 0.7 (0.4-1.3) | 0.8 (0.4-1.4) | ||
| Birthplace | 0.024 | 0.047 | ||||
| Outside US/Canada | 6 (27.3%) | 16 (72.7%) | REF | REF | ||
| US/Canada | 81 (50.9%) | 78 (49.1%) | 3.0 (1.2-8.6) | 2.7 (1.4-5.6) | ||
| Smoking status | 0.329 | 0.680 | ||||
| Never | 30 (42.3%) | 41 (57.7%) | REF | REF | ||
| Former | 42 (49.4%) | 43 (50.6%) | 1.3 (0.7-2.5) | 0.9 (0.4-1.8) | ||
| Current | 19 (57.6%) | 14 (42.4%) | 1.9 (0.8-4.3) | 1.3 (0.5-3.1) | ||
| Family history of cancer | 0.080 | 0.095 | ||||
| No | 32 (41.0%) | 46 (59.0%) | REF | REF | ||
| Yes | 63 (53.8%) | 54 (46.2%) | 1.7 (0.9-3.0) | 1.7 (0.9-3.1) | ||
| BMI | 27.4 (18.9-20.7) | 29.3 (19.2-50.7) | 1.0 (0.9-1.0) | 0.145 | 1.0 (0.9 -1.0) | 0.296 |
| Obese (BMI ≥30) | 0.039 | 0.095 | ||||
| No | 70 (53.4%) | 61 (46.6%) | REF | REF | ||
| Yes | 31 (38.8%) | 49 (61.3%) | 0.6 (0.3-0.8) | 0.6 (0.3-1.1) | ||
| History of diabetes | 0.010 | 0.003 | ||||
| No | 71 (55.0%) | 58 (45.0%) | REF | REF | ||
| Yes | 33 (37.1%) | 56 (62.9%) | 0.5 (0.3-0.8) | 0.4 (0.2-0.7) | ||
| Cirrhosis | 0.041 | 0.063 | ||||
| No | 39 (41.1%) | 56 (58.9%) | REF | REF | ||
| Yes | 63 (55.3%) | 51 (44.7%) | 1.7 (1.0-3.1) | 1.8 (1.0-3.4) | ||
| Cirrhosis/alcohol | 0.066 | 0.127 | ||||
| No | 39 (41.1%) | 56 (58.9%) | REF | REF | ||
| Yes (Nonalcoholic) | 32 (54.2%) | 27 (45.8%) | 1.7 (0.9-3.3) | 2.0 (1.0-4.3) | ||
| Yes (Alcoholic) | 25 (62.5%) | 15 (37.5%) | 2.2 (1.1-4.8) | 1.8 (0.8-4.3) | ||
| Alcohol use | 0.131 | 0.150 | ||||
| Never/former | 73 (44.8%) | 90 (55.2%) | REF | REF | ||
| Current | 28 (57.1%) | 21 (42.9%) | 1.6 (0.9-3.2) | 1.7 (0.8-3.0) | ||
| HCV | 0.192 | 0.216 | ||||
| Negative | 63 (44.1%) | 80 (55.9%) | REF | REF | ||
| Positive | 37 (53.6%) | 32 (46.4%) | 1.5 (0.8-2.6) | 1.5 (0.8-3.0) | ||
| HBV | 0.960 | 0.611 | ||||
| Negative | 79 (47.3%) | 88 (52.7%) | REF | REF | ||
| Positive | 21 (47.7%) | 23 (52.3%) | 1.0 (0.5-2.0) | 0.8 (0.4-1.8) | ||
| NAFLD | 0.217 | 0.126 | ||||
| No | 56 (52.8%) | 50 (47.2%) | REF | REF | ||
| Yes | 4 (30.8%) | 9 (69.2%) | 0.5 (0.1-1.3) | 0.3 (0.1-1.3) | ||
| No known risk factor | 0.557 | 0.997 | ||||
| No | 68 (51.1%) | 65 (48.9%) | REF | REF | ||
| Yes | 6 (42.9%) | 8 (57.1%) | 0.7 (0.2-2.2) | 1.0 (0.3-3.5) | ||
| Stage | 0.0003 | 0.008 | ||||
| I | 7 (21.2%) | 26 (78.8%) | REF | REF | ||
| II | 11 (31.4%) | 24 (68.6%) | 1.7 (0.6-5.3) | 1.9 (0.6-6.3) | ||
| III | 46 (54.8%) | 38 (45.2%) | 4.5 (1.8-12.3) | 3.5 (1.4 -10.0) | ||
| IV | 39 (60.9%) | 25 (39.1%) | 5.8 (2.3-16.3) | 4.9 (1.8-14.4) | ||
| Child-Pugh score | 0.214 | 0.527 | ||||
| A | 79 (44.9%) | 97 (55.1%) | REF | REF | ||
| B | 23 (60.5%) | 15 (39.5%) | 1.9 (0.9-3.9) | 1.5 (0.7-3.4) | ||
| C | 2 (50.0%) | 2 (50.0%) | 1.2 (0.1 -10.4) | 0.9 (0.1-7.5) | ||
| Number of tumors | 0.026 | 0.012 | ||||
| 1 | 30 (37.5%) | 50 (62.5%) | REF | REF | ||
| Multiple | 73 (53.3%) | 64 (46.7%) | 1.9 (1.1-3.4) | 2.2 (1.2-4.2) | ||
| AFP‡ | 150.5 (1-660,959) | 33.3 (1-549,987) | 1.1 (1.0-1.2) | 0.051 | 1.1 (1.0-1.2) | 0.053 |
- Data are presented as frequency (%) or as median (range). *Adjusting for age and sex; †ORs calculated for 10-year age intervals; ‡ORs are for log-transformed values.
- Abbreviations: AFP, alpha-fetoprotein; BMI, body mass index; REF, reference value.
To rule out the possibility that detection of TERT promoter mutations correlated with amounts of extracted cfDNA, we reanalyzed the data after controlling for cfDNA amounts. Only minor changes in measures of association were noted. The most extreme changes were that the OR for birthplace increased from 2.7 to 3.3 and the OR for Child score C relative to A decreased from 1.2 to 1.0, with no changes in statistical significance (Supporting Table S5).
INDEPENDENT VALIDATION IN PATIENTS WITH HCC WITH HCV AND ALCOHOLIC CIRRHOSIS
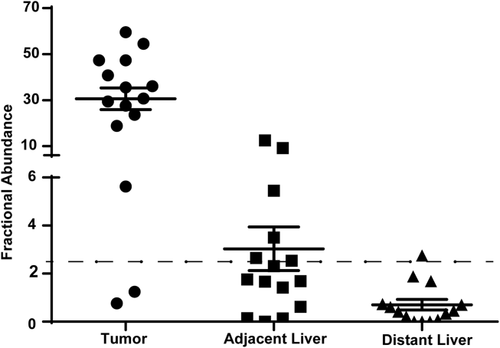
Altogether, the results from both HCC tumor and HCC cfDNA data sets suggest that the TERT promoter is very frequently mutated in male individuals with HCV and alcoholic cirrhosis. Indeed, while the prevalence of TERT promoter mutations in the HCC tumors and HCC cfDNAs analyzed above was 44.4% and 47.7%, respectively, it reached 100% and 80% in tumors and cfDNAs from male patients with HCC with combined HCV and alcoholic cirrhosis, respectively. We further confirmed this result in an independent set of 15 HCC tumors from male patients with HCV and alcoholic cirrhosis. Demographic and clinical parameters of these 15 patients are summarized in Supporting Table S3. The distribution of well-, moderately, and poorly differentiated tumors was 20%, 46.7%, and 33.3%, respectively. We used ddPCR to measure TERT promoter mutations C228T and C250T in tumors, adjacent liver, and distant liver. Using a cutoff for a mutant allele's fractional abundance of 2.5%, TERT promoter mutations were detected in 13 out of the 15 tumors (86.6%). TERT promoter mutations were also detected in six of the corresponding adjacent liver and in one distant liver (Fig. 2). Among those samples with TERT promoter mutations, the mean mutation fractional abundance in tumors, adjacent liver, and distant liver was 35.2% (range, 5.6%-59.5%), 6.0% (range, 2.5%-12.5%), and 2.75%, respectively, confirming the association of TERT mutations with hepatocarcinogenesis.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF PATIENTS WITH CIRRHOSIS AND NO HCC WITH TERT PROMOTER MUTATIONS IN PLASMA cfDNA
Finally, we measured TERT promoter mutations C228T and C250T in cfDNA isolated from plasma collected from 81 patients with cirrhosis without HCC. The demographic and clinical parameters of these 81 patients are shown in Supporting Table S4. The ethnicity/race distribution among these 81 patients was 69.1% non-Hispanic Caucasian, 19.8% Hispanic, 3.7% Asian, and 7.4% Black. Among these 81 patients, 45.7% were male individuals, 42% were positive for HCV, 2.5% were positive for HBV, 13.6% had alcoholic cirrhosis, and 38.3% had NAFLD. The prevalence of TERT promoter mutations in cfDNA of these patients with cirrhosis was 8.6%. Among the 7 patients with TERT promoter mutations, 5 were male individuals (71.4%), 3 were positive for HCV (42.8%), 2 had alcoholic cirrhosis (28.6%), and 1 had NAFLD (14.3%), demonstrating that, similar to patients with HCC, TERT promoter mutations were more prevalent in males with HCV or alcoholic disease and less prevalent in patients with NAFLD.
OVERALL SURVIVAL OF PATIENTS WITH HCC WITH TERT PROMOTER MUTATIONS IN TUMOR OR IN PLASMA cfDNA
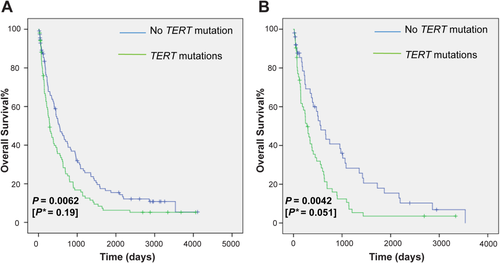
We further evaluated whether noninvasive detection of TERT promoter mutations in plasma cfDNA could have utility in prognosis. To that end, we calculated and plotted Kaplan-Meier survival curves. The overall survival of these patients, stratified by the presence or absence of TERT promoter mutations, is shown in Fig. 3A. In the HCC patient group with TERT promoter mutations measured in plasma cfDNA, overall survival decreased rapidly after diagnosis, with overall survival of patients with TERT promoter mutations significantly lower than for those without TERT promoter mutations (P = 0.0062). In multivariate analysis adjusting for tumor stage, the association was not significant (P = 0.19). When repeating a similar analysis only in patients with cirrhosis, overall survival of patients with TERT promoter mutations was again significantly lower than for those without TERT promoter mutations (P = 0.0042), and in multivariate analysis adjusting for tumor stage, the association remained significant (P = 0.051) (Fig. 3B).
TUMORAL TRANSCRIPTOMIC SIGNATURE ASSOCIATED WITH TERT PROMOTER MUTATIONS
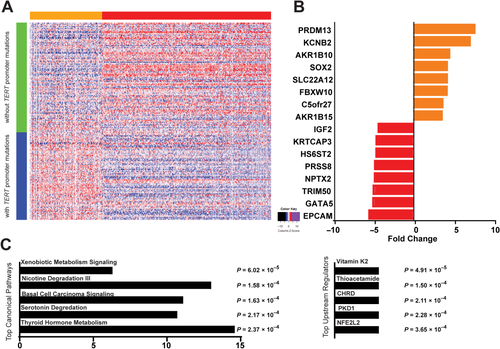
To determine whether TERT promoter mutations are associated with a specific tumor transcriptomic signature, we downloaded mRNA expression data from 193 TCGA HCCs with TERT promoter mutation information available. The expression of 499 genes was significantly changed in tumors with mutated TERT compared to tumors with no TERT mutations (350 down-regulated and 149 up-regulated) (Fig. 4A; Supporting Table S6). The largest expression changes were observed for PR/SET domain 13 (PRDM13), potassium voltage-gated channel subfamily B member 2 (KCNB2), aldo-keto reductase family 1 member B10 (AKR1B10), SRY (sex determining region Y)-box 2 (SOX2), solute carrier family 22 member 12 (SLC22A12), and F-box and WD repeat domain-containing 10 (FBXW10), all overexpressed in samples with TERT promoter mutations compared to samples without mutation (Fig. 4B). Epithelial cell adhesion molecule (EPCAM), GATA binding protein 5 (GATA5), tripartite motif containing 50 (TRIM50) neuronal pentraxin 2 (NPTX2), and protease serine 8 (PRSS8) were the strongest underexpressed genes in tumors with TERT promoter mutations (Fig. 4B). Ingenuity Pathway Analysis (IPA) of all 499 genes identified xenobiotic metabolism signaling (P = 6.02 × 10–5), nicotine degradation III (P = 1.58 × 10–4), and serotonin degradation (P = 2.17×10–4) as the top canonical pathways and vitamin K2 (P = 4.91 × 10–5) and thioacetamide (P = 1.50 × 10–4) as the top upstream regulators affected by TERT promoter mutations (Fig. 4C). Most genes included in the identified top canonical pathways were genes involved in alcohol metabolism.
Discussion
TERT promoter mutations are relatively frequent in human cancers characterized by low rates of self-renewal, such as gliomas, bladder cancers, and melanoma,13, 14 and have been identified as recurrent somatic mutation in regulatory regions of human cancer genomes.15 The aims of this study were 2-fold: (1) to determine whether TERT promoter mutations could be detected in plasma cfDNA in patients with HCC but also in patients with cirrhosis at risk for HCC; and (2) to characterize patients with HCC or cirrhosis with high frequency of TERT promoter mutations. To that end, we analyzed publicly available tumor data from 196 patients with HCC and measured two TERT promoter mutations, C228T and C250T, in plasma cfDNA by ddPCR in an independent set of 218 patients with HCC. A prior study showed that circulating tumor DNA mutants could be readily detected in patients with HCC using ddPCR with a detection limit of 0.01%.16 We also recently reported the detection of TP53 R249S mutant in plasma cfDNA of patients with HCC using ddPCR.17 In our analysis, we confirmed that the detection of TERT promoter mutations in cfDNA was not dependent on the amount of cfDNA extracted. This is consistent with a previous study showing no relationship between detection of hotspot HCC-associated mutations and cfDNA concentrations.18
TERT promoter mutations in tumors have been reported in HCC of all etiologies, including NAFLD,19 HBV,20 and HCV,21 and in HCC arising from both cirrhotic and noncirrhotic liver.7 Prior reports suggested that TERT promoter mutations were associated with older age, presence of HCV, absence of HBV, and poor overall survival.21 In HBV-associated HCC, TERT promoter mutations were observed more frequently in patients with low α-fetoprotein serum levels, advanced age, and in patients lacking HCC family history.20 In most of these studies, one specific etiology was strongly overrepresented. In TCGA, using pairwise statistical analysis, it was reported that patients with a TERT promoter mutation were older, predominantly male sex, more likely to be HCV positive, and less likely to be HBV positive.6 The statistical significance reported was weak for both HCV and HBV. Applying a logistic regression analysis to estimate AORs and after adjusting for age and sex, we confirmed an association of TERT promoter mutations with male sex, older age, HCV, and lack of HBV. In this study, we found additional associations with TERT promoter mutations, including family history of cancer and cirrhosis, particularly alcoholic cirrhosis.
We then measured TERT promoter mutations in cfDNA isolated from plasma from an independent group of 218 patients with HCC. We detected TERT promoter mutations in plasma cfDNA of 47.7% of these patients, a prevalence similar to the prevalence reported in tumors (44.4%-65%).6, 7, 9, 21 In this group of patients, we again found an association of circulating cfDNA TERT promoter mutations with male sex, cirrhosis (alcoholic cirrhosis in particular), HCV, and family history of cancer. We further found an inverse association of TERT promoter mutations with obesity or history of diabetes and confirmed the low prevalence of TERT promoter mutations in patients with NAFLD. Association with older age or inverse association with HBV was not found in this independent set of patients with HCC. Therefore, TERT promoter mutations were not only detected at a similar frequency in HCC tumors or in circulating cfDNAs from patients with HCC but were also detected in the same group of patients with HCC, namely male individuals with alcoholic cirrhosis and/or HCV and with a family history of cancer. The differences in statistical significance observed in our analysis of the two cohorts are likely due to the difference in the distribution of patients' clinical parameters. Patients with HCC in TCGA had a greater proportion of HCV or HBV etiologies, while the independent HCC patient group had a greater proportion of alcohol and NAFLD etiologies. Nevertheless, in the subgroup of male patients with HCC and HCV and alcoholic cirrhosis, the prevalence of TERT promoter mutation reached 100% and 80% in both patient groups, respectively. The results were further confirmed by an 86.6% prevalence of TERT promoter mutation in an independent set of 15 male patients with HCC with HCV and alcoholic cirrhosis.
We also showed in this study that TERT promoter mutations can be detected at increasing frequency and abundance in liver areas distant from tumors, liver areas adjacent to tumors, and in tumors, further supporting the role of mutated TERT in the early steps of hepatocarcinogenesis. Together with the results discussed above, we hypothesized that TERT promoter mutations could be detected in circulating cfDNA of male patients with cirrhosis and HCV and/or alcoholic etiology. We tested that hypothesis in a group of 81 patients with cirrhosis and no HCC and detected TERT promoter mutations in 7 of these patients, a large majority of whom were male patients with cirrhosis of HCV and/or alcoholic etiologies. Among these 7 patients, 3 received magnetic resonance imaging, 3 received computed tomography, and 1 received ultrasound. For 6 of these patients, no hepatic lesion was detected while dysplatic nodules were observed by magnetic resonance imaging in 1 patient. Additional studies are needed to determine whether detection of these mutations in circulating cfDNA could serve as early markers for risk assessment of HCC in this patient group. All 81 patients with cirrhosis continue to have imaging surveillance for HCC every 6 months.
Overwhelming lines of epidemiologic evidence have indicated that persistent infection with HCV is a major risk for the development of HCC and that heavy alcohol use is linked with earlier progression to HCC in patients with chronic hepatitis C. Our study suggests that telomerase may contribute to the molecular basis for the acceleration of HCC development by alcohol in HCV infection. Remarkably, through IPA analysis, differentially expressed genes in HCCs with TERT promoter mutations were mostly representative of canonical pathways related to alcohol metabolism. These include two members of the aldehyde dehydrogenase family, ALDH1A1 and ALDH1L1, enzymes playing major roles in alcohol metabolism.22 ALDH1L1 mRNA has been reported elevated in alcoholic cirrhosis,23 and single-nucleotide polymorphisms (SNPs) of ALDH1A1 were associated with alcohol dependence.24, 25 ALDH1 is a marker for stem cell or cancer stem-like cell, which also has high telomerase activity.26, 27 Several uridine diphosphoglucuronate-glucuronosyltransferases (UGTs), including UGT1A6, UGT2B7, and UGT1A5, were up-regulated in HCCs with TERT mutations. Glucuronidation by UGTs represents a minor detoxifying pathway for ethanol.28 Alcohol treatment up-regulates UGT1A5 expression in hepatocytes29 and moderate to severe alcohol consumption is associated with increased levels of UGT1A6 in liver.30 Other alcohol metabolism-related genes included cytochrome P450 family 2 subfamily J member 2 (Cyp2J2), involved in the oxidation pathway of ethanol into acetaldehyde at elevated ethanol concentration,31 and adenosine triphosphate binding cassette subfamily B members 1 and 2 (ABCB1 and ABCC2), involved in multidrug resistance and biliary transport. An SNP of ABCB1 has been reported to be associated with alcohol dependence.32 Heavy alcohol consumption has been associated with reduced telomere length 33, 34; however, alcohol feeding in mice resulted in increased telomerase activity and TERT expression, highlighting the complexity of the interaction between alcohol and telomere.35
The association of TERT promoter mutations with cirrhosis is also interesting. Telomerase gene delivery inhibited experimental liver fibrosis in mice,36 and an increased incidence of germline telomerase mutations was detected in patients with cirrhosis compared to noncirrhosis controls, suggesting that telomere shortening can accelerate cirrhosis formation.37, 38 It was postulated that somatic TERT promoter mutations could counterbalance germline loss-of-function mutations in pulmonary fibrosis.39 Such events should be evaluated further in liver cirrhosis and HCC.
Vitamin K2 and thioacetamide were identified as top upstream regulators by IPA analysis. Thioacetamide is a well-known hepatotoxicant and could be a liver carcinogen in humans. Vitamin K2 has been tested in trials for its effect on prevention of recurrence and survival in patients with HCC. In a prospective randomized controlled trial with 101 patients undergoing resection, vitamin K2 had a moderately suppressive effect on HCC recurrence after hepatectomy.40 However in a double-blind, randomized, placebo-controlled study of 548 patients, efficacy of vitamin K2 in suppressing HCC recurrence was not confirmed.41 Meta-analysis of five randomized controlled trials failed to confirm significantly higher disease-free survival at 1 year, and the reduced tumor recurrence at later years may be just due to insufficient data.42 Setoguchi et al.43 demonstrated that effective delivery of menahydroquinoe-4 (MKH), the active form of menaquinoe-4 (vitamin K2 homolog), was critical for regulating HCC growth. Our study suggests that vitamin K2 may be more efficient in patients with HCC with TERT promoter mutations. Therefore, the utility of TERT promoter mutation detection in cfDNA for patient stratification in clinical trials of vitamin K2 should be evaluated.
In conclusion, our study showed that TERT promoter mutations can be readily detected in plasma cfDNA of patients with HCC and of patients with cirrhosis without HCC, by ddPCR. It also identified male patients with cirrhosis of HCV and/or alcoholic etiologies as the patient group with the highest prevalence of TERT promoter mutations. Finally, vitamin K2 was identified as the top upstream regulator of genes specifically dysregulated in HCCs with TERT mutations. TERT mutations in circulating cfDNA may therefore serve as a promising risk prediction marker for the early detection of HCC in male patients with cirrhosis of HCV and/or alcoholic etiologies.
Acknowledgment
We thank Haidee Chancoco from the Biospecimen Extraction Facility of MD Anderson Cancer Center for help with DNA extraction and Erika Thompson, Denaha J. Doss, and Viju Varghese from the Sequencing and Microarray Facility for help with ddPCR and Sanger sequencing. We also thank the Center for Translational and Public Health Genomics at University of Texas MD Anderson Cancer Center, Dr. Yang Deng, Paula M. Westergren, and Jana Lee for collection of biospecimens and associated clinical information.
REFERENCES
Author names in bold designate shared co-first authorship.