Ductular reaction promotes intrahepatic angiogenesis through Slit2–Roundabout 1 signaling
Mar Coll and Silvia Ariño contributed equally to this work.
Funding information
Supported by grants from Fondo de Investigación Sanitaria Carlos III (FIS), cofinanced by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa” (FIS PI20/00765, PI17/00673, to P.S.-B; FIS 18-PI18/00862, to I.G and M.C); from the National Institute on Alcohol Abuse and Alcoholism (1U01AA026972-01 and AGAUR 2017-SGR-01456, to P.S.-B.); and from the European Foundation for Alcohol Research (EA1653, to P.S.-B.). M.C. is funded by the Ramon y Cajal program from the Ministerio de Ciencia e Innovación RYC2019-026662-I. P.G. is funded by the Agencia de Gestió d'Ajuts Universitaris i de Recerca 2014 SGR 708, Centro de Investigaciónen Red Enfermedades Hepáticas y Digestivas (CIBERehd), and Institució Catalana de Recerca i Estudis Avançats. S.A. received a grant from the Ministerio de Educación, Cultura y Deporte (FPU17/04992). B.A.-B. is funded by the Instituto de Salud Carlos III (FI16/00203)
[Correction added on December 20, 2021, after first online publication: Affiliation for Mercedes Fernandez was corrected to affiliation 1]
Abstract
Background and Aims
Ductular reaction (DR) expands in chronic liver diseases and correlates with disease severity. Besides its potential role in liver regeneration, DR plays a role in the wound-healing response of the liver, promoting periductular fibrosis and inflammatory cell recruitment. However, there is no information regarding its role in intrahepatic angiogenesis. In the current study we investigated the potential contribution of DR cells to hepatic vascular remodeling during chronic liver disease.
Approach and Results
In mouse models of liver injury, DR cells express genes involved in angiogenesis. Among angiogenesis-related genes, the expression of Slit2 and its receptor Roundabout 1 (Robo1) was localized in DR cells and neoangiogenic vessels, respectively. The angiogenic role of the Slit2–Robo1 pathway in chronic liver disease was confirmed in ROBO1/2−/+ mice treated with 3,5-diethoxycarbonyl-1,4-dihydrocollidine, which displayed reduced intrahepatic neovascular density compared to wild-type mice. However, ROBO1/2 deficiency did not affect angiogenesis in partial hepatectomy. In patients with advanced alcohol-associated disease, angiogenesis was associated with DR, and up-regulation of SLIT2–ROBO1 correlated with DR and disease severity. In vitro, human liver-derived organoids produced SLIT2 and induced tube formation of endothelial cells.
Conclusions
Overall, our data indicate that DR expansion promotes angiogenesis through the Slit2–Robo1 pathway and recognize DR cells as key players in the liver wound-healing response.
Abbreviations
-
- AH
-
- alcohol-related hepatitis
-
- ARLD
-
- alcohol-related liver disease
-
- CDE
-
- choline-deficient, ethionine-supplemented
-
- DDC
-
- 3,5-diethoxycarbonyl-1,4-dihydrocollidine
-
- DR
-
- ductular reaction
-
- EpCAM
-
- epithelial cell adhesion molecule
-
- FC
-
- fold change
-
- GO
-
- gene ontology
-
- HUVEC
-
- human umbilical cord endothelial cell
-
- KRT19
-
- keratin 19
-
- MELD
-
- Model for End-Stage Liver Disease
-
- qPCR
-
- quantitative real-time PCR
-
- Robo
-
- Roundabout
-
- SLIT-2
-
- Slit Guidance Ligand 2
-
- Sox9
-
- SRY (sex determining region Y)-box 9
-
- VWF
-
- von Willebrand factor
-
- WT
-
- wild type
-
- YFP
-
- yellow fluorescent protein
INTRODUCTION
Chronic liver injury drives a wound-healing response in order to maintain liver function and hepatic structural properties. The wound-healing response activates well-coordinated repair mechanisms including hepatocyte proliferation, extracellular matrix turnover, and angiogenesis, which induces new blood vessel formation and vascular system remodeling. In this context, the ductular reaction (DR) expands as a regenerative response of the liver, sustaining biliary compartment remodeling. DR cells are a heterogeneous population of biliary cells ranging from reactive cholangiocytes to immature liver progenitor cells. The potential of the biliary compartment to contribute to biliary and parenchymal regeneration has been a focus of intense investigation in the field of liver disease.[1-5] However, few studies have assessed the interaction of DR cells with their surrounding environment and their role in the wound-healing response of the liver.
Patients with underlying alcohol-related liver disease (ARLD) and heavy alcohol intake can develop an episode(s) of alcohol-related hepatitis (AH), which is an acute-on-chronic condition associated with poor short-term prognosis. AH is characterized by inflammatory infiltration, fibrosis, hepatocellular damage, and expansion of the DR, which has been associated with disease severity and short-term mortality.[6, 7] We have recently described that DR cells exhibit a proinflammatory profile in AH, increasing liver inflammation and participating in neutrophil chemotaxis.[8]
Slit Guidance Ligand (SLIT) ligands were first described as secreted chemorepellents of growing axons and migrating neurons that act through Roundabout (Robo) receptors. In recent years, it has been described that guidance cues, which are responsible for neuronal development, have a crucial role in vessel formation.[9-11] Among the three SLIT proteins, SLIT2 has emerged as a proangiogenic factor by inducing sprouting of new vessel formation in angiogenic tissues. SLIT2 can both positively and negatively modulate angiogenesis by binding to ROBO1 and ROBO4, respectively.[12-14] A study on skin and retina showed that Slit2 binding to Robo4 negatively regulates new vessel formation by counteracting VEGF-mediated angiogenesis.[15] The potent proangiogenic role of Slit2 has been described mainly in cancer and ischemic diseases,[16-19] in which secreted Slit2 by solid tumors binds to Robo1 and is expressed in vascular endothelial cells, promoting angiogenesis. Conversely, tumor growth can be inhibited by blocking Robo1 activity.[13, 20] There are few studies assessing the role of the Slit2–Robo1 axis in liver diseases. In this regard, the Slit2–Robo1 pathway was found to be overexpressed in liver cancer, and expression of Slit2 and Robo1 was enhanced in fibrotic liver, promoting liver fibrogenesis through HSC activation.[21] However, it remains unknown whether the Slit2–Robo1 pathway exerts a proangiogenic role in liver regeneration and chronic liver injury.
In this study we hypothesized that DR cells participate in liver tissue remodeling in ARLD. In order to identify pathways in which DR cells are involved, we analyzed the transcriptome of DR cells in a mouse model of chronic liver injury. We identified that liver angiogenesis is a significantly enriched biological process related to DR cells. Additionally, we investigated the role of the Slit2–Robo1 pathway in intrahepatic angiogenesis. The results of our study indicate that DR cells promote liver angiogenesis and that the Slit2–Robo1 pathway is a mechanism underlying this fundamental repair process.
PATIENTS AND METHODS
Human biopsies and samples
Liver tissue samples were obtained from fragments of nontumoral cirrhotic liver tissue surrounding colon metastasis collected at the time of liver resection or from explants from liver transplantation due to ARLD, NASH, and HCV. The study was approved by the Ethics Committee of the Hospital Clinic of Barcelona, and all patients included in this study provided written informed consent.
Patient cohorts
ARLD cohort
The ARLD cohort consisted of patients with AH and MELD ≥ 21 (n = 11), patients with AH and Model for End-Stage Liver Disease (MELD) score < 21 (n = 18), patients with compensated cirrhosis (n = 9), and healthy individuals (n = 10).
Chronic liver disease cohort
The chronic liver disease cohort consisted of patients with AH and MELD ≥ 21 (n = 11), patients with AH and MELD < 21 (n = 18), patients with compensated cirrhosis (n = 9), patients with ARLD and criteria of steatohepatitis (n = 12), patients with NAFLD (n = 10), noncirrhotic HCV (n = 10), and healthy individuals (n = 10).
The baseline characteristics at the time of liver biopsy of the patients included in the ARLD and chronic liver disease cohorts are described in Argemi et al.[22] Moreover, raw sequencing data are available in the Database of Genotypes and Phenotypes of the National Center for Biotechnology Information (phs001807.v1.p1).
AH cohort
The AH cohort consisted of patients with AH (n = 29). Patient characteristics are detailed in Table S1.
Cirrhotic organoids cohort
The cirrhotic organoids cohort consisted of patients with liver cirrhosis (n = 5). The etiologies of cirrhosis are described in Table S2.
Informed consent in writing was obtained from each patient, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
Animals
Because complete or partial genetic deletion of Robo1 is embryonically lethal, we explored the role of the Slit2–Robo1 pathway in mice with partial genetic deletion of both Robo1 and Robo2 which are viable. ROBO1−/+ROBO2−/+ (also referred to as ROBO1/2−/+) mice with a BALB/c background were kindly supplied by Dr. Jian-Guo Geng from the University of Michigan. Littermates of Robo1/2−/+ mice were used in all studies. Details on chronic liver injury and regeneration mouse models are described in the Supporting Information. All animal experiments were approved by the Ethics Committee of Animal Experimentation of the University of Barcelona and conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Gene ontology analysis
A microarray analysis of yellow fluorescent protein–positive (YFP+) cells isolated from HNF1βCreERYFP and wild-type (WT) mice receiving a 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) or a choline-deficient, ethionine-supplemented (CDE) diet for 3 weeks (n = 3 in all groups) was performed previously.[23] In order to assess new biological processes related to DR, we performed a functional analysis using the transcriptomic data generated and deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (GSE51389). A description of the Gene Ontology (GO) analysis (Geneontology.org) performed is detailed in the Supporting Information.
Liver tissue and organoid analysis
Details on immunostaining of liver tissue and organoids as well as gene expression, western blot, and hydroxyproline analysis of liver tissue samples are included in the Supporting Information.
In vitro angiogenesis assay
Growth factor–reduced Matrigel (BD Biosciences, San Jose, CA) was completely thawed at 4℃ for 16–24 h before conducting the tube formation assay. Liquid Matrigel was added to a previously chilled 24-well plate (300 μl/well). The plate was then incubated for 1 h at 37℃ to allow Matrigel solidification. Human umbilical cord endothelial cells (HUVECs; 60,000 cells/well) were resuspended in basal medium (DMEM-F12, 1% Glutamax, 1% 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid [Hepes], and 1% penicillin–streptomycin) or basal medium with recombinant SLIT2 protein (2 ng/ml). To further explore the role of organoid-derived SLIT2 in promoting angiogenesis, we preincubated HUVECs for 2 h with or without 10 mg/ml of sheep antihuman ROBO1 antibody (R&D Systems) in basal medium. Following this, HUVECs were stimulated with conditioned medium with and without αROBO1 antibody (10 mg/ml). In all conditions, HUVECs were incubated for 12 h at 37℃, 5% CO2. Then, the medium was removed, and the endotubes were rinsed with PBS and fixed with 4% paraformaldehyde for 15 min. Tubules were visualized by phase-contrast microscopy, and six images per well at ×4 were taken. Tube formation was evaluated using the Angiogenesis Analyzer tool (ImageJ software).
Liver organoids generated from cirrhotic liver tissues
Liver organoids were generated from cirrhotic liver tissue samples and cultured following the protocol previously described with minor modifications.[8] Briefly, liver tissue was digested using Collagenase XI (Sigma-Aldrich) and Dispase II (ThermoFisher) for 30 min at 37℃. After washing and erythrocyte lysis steps, pelleted cells were embedded in basement matrix extract (BME; Ambio, Abingdon, UK) and seeded in 24-well plates. Following BME solidification, organoid expansion medium was added. Organoid expansion medium consisted of basal medium (Advanced DMEM/F12 [ThermoFisher], 1% Glutamax [ThermoFisher], 1% Hepes [Life Technologies, Carlsbad, CA], and 1% penicillin–streptomycin [Lonza, Basilea, Switzerland]) supplemented with % N2 and 1% B7 without vitamin A (both from Life Technologies); 1.25 mM N-acetylcysteine, 10 mM nicotinamide, and 10 nM gastrin (Sigma-Aldrich); 50 ng/ml EGF, 100 ng/ml FGF-10, 25 ng/ml HGF, 25 ng/ml Noggin, and 500 ng/ml Rspo1 (Peprotech, London, UK); and 5 μM A8301, 0.5 μM CHIR99021, 10 μM forskolin, and 10 μM of Y27632 (Axon Medchem, Groningen, the Netherlands). Y27632 was added only the first 3 days after isolation. The expansion medium was replaced every 2–3 days, and cells were split once a week. At a confluence of 70%–80%, organoid expansion medium was replaced by basal medium for 18 h. Organoid conditioned medium was collected and stored at −80℃ until the HUVECs tubulogenic assay and ELISA SLIT2 quantification were performed.
Serum SLIT2 detection
SLIT2 serum levels from healthy individuals and patients with AH as well as from supernatants of liver organoids generated from patients with cirrhosis were evaluated with a specific ELISA kit for SLIT2 (Cusabio Biotech Co., Wuhan, China) following the manufacturer's protocol.
Statistical analysis
Values are expressed as mean ± SEM. Statistical differences between groups were analyzed using the Student t test, two-way ANOVA with Bonferroni correction, or the Mann-Whitney U test when appropriate with GraphPad Prism 5.0 (San Diego, CA). p ≤ 0.05 was considered statistically significant. Correlations between variables were evaluated using Spearman's rho or Pearson's r, when appropriate.
RESULTS
Mouse DR cells exhibit enriched expression of genes related to angiogenesis
In order to identify biological functions associated with DR cells, we evaluated transcriptomic data obtained from biliary cells, isolated as YFP+ cells by fluorescence-activated cell sorting, from HNF1βCreERYFP mice. DR cells were isolated from mice fed with a diet enriched in DDC, a CDE diet, or a chow diet for 3 weeks and from control mice. GO analysis revealed that the top five enriched biological processes associated with DR cells were inflammatory response (gene ratio 37/315, p = 1 × 10−9), followed by regulation of cell adhesion (gene ratio 31/315, p = 8 × 10−9), angiogenesis (gene ratio 25/315, p = 9 × 10−8), tube morphogenesis (gene ratio 33/315, p = 3.7 × 10−7), and blood vessel morphogenesis (gene ratio 26/315, p = 1.18 × 10−6) (Figure 1A). Because very little is known regarding the capacity of DR cells to participate in angiogenesis, we decided to focus on this key repair mechanism. In order to investigate the mechanism by which DR contributes to liver angiogenesis, we examined the molecules underlying the GO terms related to angiogenesis (Figure 1B). Slit2, a well-described proangiogenic factor, was the gene included in all the categories. Moreover, Slit2 was found to be significantly up-regulated in YFP+ cells isolated from the DDC and CDE models compared to those sorted from control mice (fold change [FC] = 2.2, p = 0.004 and FC = 7.48, p = 0.002, respectively) (Figure 1C).
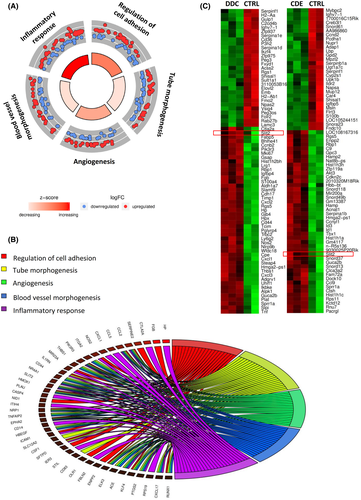
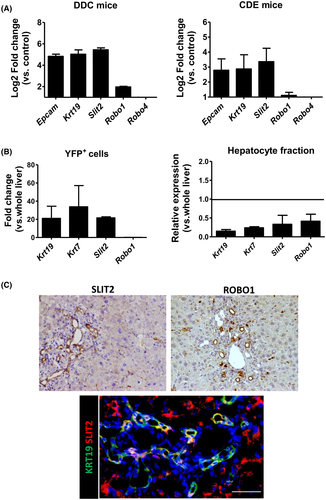
Slit2 is expressed by DR cells and Robo1 by vessels in DDC mice
Slit2 acts as a regulator of new vessel formation through interactions with either Robo1 or Robo4 receptor in the context of cancer and ischemic diseases.[16-19] In order to assess the role of Slit2 in intrahepatic angiogenesis, we first examined the levels of Slit2 expression and its receptors in liver tissue from DDC and CDE mouse models. Slit2 was found to be up-regulated in DDC and CDE compared to control livers (FC = 44.8 ± 4.6 and FC = 8.2 ± 3.3, respectively) at similar levels to the DR markers epithelial cell adhesion molecule (Epcam) and keratin 19 (Krt19). We also observed a significant up-regulation of Robo1 in both models (FC = 3.9 ± 0.15 and FC = 2.25 ± 0.3, respectively), whereas Robo4 was not found to be differentially expressed (Figure 2A). In order to confirm that the expression of Slit2 was restricted to DR cells, we analyzed the gene expression levels of Slit2 in both YFP+ isolated cells and a hepatocyte fraction from DDC mice using quantitative real-time PCR (qPCR). While Slit2 was found to be significantly enriched in DR cells compared to total liver tissue, no expression was found in the hepatocyte fraction (Figure 2B). As expected, we did not find Robo1 expression in either of the examined cell fractions. Next, by performing immunohistochemistry in DDC livers, we confirmed that SLIT2 was expressed by the ductular structures (KRT19-positive cells), whereas its receptor, ROBO1, was expressed by the endothelial cells comprising the new vessels located near the periportal areas (Figure 2C). It is important to note that Robo1 expression was not detected in endothelial cells of the portal vein or hepatic artery, indicating that its expression is specific to the neoangiogenic endothelial cells. These results indicate that, in a DDC mouse model, DR structures express the proangiogenic factor SLIT2, which might interact with ROBO1 receptors expressed by the neo-vessels.
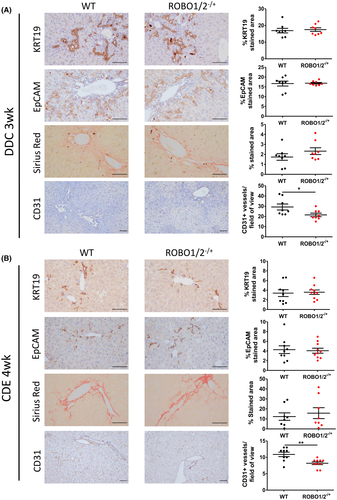
ROBO1/2−/+ mice display reduced hepatic angiogenesis compared to WT mice in response to DDC-induced injury
In order to determine the functional role of Slit2–Robo1 signaling in the pathogenesis of chronic liver injury, we used Robo1/2 partial knockout mice (ROBO1/2−/+) and WT littermates with DDC and CDE diets, both involving DR expansion, for 3 and 4 weeks, respectively. Immunohistochemistry of DR key markers such as KRT19 and EpCAM revealed that ROBO1/2−/+ mice fed the DDC or CDE diet did not display a reduction in DR structures compared to WT mice, indicating that Slit2–Robo1 signaling is not directly involved in ductular cell proliferation (Figure 3A,B). In line with this result, we did not find significant differences in the hepatic expression of genes related to DR such as Krt19, Epcam, or SRY (sex determining region Y)-box 9 (Sox9) (Figures S1A and S2A). In addition, we observed that fibrosis assessed by Sirius red staining; by gene expression of actin alpha 2 smooth muscle, collagen type I alpha 1 chain, matrix metalloproteinase 2, and tissue inhibitor of metalloproteinase 1; and by liver hydroxyproline level determination was not altered in ROBO1/2−/+ mice treated with DDC or CDE when compared to WT mice (Figures 3A,B, S1B, S2B and S3). Indeed, although a profibrogenic role of Slit2–Robo1 signaling has been previously reported in a mouse model of advanced fibrosis based on chronic CCl4 administration, it has been attributed to Slit2 expression by HSCs. Interestingly, quantification of immunohistochemistry staining for CD31 showed a marked reduction of vessels surrounding periportal areas in ROBO1/2−/+ compared to WT mice in both injury models. However, we did not observe a major gene expression reduction of key markers related to angiogenesis (Figures 3, S1C and S2C). Moreover, we did not observe any differences in liver enzymes in serum of ROBO1/2−/+ fed the DDC or CDE diet compared to WT (Figure S4A,B). These results demonstrate that Slit2–Robo1 signaling regulates hepatic angiogenesis in both models of chronic liver disease involving DR expansion.
Slit2–Robo1 signaling does not participate in modulating physiological angiogenesis in response to partial hepatectomy
After partial hepatectomy, angiogenesis and angiocrine growth factors play a key role in the correct regeneration of the liver.[25] Because Slit2–Robo1 has a role in regulating hepatic angiogenesis in chronic liver damage, we assessed whether this signaling pathway participates in the regeneration mechanisms of a healthy liver. With this objective, we first performed two-thirds partial hepatectomy in C57BL/6J mice. We assessed the hepatic gene expression of Slit2 and Robo1 together with DR markers (Epcam, Krt19, Sox9) at different stages of liver regeneration after partial hepatectomy, considering early (0, 24, 48, and 24 h) and late (Days 7 and 28) time points after surgery. Interestingly, we found increased Slit2 expression at 48 h, 72 h, and Day 7 after partial hepatectomy compared to intact liver at 0 h. Besides, liver expression of Robo1 was significantly up-regulated at 72 h after the surgery (Figure 4A). Next, to assess the potential role of Slit2–Robo1 signaling in physiologic angiogenesis, we performed partial hepatectomy in ROBO1/2−/+ mice and WT littermates. We evaluated liver regeneration at 48 h and Day 7 after partial hepatectomy. We did not find significant differences in the number of proliferating cells within the parenchyma, assessed by immunohistochemistry of KI67 between ROBO1/2−/+ and WT mice at early (48 h) and late (7 days) time points after surgery (Figures 4B and S6B). Likewise, liver regeneration index, calculated as liver weight/body weight ratio, did not change either (Figures S5A and S6A). Regarding liver progenitor cell expansion, we found a down-regulation of Epcam and Krt19 gene expression in ROBO1/2−/+ mice at early, but not at late, time points after partial hepatectomy when compared to WT mice. However, we did not see any differences in DR expansion as assessed by immunohistochemistry of KRT19 at 48 and 7 days after surgery (Figures 4B,C, S5B and S6C), suggesting that liver regeneration is not affected by Robo1 deficiency in partial hepatectomy. To specifically investigate whether angiogenesis was impaired in ROBO1/2−/+ mice, we quantified CD31-stained vessels within the liver tissue. We did not observe differences in the formation of new vessels at early or late time points after partial hepatectomy, indicating that Slit2–Robo1 signaling does not significantly contribute to physiological angiogenesis (Figure 4B,C). These results suggest that, although there is an activation of the Slit2–Robo1 pathway after partial hepatectomy, other mechanisms, possibly mediated by VEGF, are more relevant in driving angiogenesis during hepatic regeneration of healthy liver.
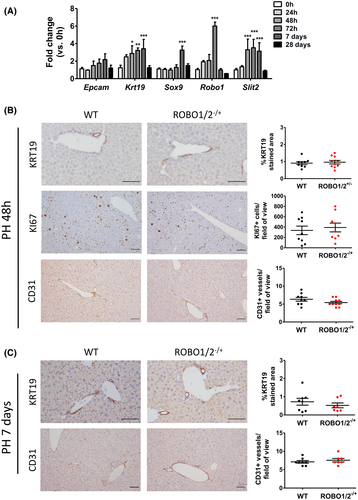
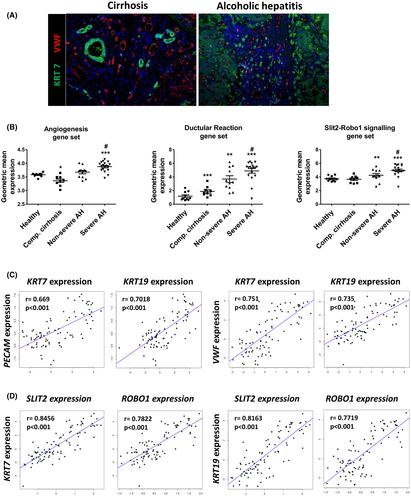
DR and the Slit2–Robo1 pathway are associated with ARLD progression
DR is a histological key feature of AH and increases along ARLD progression. Given the proangiogenic role of SLIT2 in mouse models involving DR expansion, we hypothesized that DR contributes to tissue repair along ARLD progression by promoting angiogenesis through Slit2–Robo1 signaling. In order to assess this hypothesis, we first showed that neoangiogenesis was taking place near the DR structures in liver tissues from patients with cirrhosis and AH by performing a double immunohistochemistry of von Willebrand factor (VWF) and KRT7 (Figure 5A). Next, we examined the levels of SLIT2 and ROBO1 expression as well as the gene expression profile of gene sets related to angiogenesis and DR in RNA sequencing data from total liver tissue from a cohort of patients encompassing the whole spectrum of ARLD.[22] The patients included in this cohort were grouped according to ARLD stage: severe AH, patients with AH and MELD ≥ 21 (n = 11); nonsevere AH, patients with AH and MELD < 21 (n = 18); patients with compensated cirrhosis (n = 9); and healthy individuals (n = 10). Interestingly, the expression profile of the angiogenesis gene set markedly increased with ARLD progression, suggesting that intrahepatic angiogenesis is a relevant regeneration mechanism underlying ARLD. As expected, the DR gene signature increased in ARLD progression, and we found that it followed a similar gene expression pattern as angiogenesis. In addition, SLIT2 and ROBO1 expression displayed a similar trend to DR and angiogenesis sets, showing significant up-regulation in severe compared to nonsevere AH (Figure 5B). Next, we sought to determine if DR and angiogenesis were linked biological processes in patients with chronic liver diseases of different etiologies. For this analysis we included a group of patients with nonadvanced chronic liver disease with the following etiologies: ARLD with histologic criteria of steatohepatitis (n = 12), NAFLD (n = 10), and HCV-infected patients without cirrhosis (n = 10). The hepatic expression of DR markers (KRT7 and KRT19) strongly correlated with the expression of angiogenesis markers such as platelet/endothelial cell adhesion molecule and VWF (Figure 5C) as well as with SLIT2 and its receptor ROBO1 (Figure 5D). This result suggests that DR expansion and angiogenesis are activated not only along ARLD progression but also in chronic liver diseases of other etiologies. Moreover, our findings indicate that the Slit2–Robo1 pathway is associated with fundamental repair processes in chronic liver disease such as DR and angiogenesis. In addition, the expression of the angiogenic signaling factor VEGFA and the kinase insert domain receptor and Fms-related receptor tyrosine kinase 1 did not correlate with the hepatic expression of DR markers (Figure S7), suggesting that in chronic liver disease DR-mediated angiogenesis may not be related to VEGFA signaling.
The Slit2–Robo1 pathway is enhanced in patients with AH
To confirm the association of DR with the Slit2–Robo1 pathway, we evaluated the hepatic expression of SLIT2 and its receptor ROBO1 in a cohort of patients with AH (n = 29) and healthy individuals (n = 5). The characteristics of the patients are described in Table S1. SLIT2 and ROBO1 expression was increased in patients with AH compared to controls (FC = 3.4 ± 0.4, and FC = 2.35 ± 019, respectively) (Figure 6). As expected, the hepatic expression of SLIT2 significantly correlated with the expression of its receptor, ROBO1. Interestingly, in the same cohort of patients with AH, SLIT2 expression strongly correlated with the hepatic expression of KRT7, suggesting that DR cells are a cell source of SLIT2 in patients with AH (Figure 6A). Next, we investigated the serum levels of SLIT2 in a subset of patients with AH (n = 16) and healthy individuals (n = 6). We observed that circulating SLIT2 levels were significantly increased in patients with AH compared to healthy individuals (9.8 ± 0.9 versus 3.4 ± 1.1 ng/ml) (Figure 6B). Additionally, circulating levels of SLIT2 positively correlated with serum levels of aspartate aminotransferase and alkaline phosphatase, suggesting that SLIT2 increases together with liver injury in AH.
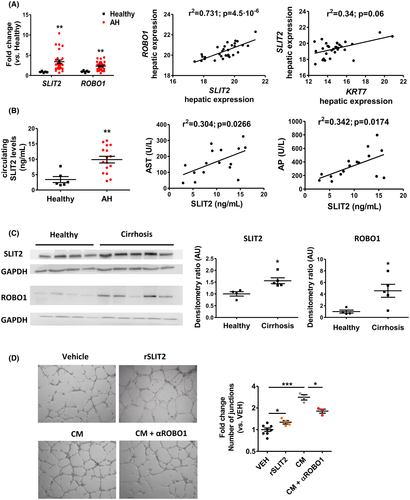
DR cells from cirrhotic livers secrete SLIT2 and induce tubulogenesis
We recently reported that liver organoids derived from cirrhotic liver tissue mimic DR cells and represent a relevant in vitro model to study DR.[8] In order to confirm that human DR cells secrete SLIT2, we generated liver biliary organoids from patients with cirrhosis of different etiologies (NASH, n = 1; ARLD, n = 2; and HCV, n = 2). The cirrhotic tissues used to generate organoids expressed higher levels of SLIT2 and ROBO1 proteins compared to control livers (Figure 6C). We also confirmed the presence of DR in all the cirrhotic tissue samples examined by KRT7 immunohistochemistry (Figure S8A). As expected, we detected the SLIT2 protein in all the supernatants of the organoids generated (17 ± 2.8 ng/ml) (Table S2 and Figure S8B), and we observed SLIT2 expression by immunofluorescence in the liver organoid (Figure S8C). Next, with the purpose of demonstrating the proangiogenic potential of DR cells, we performed a tubulogenic assay by exposing HUVECs to conditioned medium derived from cirrhotic liver organoids (n = 3). As a positive control, we incubated HUVECs with recombinant SLIT2 protein (2 ng/ml). Compared to the basal medium, liver organoid conditioned medium significantly increased angiogenesis, as assessed by the quantification of the number of junctions formed by HUVECs. To further demonstrate that Slit2 secreted by DR cells mediates angiogenesis, we treated HUVECs with conditioned medium containing αROBO1 blocking antibody. Interestingly, we observed that the proangiogenic ability of DR cells was significantly attenuated after ROBO1 blockade (Figures 6D and S8D). Altogether, these results suggest that DR cells release the proangiogenic factor SLIT2, thus contributing to angiogenesis.
DISCUSSION
In this study we show that DR may promote intrahepatic angiogenesis in chronic liver diseases. We show that DR structures express SLIT2, whereas its receptor ROBO1 is expressed by endothelial cells of small vessels. We describe that the Slit2–Robo1 pathway regulates intrahepatic angiogenesis in chronic liver injury but not in physiological liver regeneration induced by loss of liver cell mass. Moreover, we describe that intrahepatic angiogenesis and DR expansion are concomitant mechanisms taking place along ARLD progression. With the use of a human in vitro model of DR cells, we demonstrate the capacity of DR cells to produce SLIT2 and induce angiogenesis. Collectively, these data indicate that DR cells trigger intrahepatic liver angiogenesis through the Slit2–Robo1 pathway.
Under chronic liver damage, the intrahepatic vascular bed undergoes a critical structural modification by enhancing the number of sinusoidal vessels and producing shunts connecting central and portal venules to reduce sinusoidal vascular resistance.[26] In the present study, we show that intrahepatic angiogenesis increases with disease progression, indicating that liver angiogenesis behaves as a dynamic process following the same pattern as other repair mechanisms such as fibrogenesis. In this regard, previous studies have evaluated the therapeutic potential of preventing hepatic angiogenesis as a strategy to reduce disease progression. However, the beneficial effects of the administration of antiangiogenic drugs in animal models of chronic liver injury is still controversial.[27, 28] In fact, angiogenesis is a fundamental mechanism underlying liver injury repair and regeneration, and therefore, it may play a beneficial role in the wound-healing response to injury. To understand to what extent the intrahepatic angiogenesis contributes to disease progression and tissue healing in chronic liver diseases is a very important aspect with clinical implications that should be directly investigated.
DR cells are known to play a role in liver fibrosis and inflammation.[8, 29, 30] However, there is a lack of information regarding the potential of DR in promoting angiogenesis. In order to elucidate whether DR participates in angiogenesis, we performed a GO analysis with the transcriptomic data generated from DR cells isolated from mice subjected to chronic injury involving DR expansion (3 weeks of DDC and CDE diets). Interestingly, angiogenesis was found to be one of the most enriched biological processes associated with mouse DR. In humans, in a large cohort encompassing the whole spectrum of ARLD, we confirmed that DR expansion correlates with intrahepatic expression of angiogenic factors and markers and that both mechanisms paralleled disease progression.
Intrahepatic angiogenesis takes place under the physiological context of liver regeneration and under pathological conditions.[31, 32] To investigate whether the Slit2–Robo1 pathway promotes angiogenesis in these contexts, we used chronic injury (3 and 4 weeks of DDC and CDE diets, respectively) and liver regeneration (partial hepatectomy) models in mice with a partial deletion of Robo1 and Robo2 genes because complete deletion of Robo1 is embryonically lethal. Interestingly, we show that the Slit2–Robo1 pathway promotes liver angiogenesis in response to chronic injury but does not contribute to remodeling the vascular bed during liver regeneration after partial hepatectomy. These data indicate that angiogenesis driven by the Slit2–Robo1 pathway may be taking place only in the context of chronic liver injury where DR expansion is present but not in a regenerative response in a healthy liver, where there is no involvement of the DR. These results suggest that the Slit2–Robo1 pathway may be a potential target to enhance liver wound healing in chronic liver disease. In fact, it is known that Slit2–Robo1 signaling participates in promoting angiogenesis in several pathological contexts such as retinopathy,[33] ischemia,[18] or endometriosis.[34] In addition, Slit2 expression in solid tumors promotes tumor-induced angiogenesis by acting through Robo1 expressed in vascular endothelial cells.[13] Further studies are needed to elucidate whether intrahepatic Slit2 contributes to angiogenesis in other pathological liver settings such as tumor angiogenesis.
Little is known regarding the mechanisms that trigger pathological intrahepatic angiogenesis. To date, the main cell type inducing liver angiogenesis in a pathological setting is liver endothelial sinusoidal cells, although HSCs also directly contribute to new vessel formation by producing proangiogenic factors such as VEGF, platelet-derived growth factor, and angiopoietin-1 or in a paracrine manner by activating liver sinusoidal endothelial cells.[35, 36] Our study focused on intrahepatic angiogenesis underlying chronic liver diseases with DR expansion such as ARLD. In this context, we showed that the neoangiogenesis, which is histologically visualized as small vessels with varying diameter and positive staining for VWF or CD31, takes place near the proliferative ductular structures. To confirm the angiogenic role of DR cells, we generated biliary three-dimensional organoids derived from cirrhotic liver tissue, which have been shown to retain the phenotypic and transcriptomic features of DR cells mimicking in vitro DR.[8] In this regard, we demonstrate that all DR organoids generated from different etiologies (ARLD and NASH), secrete SLIT2 and induce HUVEC tube formation.
Interestingly, serum SLIT2 levels are increased and correlate with the severity of liver injury in patients with AH, the most severe form of ARLD that is characterized by extensive DR. These results are in agreement with previous data showing increased circulating levels of SLIT2 in patients with fibrosis.[21] In addition, activated HSCs produce and are also targets of SLIT2, contributing to fibrogenesis. These results indicate that DR cells are not the only cell source of Slit2 in chronic liver disease and suggest that Slit2 secreted by DR cells could contribute to wound healing not only by inducing angiogenesis but also by targeting HSCs and promoting fibrogenesis.
Although we have shown that DR cells promote angiogenesis through the Slit2–Robo1 pathway, DR may also produce other proangiogenic factors. Histologically, it has been shown that liver progenitor cells derived from patients with primary biliary cholangitis express VEGFA and VEGFC at the protein level.[37] However, in the ARLD cohort, we did not find a correlation between the hepatic expression of VEGFA and the expression pattern of genes related to DR, suggesting that intrahepatic expression of VEGFA does not occur in parallel with DR expansion.
DR cells have the potential to contribute to liver regeneration by giving rise to new hepatocytes when their replication capacity is hampered. In this study, we provide evidence to support that DR cells contribute to tissue remodeling by promoting angiogenesis. In addition, we have recently reported that in chronic liver damage DR cells produce chemoattractant agents to induce neutrophil recruitment that might also participate in liver tissue repair.[8] Taken together, these results suggest that DR has a major role in liver wound healing, driving fundamental repair mechanisms.
Overall, this report provides evidence that DR cells mediate intrahepatic angiogenesis through the Slit2–Robo1 pathway and recognizes DR cells as important contributors to wound-healing processes in chronic liver disease.
ACKNOWLEDGMENTS
This work was performed in the Centre Esther Koplowitz. We thank Dr. Jian-Guo Gen from the University of Michigan for kindly providing the ROBO1–/+ROBO2–/+ mice and for his scientific support. We thank Cristina Millán for her excellent technical support, especially in immunohistochemistry. We are indebted to the Cytomics Unit, Genomics Unit, and Biobank core facility of the Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS) for technical help.
CONFLICT OF INTEREST
Dr. Gines consults for and received grants from Gilead and Grifols. He consults for Martin Pharmaceuticals, Novartis, and Rallybio. He received grants from Mallinckrodt.
AUTHOR CONTRIBUTIONS
Mar Coll participated in the design of the study, performed experiments, and drafted the manuscript. Silvia Ariño participated in all the experiments and critically reviewed the manuscript. Ester Garcia-Pras and Javier Gallego performed angiogenesis in vitro assays. Celia Martínez-Sánchez performed data analysis. Beatriz Aguilar-Bravo participated in liver organoid generation. Delia Blaya, Julia Vallverdú, and Teresa Rubio-Tomás helped with the maintenance and genotyping of the ROBO1/2+/− mouse colony. Juan Jose Lozano performed bioinformatics analysis. Elisa Pose and Isabel Graupera recruited the patients and critically reviewed the manuscript. Andrea Fernández-Vidal performed the hepatic hepatectomy surgeries. Albert Pol contribute to the critical review of the manuscript. Ramón Bataller provided the ARLD sequencing data set and critically reviewed the manuscript. Jian-Guo Geng provided the ROBO1/2+/− mice and critically reviewed the manuscript. Mercedes Fernandez and Pere Ginès interpreted data and contributed to the critical review of the manuscript. Pau Sancho-Bru conceived and designed the study, critically reviewed the manuscript, and supervised the study.