The RNA binding protein human antigen R is a gatekeeper of liver homeostasis
Pallavi Subramanian and Sofia Gargani equal contribution as first authors.
Dimitris L. Kontoyiannis and Triantafyllos Chavakis equal contribution as senior authors.
Funding information
Supported in part by grants from the European Research Council (DEMETINL, to T.C.); the Else Kröner Fresenius Stiftung (2014_A30, to P.S.); the Medical Faculty, Technische Universität Dresden (MeDDriveGrant-60409, to P.S.); and the InfrafrontierGR/Phenotypos (MIS 5002135) project, Operational Programme Competitiveness, Entrepreneurship and Innovation (NSRF 2014-2020).
Abstract
Background and Aims
NAFLD is initiated by steatosis and can progress through fibrosis and cirrhosis to HCC. The RNA binding protein human antigen R (HuR) controls RNAs at the posttranscriptional level; hepatocyte HuR has been implicated in the regulation of diet-induced hepatic steatosis. The present study aimed to understand the role of hepatocyte HuR in NAFLD development and progression to fibrosis and HCC.
Approach and Results
Hepatocyte-specific, HuR-deficient mice and control HuR-sufficient mice were fed either a normal diet or an NAFLD-inducing diet. Hepatic lipid accumulation, inflammation, fibrosis, and HCC development were studied by histology, flow cytometry, quantitative PCR, and RNA sequencing. The liver lipidome was characterized by lipidomics analysis, and the HuR–RNA interactions in the liver were mapped by RNA immunoprecipitation sequencing. Hepatocyte-specific, HuR-deficient mice displayed spontaneous hepatic steatosis and fibrosis predisposition compared to control HuR-sufficient mice. On an NAFLD-inducing diet, hepatocyte-specific HuR deficiency resulted in exacerbated inflammation, fibrosis, and HCC-like tumor development. A multi-omic approach, including lipidomics, transcriptomics, and RNA immunoprecipitation sequencing revealed that HuR orchestrates a protective network of hepatic-metabolic and lipid homeostasis–maintaining pathways. Consistently, HuR-deficient livers accumulated, already at steady state, a triglyceride signature resembling that of NAFLD livers. Moreover, up-regulation of secreted phosphoprotein 1 expression mediated, at least partially, fibrosis development in hepatocyte-specific HuR deficiency on an NAFLD-inducing diet, as shown by experiments using antibody blockade of osteopontin.
Conclusions
HuR is a gatekeeper of liver homeostasis, preventing NAFLD-related fibrosis and HCC, suggesting that the HuR-dependent network could be exploited therapeutically.
Abbreviations
-
- AFP
-
- alpha-fetoprotein
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BA
-
- bile acid
-
- CD
-
- cluster of differentiation
-
- CD-HFD-45%
-
- choline-deficient HFD with 45% kcal from fat
-
- CE
-
- cholesterol ester
-
- CM
-
- conditioned medium
-
- Col1a1
-
- collagen type I alpha 1 chain
-
- Cre
-
- cyclization recombination
-
- Elavl1
-
- embryonic lethal abnormal vision like 1
-
- ER
-
- endoplasmic reticulum
-
- FXR
-
- farnesoid X receptor
-
- GSEA
-
- gene set enrichment analysis
-
- Hes1
-
- Hes family basic helix-loop-helix transcription factor 1
-
- HFD
-
- high-fat diet
-
- 60%-HFD-CD
-
- 60% kcal from fat, 0.1% methionine and choline–deficient
-
- HuR
-
- human antigen R
-
- IPA
-
- Ingenuity Pathway Analysis
-
- Jag1
-
- jagged-1
-
- KO
-
- knockout
-
- ND
-
- normal diet
-
- NK
-
- natural killer
-
- OPN
-
- osteopontin
-
- PDGF
-
- platelet-derived growth factor
-
- RBP
-
- RNA binding protein
-
- RIP-seq
-
- RNP-immunoprecipitation and RNA sequencing
-
- RNA-seq
-
- RNA sequencing
-
- RNP
-
- ribonucleoprotein
-
- RXR
-
- retinoid X receptor
-
- Slc27a2
-
- solute carrier family 27 member 2
-
- Slco1b2
-
- solute carrier organic anion transporter family member 1b2
-
- Sox9
-
- SRY (sex-determining region Y)-box 9
-
- Spp1
-
- secreted phosphoprotein 1
-
- TAG
-
- triglycerides
-
- TCDCA
-
- taurochenodeoxycholic acid
-
- Timp2
-
- tissue inhibitor of metalloproteinase 2
-
- TUDCA
-
- tauroursodeoxycholic acid
-
- UPR
-
- unfolded protein response
-
- WT
-
- wild type
INTRODUCTION
NAFLD, affecting up to 30% of the adult population and 70%–80% of obese and diabetic individuals, is associated with dysfunctional hepatic metabolism.[1, 2] NAFLD ranges from simple intrahepatic fat accumulation (steatosis) to NASH, which is characterized by hepatocyte death and inflammation and may progress to fibrosis, cirrhosis, and HCC.[1, 3] Hepatic fibrosis is the main factor determining liver-related mortality in patients with NASH.[4]
Excessive triglyceride (TAG) accumulation is the initiating process of NAFLD.[5] Both plasma nonesterified fatty acids and fatty acids from de novo lipogenesis are used for TAG synthesis in the liver.[5] Liver TAG can be stored as lipid droplets in hepatocytes, secreted as very-low-density lipoprotein particles, or hydrolyzed.[6] A network of enzymes and transcription factors controls hepatic metabolic pathways.[2] RNA binding proteins (RBPs) are integral to the posttranscriptional regulation of gene expression in the liver under physiological and pathological conditions.[7, 8] However, less is known about the cell type–specific function of RBPs in the liver, particularly in hepatocytes.
The RBP human antigen R (HuR; encoded by embryonic lethal abnormal vision like 1 [Elavl1]) regulates gene expression posttranscriptionally by altering the abundance and use of RNAs that it binds to. HuR is present in the nucleus and in the cytoplasm; however, the most predominant functions of HuR are associated with its cytoplasmic localization.[9, 10] Cytoplasmic HuR can promote the stability and translation of mRNAs containing uridine-rich or adenine/uridine-rich elements in their untranslated termini, although in some cases HuR may act in an opposite manner.[10, 11] Short hairpin RNA–mediated HuR silencing in the liver attenuates HSC activation and fibrosis development following bile duct ligation,[12] and HuR is involved in TGF-β1-induced HSC activation.[13] Hepatocyte HuR regulates hepatic steatosis development in response to high-fat diet (HFD) feeding.[14] However, an in-depth analysis of the role of hepatocyte HuR in regulating NAFLD progression to fibrosis and HCC development is missing.
We identified HuR here as a master regulator of hepatic homeostasis. Hepatocyte-specific HuR deletion led to spontaneous steatosis and fibrosis predisposition. Upon feeding an NAFLD-inducing diet, hepatocyte-specific, HuR-deficient mice developed heightened inflammation and fibrosis, which resulted in HCC-like tumor development. Mechanistic analysis revealed that HuR regulates a protective hepatic network of mRNAs, thereby sustaining hepatic metabolic and lipid homeostasis.
METHODS AND MATERIALS
Mouse experiments
Mice with hepatocyte-specific knockout of HuR (HuRHepKO) in the C57BL/6 background were derived by crossing mice with a floxed Elavl1 allele with mice expressing cyclization recombination (Cre) recombinase under the control of albumin promoter (albumin-Cre). Cre-negative littermate wild-type mice with Elavl1 floxed alleles (HuRWT) were used as controls.
HuRWT or HuRHepKO mice were fed a methionine-low, choline-deficient HFD (60% kcal from fat, 0.1% methionine and choline–deficient diet; 60%-HFD-CD; A06071302; Research Diets) for 2 or 6 weeks or a normal diet (ND; 10% kcal fat, normal methionine and choline; A08051501; Research Diets) for 6 weeks. Long-term feedings were performed with a choline-deficient HFD (45% kcal from fat; CD-HFD-45%; D05010402; Research Diets) for up to 14 months. Mice of both genders (7–10 weeks old) were used. Animal experiments were approved by the prefecture of Attica, Greece, and by the Landesdirektion Sachsen, Germany; animals received humane care according to the Guide for the Care and Use of Laboratory Animals.
Statistical analysis
For analysis, GraphPad Prism software (GraphPad Inc., La Jolla, CA) was used, unless otherwise stated in Materials and Methods. For comparison of two groups, the Mann-Whitney U test was used. Statistics for tumor incidence were calculated using two-tailed Fisher's exact test. Statistical analysis for lipidomics and RNA sequencing (RNA-seq) is described in the respective sections (in the Supporting Information). Data are expressed as mean ± SEM. Significance was set at p < 0.05.
Detailed methods are in the Supporting Information.
RESULTS
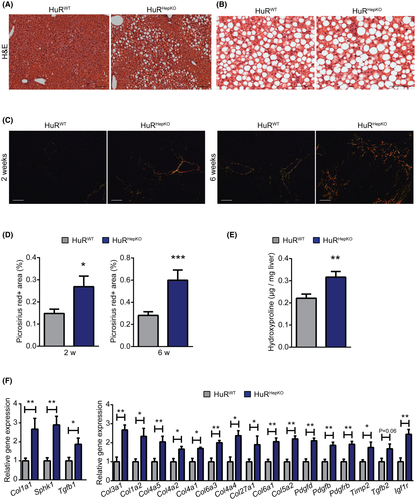
Hepatocyte-specific HuR deletion in mice promotes hepatic steatosis and fibrosis
To assess the function of HuR in hepatocytes, we generated HuRHepKO mice. Littermate Cre-negative Elavl1 floxed mice (HuRWT) were used as controls. Efficient HuR deletion was found in livers of HuRHepKO mice (Figure S1). Mice were fed the ND for 6 weeks (designated “steady state”) or an NAFLD-inducing diet. Hepatocyte-specific HuR deficiency resulted in spontaneous hepatic steatosis at steady state (Figure 1A). To clarify if the enhanced steatosis in HuRHepKO mice at steady state was metabolically benign[15] or predisposed to NASH and fibrosis, we fed HuRWT and HuRHepKO mice an NAFLD-inducing diet (60%-HFD-CD) for 2 or 6 weeks, representing early and advanced NAFLD/NASH development, respectively. Both HuRWT and HuRHepKO mice on the 60%-HFD-CD diet developed steatosis (Figure 1B). However, hepatocyte-specific HuR deficiency exacerbated NAFLD-related fibrosis, as assessed by picrosirius red staining of livers at 2 and 6 weeks of 60%-HFD-CD feeding (Figure 1C,D). Moreover, hydroxyproline concentration was increased in livers of HuRHepKO mice compared to control mice at 6 weeks of 60%-HFD-CD feeding (Figure 1E). We found enhanced mRNA expression of fibrosis-related factors, such as genes encoding different collagen subtypes as well as sphingosine kinase 1, platelet-derived growth factor D (Pdgfd), Pdgfb, Pdgfrb, tissue inhibitor of metalloproteinase 2 (Timp2), insulin-like growth factor 1 receptor, Tgfb2, and Tgfb1 in HuRHepKO livers compared to HuRWT livers (Figure 1F).
Analysis of fibrosis development owing to hepatocyte-specific HuR deletion
To provide mechanistic insights for the fibrosis development resulting from hepatocyte HuR deletion, we performed RNA-seq of livers from HuRHepKO and HuRWT mice at steady state. Hepatocyte HuR deficiency resulted in down-regulation of 456 genes and up-regulation of 593 genes (Figure 2A). Ingenuity Pathway Analysis (IPA) revealed that the hepatic fibrosis/HSC activation pathway was the most prominently enriched one in the significantly up-regulated genes of HuRHepKO livers (Figure 2B). Significantly enhanced expression of several genes involved in the hepatic fibrosis/HSC activation pathway was found in HuRHepKO livers compared to HuRWT livers (Figure 2C and S2A). Several pathways related to hepatic fibrosis, such as the PDGF signaling pathway, the epithelial–mesenchymal transition pathway, and the hepatic fibrosis signaling pathway, were enriched in the significantly up-regulated genes of HuRHepKO livers relative to HuRWT livers (Figure 2B). Gene set enrichment analysis (GSEA) over the Molecular Signatures Database displayed a positive correlation between hepatocyte-specific HuR deficiency and extracellular matrix–associated gene expression and epithelial–mesenchymal transition–associated gene expression in the liver (Figure 2D,E and Table S1). Moreover, we assessed the crosstalk between hepatocytes and HSCs in vitro. Conditioned medium (CM) derived from HuR-deficient hepatocytes promoted the expression of profibrotic genes, such as actin alpha 2, collagen type I alpha 1 chain (Col1a1), Col3a1, Col6a3, and Timp2, in WT HSCs compared to treatment with CM from HuRWT hepatocytes (Figure S2B). Together, hepatocyte-specific HuR deficiency promotes HSC activation, thereby contributing to fibrosis predisposition of HuRHepKO livers.
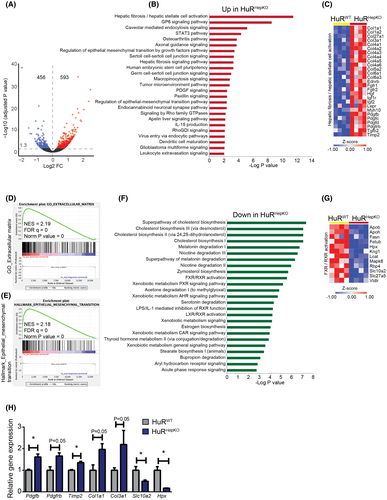
IPA revealed overrepresentation of several pathways in the significantly down-regulated genes of HuRHepKO livers, including cholesterol biosynthesis pathways (Figure 2F). The protective bile acid (BA)–activated farnesoid X receptor (FXR)/retinoid X receptor (RXR) pathway was also enriched in the significantly down-regulated genes of HuR-deficient livers (Figure 2F,G). The FXR pathway regulates lipid homeostasis and protects against liver fibrosis.[1, 16-19] FXR activation in hepatocytes increases TAG clearance and blocks sterol regulatory element binding protein 1–mediated lipogenesis, whereas FXR signaling in HSCs inhibits their activation and protects against fibrogenesis.[1, 17, 20] IPA also revealed enrichment of the retinol biosynthesis pathway in the significantly down-regulated genes of HuR-deficient livers (not shown); hence, potentially decreased retinoic acid levels may further contribute to reduced FXR/RXR activation in HuR-deficient hepatocytes.[21]
Analysis by quantitative RT-PCR for selected genes, identified as differentially expressed due to hepatocyte-specific HuR deficiency by RNA-seq, confirmed up-regulation of expression of genes involved in the hepatic fibrosis/HSC activation pathway and down-regulation of expression of genes involved in the FXR/RXR activation pathway (Figure 2H).
Collectively, hepatocyte HuR deficiency promotes transcriptomic alterations culminating in HSC activation and fibrosis (Figure 2C–E), thereby underlying the fibrosis predisposition of HuRHepKO mice at steady state and leading to exacerbated fibrosis upon feeding an NAFLD-inducing diet.
Hepatocyte-specific HuR deletion promotes HCC-like tumor development
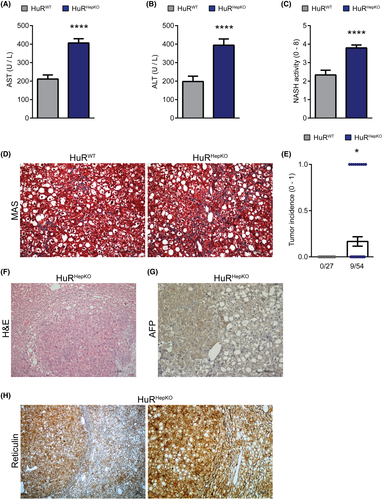
NAFLD-related fibrosis predisposes to HCC.[3] We found that the tumor microenvironment pathway was enriched in the significantly up-regulated genes in HuRHepKO livers compared to HuRWT livers at steady state (Figure 2B). Furthermore, the RNA-seq data obtained from HuRWT and HuRHepKO livers of steady-state mice were subjected to upstream regulator analysis using the IPA software (Table S2 and Figure S3). The β-catenin pathway, involved in HCC pathogenesis,[22] was predicted to be activated in HuRHepKO livers compared to HuRWT livers (Table S2 and Figure S3). Although HuR expression is increased in HCC and various other cancers and correlates with parameters of tumor progression,[23] our data show that at steady state and in NAFLD, HuR contributes to the maintenance of hepatocyte homeostasis.
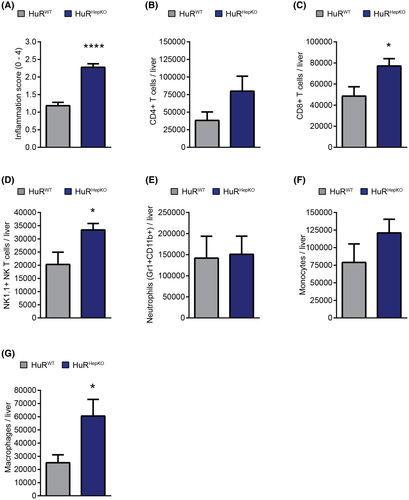
To study whether the predisposition toward hepatic steatosis and fibrosis due to hepatocyte-specific HuR deficiency may promote NASH-related cancer development, we fed HuRHepKO and HuRWT mice with another NAFLD-inducing, choline-deficient HFD (CD-HFD-45%) for up to 14 months. We chose the milder CD-HFD-45% model[24] as it displays a slower progression of liver disease pathogenesis, resembling the chronicity that governs the development of sequelae of human NAFLD, such as HCC. Plasma levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were elevated in HuRHepKO mice compared to HuRWT mice following the CD-HFD-45% feeding (Figure 3A,B), indicating enhanced liver injury. HuRHepKO livers showed an increased NASH activity score and exacerbated fibrosis compared to HuRWT livers (Figure 3C,D). Strikingly, a portion of HuRHepKO (9/54), but no HuRWT (0/27), mice developed HCC-like tumors, as assessed histopathologically (Figure 3E,F). Positive alpha-fetoprotein (AFP) staining (Figure 3G), together with the characteristic absence of reticulin staining (Figure 3H) in the tumor tissues of HuRHepKO mice, further underlined the resemblance to HCC.[25, 26] Hepatic inflammation was increased in HuR-deficient mice compared to control mice, as assessed histologically (Figure 4A). Flow-cytometric analysis for immune cell populations in the liver revealed significant increases in the numbers of cluster of differentiation 8–positive (CD8+) T cells (CD8+CD4− cells), NK1.1+ natural killer (NK) T cells (CD4−CD8−NK1.1+), and infiltrating macrophages (Gr1−CD11b+CD11c−MHCII+Ly6c+) (Figures 4B–G and S4). HuRHepKO mice on long-term feeding with the CD-HFD-45% diet exhibited more inflammation and fibrosis, ultimately leading to HCC-like tumor development, hence recapitulating the complete sequence of NAFLD-related pathogenesis and sequelae.
An NAFLD-like hepatic lipid signature owing to hepatocyte HuR deficiency
To further discern the spontaneous steatosis predisposing to fibrosis due to hepatocyte-specific HuR deficiency, we performed shotgun lipidomic analysis of livers obtained from HuRHepKO and HuRWT mice fed the ND or the 60%-HFD-CD diet. Shotgun lipidomics allow for detailed analysis of storage (TAG; cholesterol ester [CE]) and nonstorage lipids (all lipid classes other than TAG and CE). Consistent with the histological analysis (Figure 1A), the percentage of storage lipids was higher in HuRHepKO livers compared to HuRWT livers fed the ND (Figure 5A). Analysis of the lipid classes demonstrated that CEs and TAGs were enhanced in HuRHepKO livers compared to HuRWT livers (Figure 5B). Both nonstorage lipids and storage lipids of the two groups on the ND separated from each other in the principal component analysis (Figure 5C,D). Because the storage lipids were enhanced in the absence of hepatocyte HuR, we further studied alterations in storage lipid species. The abundance of several TAG and CE species was up-regulated in the absence of HuR (Figure 5E). When comparing the lipidome of livers of HuRWT mice, which were fed either the 60%-HFD-CD diet or the ND, we found that 60%-HFD-CD feeding also led to increased TAG and CE levels (Figure S5A–C). Furthermore, several TAG and CE species were enriched in NAFLD livers compared to healthy livers of HuRWT mice (Figure 5F).
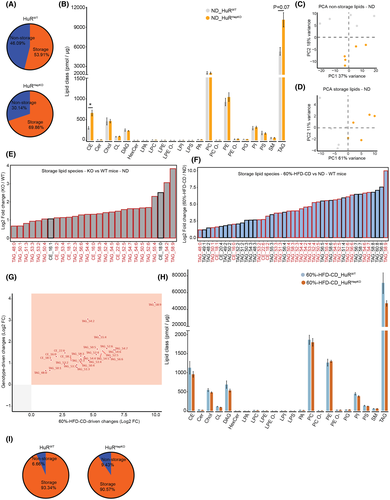
All TAG species and some CE species which were significantly increased at steady state in HuRHepKO livers (Figure 5E, bars outlined in red) were also up-regulated in HuRWT livers upon 60%-HFD-CD feeding (Figure 5F, bars outlined in red). Specifically, TAG_48:0, TAG_50:1, TAG_51:3, TAG_53:3, TAG_53:2, TAG_50:4, TAG_53:4, TAG_52:3, TAG_56:6, TAG_54:4, TAG_58:3, TAG_52:4, TAG_54:5, TAG_52:5, TAG_54:7, TAG_54:6, TAG_50:5, TAG_52:6, TAG_55:4, TAG_54:2, TAG_58:9, CE_18:2, CE_18:1, CE_16:0, and CE_22:6, which were increased due to hepatocyte-specific HuR deletion at steady state, were also altered in the same manner in livers of HuRWT mice upon feeding with the NAFLD-inducing diet (Figure 5E–G). In other words, the TAG lipidomic phenotype of HuR-deficient livers at steady state (genotype-driven change) is very similar to that of HuRWT livers with NAFLD (diet-driven change; Figure 5G). In addition to phenocopying the TAG microenvironment of WT NAFLD livers (Figure 5G), the HuR-deficient livers at steady state displayed a TAG signature similar to that of human NAFLD livers.[27, 28] During human NAFLD, TAG_50:1 is enriched[28-31]; similarly, we found that this TAG was increased in HuR-deficient livers at steady state (Figure 5E). Furthermore, TAG_48:0, TAG_52:3, TAG_54:4, TAG_52:4, TAG_54:5, TAG_56:6, TAG_52:5, TAG_54:7, TAG_54:2, CE_22:6, and CE_18:2, which were enhanced in HuR-deficient livers at steady state (Figure 5E), are up-regulated either in the liver or in circulation in human NAFLD.[27, 28, 30, 31] Therefore, hepatocyte-specific HuR deficiency leads to a spontaneous phenotype that phenocopies the TAG signature within the hepatic microenvironment characteristic of human NAFLD. Lipidomic analysis of NAFLD livers (60%-HFD-CD feeding) revealed no differences in storage lipids between the two genotypes (Figure 5H,I). Together, hepatocyte HuR deficiency promotes the accumulation of storage lipids in the liver under steady-state conditions, resulting in a lipidomic signature similar to that of WT livers with NAFLD as well as of human NAFLD livers.
The HuR-dependent hepatoprotective network
Our phenotypic and multi-omic analysis demonstrated that hepatocyte-specific HuR deficiency promotes liver steatosis associated with a profibrotic predisposition at steady state, thereby leading to exacerbated fibrosis and HCC-like tumor development upon feeding an NAFLD-inducing diet. To mechanistically connect HuR functions as an RBP to RNA changes incurred by its deficiency in the liver, we next mapped the RNA interactors of HuR ribonucleoprotein (RNP) complexes in mouse livers by RNP immunoprecipitation and RNA-seq (RIP-seq). RNP immunoprecipitation was performed in cytoplasm-enriched extracts from healthy livers of WT mice using an anti-HuR antibody or an isotype-matched IgG as control (Figure S6A). Sequencing analysis of immunoprecipitated RNAs normalized to their expression values revealed that 1,380 mRNAs were enriched in HuR-RNPs with a fold change > 1.5 and p < 0.05 over the IgG controls (Table S3). The quality of the RIP-seq was evaluated by the successful validation of 7 out of 8 mRNAs tested as HuR–RNP interactors in confirmatory quantitative RT-PCRs from similarly immunoprecipitated samples (Figure S6B). Putative HuR targets were used to create functional pathway networks (Figure 6 and Tables S4–S6).
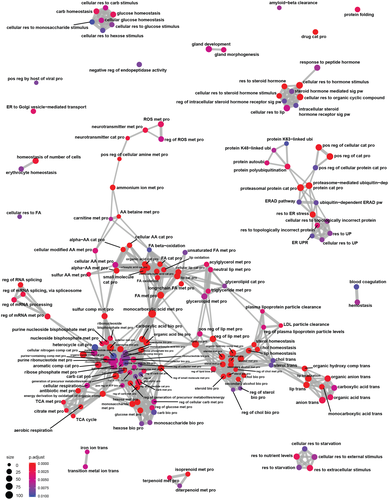
Cytoplasmic HuR binds to its target mRNAs and promotes their use by augmenting mRNA stability or translation, although in some cases it may act in a negative fashion.[11, 32] Several target mRNAs from the RIP-seq were associated with hepatic metabolism, including glycerolipid, cholesterol, long chain fatty acid, monocarboxylic acid, and carbohydrate metabolism (Figure 6; Supporting Tables S4–S6). The most significant “biological process” identified by Gene Ontology analysis of the RIP-seq data was the monocarboxylic acid metabolic process, which contained 102 targets (Figure 6; Supporting Table S5). In Gene Ontology, the monocarboxylic acid metabolic process is a parent term for the BA metabolic process, among others. Thus, the RIP-seq findings are in agreement with the RNA-seq data, which showed that expression of genes involved in cholesterol biosynthesis and in the BA-activated FXR/RXR pathway were diminished due to hepatocyte HuR deficiency (Figure 2F,G).
The hepatic targets of HuR identified here are involved in various facets of BA metabolism and recycling. Besides mRNAs of genes mediating BA synthesis, such as cytochrome P450s 7a1 (Cyp7a1) and 7b1, HuR binds to mRNAs of transmembrane transporters of BA, such as ATP binding cassette subfamily C member 2, solute carrier organic anion transporter family member 1b2 (Slco1b2), ATP binding cassette subfamily B member 11 (Abcb11), and Slco1a4, and of BA conjugating enzymes, such as solute carrier family 27 member 2 (Slc27a2) (Table S3). We therefore measured BA levels in livers of HuRHepKO and HuRWT mice at steady state. From the BAs that could be detected, taurochenodeoxycholic acid (TCDCA) and tauroursodeoxycholic acid (TUDCA) were down-regulated in HuR-deficient livers compared to HuR-sufficient livers (Figure S7A,B). Noteworthy, both TUDCA and TCDCA have been shown to activate FXR.[33, 34] Contrastingly, other BAs, such as tauromuricholic acid (alpha+beta) or taurocholic acid, were not altered due to HuR deficiency (Figure S7C,D).
Additionally, mRNAs regulating the function of the endoplasmic reticulum (ER), protein processing in the ER, and the ER stress response pathway were HuR targets (Figure 6; Supporting Tables S3–S6). Inositol-requiring enzyme 1α (encoded by ER to nucleus signaling 1), which is one of the arms of the unfolded protein response (UPR), and cAMP responsive element binding protein 3 like 2, which is a transcriptional activator operative in the UPR, were HuR targets (Tables S3–S6). Moreover, homocysteine inducible ER protein with ubiquitin like domain 1 (Herpud1), stress-associated ER protein 1, autocrine motility factor receptor, bifunctional apoptosis regulator, Herpud2, activating transcription factor 4, stress-induced phosphoprotein 1 homology and U-box containing protein 1, vesicle-associated membrane protein–associated protein B and C, eukaryotic translation initiation factor 2 alpha kinase 2, E1A binding protein P300, and heat shock protein family A member 13, which are genes that function in the ER’s UPR, are HuR targets (Tables S3–S6). Hence, several of the factors and pathways identified here to be regulated by HuR in the liver, including the ER stress response, BA metabolism, and FXR pathways, have been implicated in hepatic homeostatic metabolism and function, thereby portraying a central role of HuR in liver homeostasis.[16-18, 35]
Continuing our search for protective pathways, which could fail in the absence of HuR, we sought to identify mRNAs that were bound to and regulated by HuR. Therefore, we compared the RNA-seq (Figure 2) with the RIP-seq (Figure 6) data. Out of the 1,049 genes that were significantly deregulated in HuR deficiency (Figure 2A), mRNAs of 110 genes were identified as HuR targets by RIP-seq (Figure S8A), suggesting that HuR may bind to and regulate their use. Analysis of these 110 targets using the online automated meta-analysis tool Metascape revealed that the steroid biosynthetic process, cofactor metabolic process, biological oxidations, lipid homeostasis, and protein folding were the top five enriched pathways (Figure S8B). The steroid biosynthetic process, which was the top significant pathway, further consisted of cholesterol metabolic process, sterol metabolic process, lipid biosynthetic process, metabolism of lipids, fatty acid metabolic process, and monocarboxylic acid metabolic process as subcategories, among others. The lipid homeostasis pathway consisted of plasma lipoprotein remodeling, lipid storage, lipid localization, cholesterol homeostasis, cholesterol efflux, TAG metabolic process, lipid transport, and regulation of lipid localization as subcategories, among others. However, pathways that are known to directly contribute to hepatic fibrosis development were not enriched (Figure S8B), thereby suggesting that the overrepresented hepatic fibrosis/HSC activation pathway due to hepatocyte-specific HuR deficiency (Figure 2B,C) is likely secondary to other alterations (e.g., in lipid metabolism) in hepatocytes. Hence, HuR regulates a hepatoprotective lipid metabolic network in the liver, thereby maintaining lipid homeostasis and inhibiting NAFLD development and progression.
Osteopontin partially mediates fibrosis development in hepatocyte-specific HuR deficiency
Our data so far suggest that alterations in HuR-deficient hepatocytes may trigger HSC activation and fibrosis, possibly in a paracrine fashion. We therefore examined the RNA-seq data of HuRWT and HuRHepKO livers (Figure 2) in order to identify up-regulation in HuR-deficient hepatocytes of mRNA of secreted factors that could contribute to HSC activation and fibrosis. Among other genes implicated in fibrosis development that were up-regulated by hepatocyte-specific HuR deficiency, we chose to analyze the potential role of secreted phosphoprotein 1 (Spp1) and its product osteopontin (OPN) as Spp1 was within the top five significantly up-regulated genes (ranked based on the adjusted p value) in HuRHepKO livers compared to HuRWT livers (not shown) and as OPN is known to promote HSC activation and fibrogenesis.[36, 37] Consistent with the RNA-seq data, quantitative RT-PCR analysis verified that Spp1 mRNA was increased in HuRHepKO compared to HuRWT livers, both at steady state and upon 60%-HFD-CD diet feeding (Figure 7A,B). Additionally, primary hepatocytes from HuRHepKO mice displayed higher OPN protein secretion than cells from HuRWT mice (Figure 7C).
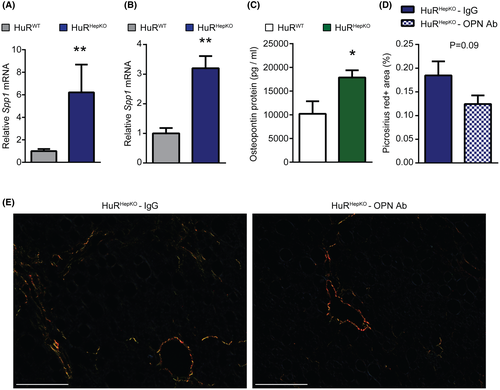
Previously, hepatocyte Notch activation was shown to induce fibrosis by increasing SRY (sex-determining region Y)-box 9 (Sox9)–dependent hepatocyte expression of OPN, which promotes HSC activation.[36] Consistent with this study, Hes family basic helix-loop-helix transcription factor 1 (Hes1) and Sox9, which are downstream targets of the Notch pathway, and Jag1, which encodes the Notch ligand jagged-1, were significantly up-regulated in HuRHepKO livers compared to HuRWT livers, based on RNA-seq (not shown). The up-regulation of Hes1, Sox9, and Jag1 expression in HuRHepKO livers was confirmed by quantitative RT-PCR (Figure S9A–C). According to the RIP-seq analysis, Jag1 is a direct target of HuR in the liver (Table S3), suggesting that HuR may negatively regulate Jag1 expression. Contrastingly, expression of other Notch pathway components, such as Notch receptors 1-4, was unchanged in HuR-deficient livers (Figure S9D–G). These findings indicate that due to the potential direct regulation of Jag1 expression by HuR, hepatocyte-specific HuR deficiency results in up-regulation of Jag1 and the Jag1/Notch-signaling pathway and consequently of Spp1 expression and OPN secretion.
Therefore, we next addressed whether the increase in OPN may promote HSC activation and fibrosis owing to HuR deficiency. To this end, HuRHepKO mice were treated with an OPN-neutralizing antibody or control antibody while being fed the 60%-HFD-CD diet. OPN blockade resulted in a tendency toward reduced fibrosis in HuRHepKO mice compared to control antibody treatment, as shown by picrosirius staining (Figure 7D,E). Additionally, quantitative RT-PCR analysis for expression of fibrosis-related genes (Figure S10) supported the partial reduction in fibrosis in HuRHepKO mice by treatment with the OPN antibody. Together, increased OPN may, at least partially, mediate fibrosis development in hepatocyte-specific HuR deficiency.
DISCUSSION
We identified here hepatocyte HuR as an integral factor to the maintenance of liver metabolic homeostasis. We show that HuR regulates a network of homeostatic hepatic processes, including lipid and BA metabolism, thereby protecting against hepatic steatosis, fibrosis, and HCC development. Strikingly, hepatocyte-specific HuR deficiency resulted in spontaneous hepatic steatosis, accumulation of a TAG signature resembling that of human NAFLD livers, HSC activation, and predisposition to fibrosis. Hepatocyte-specific, HuR-deficient mice on NAFLD-inducing diets displayed aggravated hepatic fibrosis and HCC-like tumor development. Hence, hepatocyte-specific HuR deficiency recapitulates the whole sequence of NAFLD pathology up to HCC development. Our findings unequivocally position HuR as a central player of hepatic homeostasis.
Cytoplasmic HuR interacts with its target mRNAs and either promotes mRNA stability, up-regulates mRNA translation, or can suppress either of those functions in specific tissues.[10, 11, 32, 38] Here, we show that in cytoplasm-enriched liver fractions, HuR interacts with mRNAs of genes involved, among other things, in lipid transport/metabolism, BA transport/metabolism, cholesterol metabolism, or the ER stress response pathway, hence potentially regulating several homeostatic hepatic pathways. A comprehensive analysis involving transcriptomics, lipidomics, and mass spectrometric BA measurement verified that HuR is a regulator of the aforementioned metabolic pathways.
Lipidomic analysis revealed that the TAG species that were significantly increased at steady state due to hepatocyte HuR deficiency were also increased in livers of WT mice upon feeding an NAFLD-inducing diet. Importantly, substantial similarities were observed between the storage lipid signature of HuR-deficient livers at steady state and the lipidomic alterations in human NAFLD.[27-31] TAG_48:0, TAG_50:1, TAG_52:3, TAG_54:4, TAG_52:4, TAG_54:5, TAG_56:6, TAG_52:5, TAG_54:7, TAG_54:2, CE_22:6, and CE_18:2, which were up-regulated in HuR-deficient livers at steady state, are up-regulated in human NAFLD as well.[27-31] Together, our findings demonstrate that the lipidomic signature of mouse NAFLD induced by the 60%-HFD-CD diet has substantial overlap with that from human NAFLD and is strikingly phenocopied by deletion of a single RBP at steady state. This finding suggests that HuRHepKO mice may represent an appropriate model for studying NAFLD in the future.
RIP-seq analysis showed that mRNAs of genes involved in BA synthesis, conjugation, and transport are targets of HuR-RNPs. Specifically, the rate-limiting enzyme in BA synthesis, Cyp7a1; the enzyme responsible for BA conjugation, Slc27a2; and the BA transporters Abcb11, Slco1a4, and Slco1b2, among others, were HuR targets. We corroborated the RIP-seq data by measuring hepatic BA levels. The BAs TCDCA and TUDCA were diminished in HuR-deficient livers at steady state compared to HuRWT livers. Previous studies have described the hepatoprotective role of TUDCA in the context of NAFLD by inhibiting ER stress,[39, 40] and treatment of genetically obese mice with TUDCA reduced hepatic steatosis by regulating genes involved in de novo lipogenesis.[41] Both TUDCA and TCDCA are activators of FXR[33, 34]; consistently, the BA-activated FXR/RXR pathway was overrepresented in the significantly down-regulated genes in HuR-deficient livers, as assessed by RNA-seq. Hepatic FXR is a master regulator of lipid homeostasis and protects against NASH, and its expression is inversely correlated to NASH severity.[1] Together with previous findings that activation of FXR in hepatocytes leads to HuR up-regulation,[42] our present data may point to a possible feed-forward hepatoprotective loop involving the FXR pathway and HuR.
Genes involved in the hepatic fibrosis/HSC activation pathway were up-regulated in HuR-deficient livers at steady state, reflecting the fibrosis predisposition due to HuR deficiency. However, mRNAs contributing to the hepatic fibrosis/HSC activation pathway that were up-regulated in HuR-deficient livers were not cytoplasmic targets of HuR in the liver. This suggests that the up-regulated hepatic fibrosis/HSC activation pathway due to hepatocyte-specific HuR deficiency is likely secondary to the metabolic alterations in hepatocytes as lipid accumulation in hepatocytes can promote HSC activation in a paracrine manner.[4, 43-45] In support of the hypothesis that hepatocyte HuR regulates HSC activation and fibrosis in a paracrine fashion were our findings that HuR-deficient hepatocytes displayed higher Spp1 expression and increased secretion of OPN, which promotes HSC activation and fibrosis[36] and that pharmacological OPN blockade in HuRHepKO mice partially reduced liver fibrosis compared to control antibody treatment. A recent study demonstrated that profibrotic OPN expression by hepatocytes is enhanced by hepatocyte Notch activation in a Sox9-dependent manner.[36] Consistently, we found that expression of the downstream targets of the Notch pathway Hes1 and Sox9 was up-regulated in HuR-deficient livers. Furthermore, expression of Jag1 was elevated in HuR-deficient livers. Interestingly, we identified Jag1 as a direct target of HuR in the liver by RIP-seq, suggesting that HuR may negatively regulate Jag1 expression. Therefore, we conclude that activation of the Jag1/Notch-signaling pathway resulting in increased Spp1 expression and OPN secretion due to hepatocyte HuR deficiency may, at least partially, mediate the increased HSC activation and fibrosis of HuRHepKO mice compared to their HuR-sufficient littermates, although the contribution of further profibrotic pathways cannot be excluded and merits further investigation. Furthermore, whether the disrupted lipid metabolism and/or the reduced FXR/RXR activation in HuR-deficient hepatocytes are functionally linked to the increased profibrotic Jag1/Notch/OPN pathway requires future investigation.
Interestingly, long-term feeding with an NAFLD-inducing diet not only aggravated inflammation and fibrosis in HuRHepKO mice but, importantly, led to NASH-related HCC development in a portion of HuRHepKO mice. Previous studies have shown that HuR was up-regulated in livers from patients with cirrhosis and HCC and that HuR inhibits apoptosis and promotes cell proliferation, cell cycle progression, and hypoxia-induced glycolytic switch in HCC cells.[23, 46-48] However, those studies have only investigated the function of HuR in HCC cells or in the context of established HCC. Our data show that hepatocyte-specific HuR deletion results in a predisposition to NAFLD-related HCC. Hence, at steady state, HuR exerts a protective function, supporting hepatocyte homeostasis.
The two NAFLD-inducing diets used in the present study (60%-HFD-CD and CD-HFD-45%) are based on choline deficiency and have therefore some limitations with regard to recapitulating human NAFLD pathology. It would be worth investigating the role of hepatocyte-specific HuR deficiency in the context of NAFLD development and progression by using alternative NAFLD/NASH-inducing diets, such as a high-cholesterol diet complemented with fructose-containing drinking water,[36, 44] in future studies. Notwithstanding these limitations, our present work defines HuR as a master regulator of liver homeostasis, contributing to deceleration of diet-induced NASH and HCC development by regulating a hepatoprotective metabolic network. Therefore, appropriate modulation of HuR actions could be harnessed therapeutically in the context of prevention of NAFLD-related sequelae.
ACKNOWLEDGMENT
We thank Katharina Bär, Marta Prucnal, Sylvia Grossklaus, and Stephan Friebe (Technische Universität Dresden) for technical assistance; Vaggelis Harokopos (BSRC “Alexander Fleming”) for next-generation sequencing; Martin Reczko for initial bioinformatics analyses; Sofia Grammenoudi and Kleopatra Dagla for help with flow cytometry and biochemical analysis; Meropi Gennadi for help with histology; and the InfrafrontierGR Infrastructure for providing animal housing and transgenic and genomic services at Fleming. Open access funding enabled and organized by ProjektDEAL.
CONFLICT OF INTEREST
Dr. Domingues is employed by Dewpoint Therapeutics. Dr. Henry is employed by and owns stock in AstraZeneca.
AUTHOR CONTRIBUTIONS
Pallavi Subramanian was responsible for study design, performance of experiments, analysis and interpretation of data, and writing–manuscript; Sofia Gargani was responsible for performance of experiments, analysis and interpretation of data, and editing–manuscript; Margarita Chatzimike, Mirko Peitzsch, were responsible for performance of experiments and analysis of data; Michal Grzybek, Bettina Gercken, Marina Nati, Veera Raghavan Thangapandi, Anke Witt, Ralph Burkhardt, Nicola Zamboni, Peter Mirtschink, Kyoung-Jin Chung, Margarita Andreadou, Iryna Pyrina, Vasileios Ntafis were responsible for performance of experiments. Alessandra Palladini, Mathias Lesche, Andreas Petzold, Anupam Sinha, Ioannis Kourtzelis, Andreas Dahl, Robert Haase, António Miguel de Jesus Domingues, Ian Henry, Anastasios D. Papanastasiou, were responsible for data analysis. Jochen Hampe, Ünal Coskun, were responsible for data interpretation. Dimitris L. Kontoyiannis, was responsible for codesigning the project, data interpretation, supervising research, and editing–manuscript. Triantafyllos Chavakis was responsible for codesigning the project, data interpretation, supervising research, and writing–manuscript.
Open Research
DATA AVAILABILITY STATEMENT
RNA-seq data and RIP-seq data are available at the Gene Expression Omnibus with the accession numbers GSE143358 and GSE143703.