Evaluating the Prevention Benefit of HCV Treatment: Modeling the SToP-C Treatment as Prevention Study in Prisons
Supported by Australian National Health and Medical Research Council Partnership Project Grant (1092547), Gilead Sciences, and the UK National Institute for Health Research (NIHR). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Potential conflict of interest: Dr. Martin received grants from Gilead and Merck. Dr. Grebely is on the speakers’ bureau for and received grants from AbbVie, Cepheid, Gilead, and Merck. He received grants from Hologic. Dr. Dore received grants from Gilead and AbbVie. Dr. Lloyd advises for and received grants from Gilead. He received grants from AbbVie.
Abstract
Background and Aims
Between 2014 and 2019, the SToP-C trial observed a halving in HCV incidence in four Australian prisons following scale-up of direct-acting antiviral (DAA) therapy. However, the contribution of HCV treatment to this decline is unclear because the study did not have a control group. We used modeling to consider this question.
Approach and Results
We parameterized and calibrated a dynamic model of HCV transmission in prisons to data from each SToP-C prison on incarceration dynamics, injecting drug use, HCV prevalence trends among prison entrants, baseline HCV incidence before treatment scale-up, and subsequent HCV treatment scale-up. The model projected the decrease in HCV incidence resulting from increases in HCV treatment and other effects. We assessed whether the model agreed better with observed reductions in HCV incidence overall and by prison if we included HCV treatment scale-up, and its prevention benefits, or did not. The model estimated how much of the observed decrease in HCV incidence was attributable to HCV treatment in prison. The model projected a decrease in HCV incidence of 48.5% (95% uncertainty interval [UI], 41.9-54.1) following treatment scale-up across the four prisons, agreeing with the observed HCV incidence decrease (47.6%; 95% CI, 23.4-64.2) from the SToP-C trial. Without any in-prison HCV treatment, the model indicated that incidence would have decreased by 7.2% (95% UI, −0.3 to 13.6). This suggests that 85.1% (95% UI, 72.6-100.6) of the observed halving in incidence was from HCV treatment scale-up, with the remainder from observed decreases in HCV prevalence among prison entrants (14.9%; 95% UI, −0.6 to 27.4).
Conclusions
Our results demonstrate the prevention benefits of scaling up HCV treatment in prison settings. Prison-based DAA scale-up should be an important component of HCV elimination strategies.
Abbreviations
-
- Ab
-
- antibody
-
- DAA
-
- direct-acting antiviral
-
- DIL
-
- Dillwynia
-
- GLB
-
- Goulburn
-
- IDU
-
- injecting drug use
-
- JH&FMHN
-
- Justice Health & Forensic Mental Health Network
-
- LGW
-
- Lithgow
-
- NSPs
-
- needle and syringe programs
-
- NSW
-
- New South Wales
-
- OAT
-
- opioid agonist therapy
-
- OMMPC
-
- Outer Metropolitan Multi-Purpose Correctional Centre
-
- PWID
-
- people who inject drugs
-
- py
-
- person-years
-
- SVR
-
- sustained virological response
-
- UI
-
- uncertainty interval
The HCV infection burden is high in prisons worldwide, primarily attributable to the criminalization of injecting drug use (IDU), with the global HCV seroprevalence among prisoners being 26%.(1) This situation is mirrored in Australia, where 53% of people who inject drugs (PWID) have ever been incarcerated,(2) and the HCV seroprevalence among prison entrants in 2016 was 24%.(3) Furthermore, continued IDU in Australian prisons, coupled with suboptimal coverage of prison-based harm reduction interventions, contribute to considerable HCV transmission in prisons.(4, 5)
Since 2014, highly effective direct-acting antiviral (DAA) treatments have become available.(6, 7) Mathematical modeling studies predict that HCV treatment should prevent onward HCV transmission by reducing the pool of prevalent HCV infections, a concept known as treatment-as-prevention.(8) However, empirical studies demonstrating such a prevention benefit are sparse(9-11) and limited to associations in observational studies between increases in HCV treatment and concurrent decreases in HCV incidence.(12) Just as empirical evidence for HIV treatment-as-prevention led to a large scale-up in antiretroviral therapy (ART),(13, 14) it is hoped that establishing the evidence base for HCV treatment-as-prevention will encourage expansion of DAA treatment.
To improve this empirical evidence base, the Surveillance and Treatment of Prisoners with hepatitis C (SToP-C) intervention study was conducted between 2014 and 2019 to investigate the feasibility and effectiveness of HCV treatment-as-prevention in four prisons in New South Wales (NSW), Australia.(15) SToP-C assessed whether a large scale-up in HCV treatment in these prisons would substantially reduce HCV incidence. Treatment was scaled up from 90 persons being treated in the pretreatment scale-up phase (2014 to mid-2017), to 534 in the treatment scale-up phase (mid-2017 to 2019), with this being associated with a 47.6% decrease in HCV incidence (95% CI, 23.4-64.2) across the four prisons.(15)
Although these results are very encouraging, the absence of control prisons meant that it was difficult to confidently ascribe the decrease in HCV incidence to the scale-up in HCV treatment. Indeed, other factors may have played a role, including documented decreases in HCV prevalence among new prison entrants(16, 17) resulting from increased community treatment.(18) In this analysis, we use mathematical modeling to estimate the contribution that DAA treatment scale-up made toward the observed decline in HCV incidence during the SToP-C study, allowing us to evaluate the impact of HCV treatment-as-prevention in four prison settings in Australia.
Materials and Methods
Data Sources
Detailed individual-level data were collected from 3,691 prisoners enrolled in the SToP-C study across four prisons in NSW, Australia from 2014 to 2019. The methodology of SToP-C has been reported in detail.(15) Briefly, the study included four prison sites, two being high-security (Goulburn [GLB] and Lithgow [LGW]) and two being medium-security prisons, one for males (Outer Metropolitan Multi-Purpose Correctional Centre; OMMPC) and one for females (Dillwynia; DIL). The study assessed risk behavior and screened for HCV antibody (Ab) and HCV RNA at study enrollment, and then every 6 months to estimate the incidence of HCV infection and reinfection throughout the study. The SToP-C study included two phases within a prospective cohort: (1) pretreatment scale-up and (2) treatment scale-up, when DAA treatment scale-up occurred. The pretreatment scale-up phase was used to estimate the baseline HCV incidence and “business-as-usual” uptake of DAA treatment, undertaken by the prison-based hepatitis service of the Justice Health & Forensic Mental Health Network (JH&FMHN).(19) This phase ran from October 2014 to mid-2017. The treatment scale-up phase started in mid-2017 and increased uptake of DAA treatment (sofosbuvir/velpatasvir for 12 weeks) among enrolled prisoners who tested HCV-RNA positive. Because DAA treatment scale-up was staggered across the four prisons, the treatment scale-up phase for the study evaluation was designated to commence in January 2018, whereas the pretreatment scale-up phase ended in December 2017. The 3,691 prisoners enrolled in the study accounted for one third of all persons entering the four prisons over the study period (Supporting Table S2). Empirical estimates of HCV incidence from the pretreatment scale-up and treatment scale-up phases, overall and stratified by history of IDU, were used to assess changes in HCV incidence. The study closed in November 2019.
Information on the date of enrollment, IDU status (ever or never), and HCV status (Ab negative, Ab positive/RNA negative, and RNA positive) were recorded for each prisoner at enrollment. Participants testing HCV-RNA positive, either at enrollment or at follow-up visits, and with at least one follow-up assessment after diagnosis, were eligible for HCV treatment through SToP-C. Their treatment start date and sustained virological response (SVR; effective cure) outcomes were recorded. Data were also available on HCV treatments initiated by JH&FMHN for each prison. Individual-level prisoner entry and exit dates were available for all prisoners.
Model Structure
We developed a deterministic, compartmental model to simulate the dynamics of incarceration, IDU, incoming HCV prevalence among prison entrants, ongoing HCV transmission within prisons, SToP-C enrollment, and HCV treatment provided by SToP-C or JH&FMHN. Model stratifications are shown in Fig. 1, and model equations are in the Supplementary Materials. In-prison opioid agonist therapy (OAT) was not included in the baseline model because it has uncertain efficacy in this setting,(4) but was included in a sensitivity analysis.
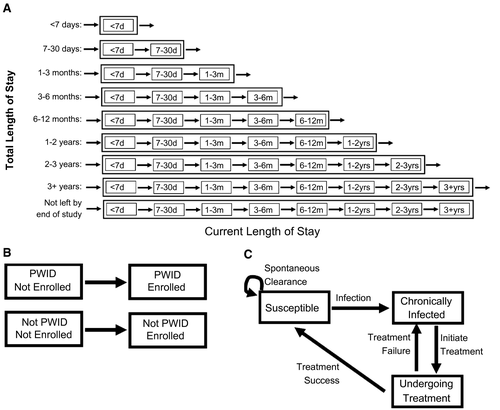
Briefly, a time-varying number of persons enter the model as new prisoners and not yet enrolled in SToP-C, with a specified IDU status, HCV status, and total prison stay as determined by monthly data from the study, which fluctuates over time (see next section). Prisoners transition progressively through successive current length-of-stay compartments until they exit the compartment corresponding to their total prison stay, except for those who have “not left by end of study,” who remain in the prison until the study end. Susceptible persons acquire HCV infection at a rate dependent on the current HCV prevalence among prisoners. We assume an IDU-related HCV infection rate among prisoners who have ever injected drugs and a non-IDU-related HCV infection rate across all prisoners (to capture the low HCV incidence in persons who report never injecting drugs). The majority of new infections become chronic.(20) Throughout the study, a time-varying number of prisoners, which depends on current and total length of stay, IDU status, and HCV infection status, are enrolled in SToP-C, with those HCV-RNA positive initiating HCV treatment at a constant rate dependent on their IDU status. HCV-infected prisoners can also be initiated onto HCV treatment by JH&FMHN at a different rate. Treatment either leads to SVR, or fails, following which retreatment can occur. Reinfection is modeled at the same rate as primary infection.(6)
Model Parameterization and Calibration
The model was parameterized and calibrated for each prison using entry and exit data for all prisoners, and SToP-C enrollment and follow-up data over 2014-2019. Model inflow rates for each prison were determined using prison entry data. Among those ever-enrolled in SToP-C, new entrants were assigned an IDU and HCV status according to their enrollment data. For those never enrolled in SToP-C, or those enrolled but with unknown IDU or HCV status, new entrants were assigned a similar prevalence of IDU (stratified by prison stay) and HCV prevalence over time as for enrolled prisoners with known data from each prison. Among ever-enrolled prisoners, prevalence of IDU was similar across SToP-C prisons, fluctuating between 44% and 62% over time (Supporting Fig. S2). Chronic HCV prevalence was low among enrolled non-PWID (0%-9%), but much higher among PWID (41%-53%), at study commencement, with this declining over time in all prisons (the odds of being HCV-RNA positive at prison entry among PWID decrease on average by a relative 1%-5% per month across prisons; Supporting Table S3; Supporting Fig. S2). The model was initialized according to the distribution of prisoners by current and total prison stay for each SToP-C prison at the start of the pretreatment scale-up phase. For simplification, ever-enrolled prisoners who exited and re-entered the model were assumed to remain in the model. This should not have affected our model projections because their estimated time periods out of prison represented only 7.4% of their total time in prison (Supplementary Materials). All persons entered prison as not enrolled in SToP-C, with enrollment determined by monthly enrollment numbers for each prison. Parameters used in the model are summarized in Table 1, with full values in Supporting Table S4.
| Meaning and Data Used for Parameters | |
|---|---|
| Incarceration and SToP-C Enrollment parameters | |
| Entry rate into prison | Time-varying rate of entry into the model, stratified by total prison stay, IDU status (PWID or non-PWID), and HCV status (infected or not), for ever and never enrolled. Individual-level entry rate data are used for each prison. Linkage of these data with enrollment data on IDU and HCV infection status are used to stratify the data for individuals ever enrolled in SToP-C. Stratifications by IDU and HCV status among never-enrolled persons entering the model are assumed to follow similar distributions as those ever enrolled. The model entry rate is parameterized for each month. |
| Enrollment rate into SToP-C | Time-varying rate of enrollment into SToP-C, which is dependent on current and total length of stay, IDU status, and HCV status. The model is parameterized using individual-level enrollment data for each month. |
| Average duration in current length-of-stay compartments (not final before release from prison) | Average transition rates through each current length-of-stay compartment are assumed to be the reciprocal of the duration of that compartment. The average duration in the 3+ years current length-of-stay compartment is assumed to be 7 years, with adjustment to the final leaving rate. |
| Average duration in final current length-of-stay compartment before release from prison | Calculated to agree with individual-level prisoner exit data, for ever and never enrolled, among those leaving prison. The final leaving rate is then the reciprocal of the mean duration in the final current length-of-stay compartment. |
| Final leaving-rate adjustment factor | Fitted to the number of prisoners with total prison stay of 3+ years before and after January 2018 for each prison. |
| HCV transmission parameters | |
| Non-IDU-related HCV transmission rate | Transmission rate parameters attributable to non-IDU-related risk and IDU-related risk are calibrated to fit each prison model to empirical HCV incidence estimates among non-PWID and PWID for each prison in the pre-treatment scale-up phase. |
| IDU-related HCV transmission rate | |
| Spontaneous clearance rate | Proportion of acute HCV infections that spontaneously clear; assumption based on the findings of a systematic review |
| HCV treatment parameters | |
| SToP-C HCV treatment rate per capita | SToP-C HCV treatment rate, split by IDU status, starting from first treatment date in prison. For each prison, this is fitted to the total number of SToP-C HCV treatments given over the treatment scale-up phase by IDU status. |
| JH&FMHN HCV treatment rate | JH&FMHN HCV treatment rate starting from first treatment date in each prison. For each prison, this is fitted to the total number of JH&FMHN HCV treatments given over the entire study. |
| Average duration on HCV treatment | The reciprocal is the average rate of undergoing treatment. The duration of treatment is assumed to be 12 weeks. |
| SVR rate | Proportion of persons achieving SVR following DAA treatment; assumption based on efficacy of DAA treatment. |
- Detailed parameter values and distributions are in Supporting Table S4.
To characterize HCV transmission and the HCV treatment rate in each prison, the model was calibrated to empirical estimates of HCV incidence from the pretreatment scale-up phase among PWID and non-PWID, and simultaneously to the total number of HCV treatments provided by SToP-C (by IDU status) and JH&FMHN over the study period (Supporting Table S5). Empirical HCV incidence estimates from the pretreatment scale-up phase encompassed both primary infection and reinfection data, were much higher among PWID, and varied across prisons. For example, HCV incidence among PWID ranged across the prisons from 3.0 (95% CI, 0.4-21.4) to 20.4 (95% CI, 14.3-29.2) per 100 person-years (py), whereas it ranged from 0.0 (95% CI, 0.0-6.8) to 8.3 (95% CI, 2.1-33.0) per 100 py among non-PWID. Total HCV treatment numbers also varied by prison (Supporting Table S5). Altogether, SToP-C initiated 324 HCV treatments over 2017-2019, with 80% occurring in the larger prisons (GLB and LGW). JH&FMHN initiated 301 HCV treatments over 2014-2019, including 123 among persons enrolled in SToP-C.
Uncertainty distributions were associated with most parameters and calibration data, including IDU and HCV prevalence among those with unknown status, and HCV incidence by IDU status. Uncertainty was not applied to quantities where individual-level data were available, including the prison entry and exit data, SToP-C enrollment data, the self-reported IDU and tested HCV status of SToP-C enrolees, and total number of HCV treatments given by SToP-C and JH&FMHN.
To calibrate the model to each prison, 1,000 paired sets of parameters and calibration data were sampled. For each set, unknown model parameters representing HCV transmission rates and the rate of initiating HCV treatment for each prison were varied to minimize the difference between the model projections and sampled values from the empirical HCV incidence estimates for PWID and non-PWID averaged over the pretreatment scale-up phase, and the HCV treatment data. This was done using a non-linear least squares optimisation algorithm. Modeled HCV incidence outputs were then validated by comparing the overall modeled HCV incidence for each prison to empirical estimates from SToP-C, both considering the average over the pretreatment scale-up phase. All results are reported as the median and 95% uncertainty interval (UI) across the 1,000 model simulations for each prison.
Model Analyses
The calibrated model was used to project whether the modeled decrease in HCV incidence across each prison and overall, resulting from the scale-up in HCV treatment and other changes, agreed with empirical data on how incidence declined in the SToP-C prisons during the treatment scale-up phase, noting that the model was not fitted to these data. To mimic outcomes from the trial, this was done by comparing the modeled and empirical HCV incidence estimates across the pretreatment scale-up and treatment scale-up phases. We also considered whether a counterfactual model that did not include any HCV treatment could also agree with these empirical data. A “goodness-of-fit” metric compared how well each model agreed with empirical incidence data, determined as the percentage of modeled estimates that lie within the 95% CI of the empirical estimates for the treatment scale-up phase (by PWID status and overall). We then estimated the contribution of HCV treatments initiated by JH&FMHN and/or SToP-C to the observed reduction in HCV incidence by removing these treatments from the model for each prison and re-estimating the relative change in incidence for that prison and overall. Effects of observed declines in HCV prevalence among prison entrants on decreasing incidence were also determined. Model analyses were conducted in MATLAB (version R2019a; The MathWorks, Inc., Natick, MA).
Sensitivity Analyses
We undertook univariate sensitivity analyses to determine how model-predicted reductions in HCV incidence attributable to SToP-C and JH&FMHN HCV treatments would vary by changes in model assumptions. We evaluated the effect of incorporating changes in OAT coverage among PWID (OAT was not included in the baseline model), which decreased from 42.4% in 2014 to 14.5% by 2019 among SToP-C enrollees who reported injecting in the current imprisonment.(15) For this sensitivity analysis, we assumed that in-prison OAT is associated with a 50% reduction in HCV acquisition risk.(21) We also investigated how our assumptions on the prevalence of IDU and HCV among prisoners with unknown status could affect our model projections by varying IDU prevalence or HCV prevalence by ±20% of their baseline values.
Ethics Statement
The SToP-C study including this modeling analysis was approved by the Human Research Ethics Committee of the Justice Health & Forensic Mental Health Network of NSW (G621/13). Each patient provided informed written consent to participate in the SToP-C study. The SToP-C study protocol was done according to the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice (ICH/GCP) guidelines, and is registered with ClinicalTrials.gov, NCT02064049.
Results
Baseline model comparison
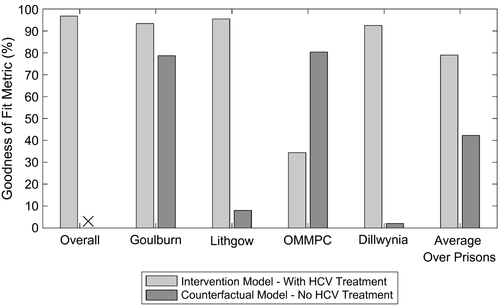
The calibrated model replicated the incarceration dynamics for each prison (Supporting Fig. S3). Because of variability in the monthly number of new entrants and their characteristics (total length of stay, chronic HCV prevalence, and proportion that inject drugs), the model for each prison exhibited fluctuations in the total number of prisoners (Supporting Fig. S3) and in prevalence and incidence of HCV (Supporting Figs. S6 and S7). However, despite this, the model accurately reproduced empirical estimates of HCV incidence by IDU status across the pretreatment scale-up phase (Fig. 2) and HCV treatment by SToP-C and JH&FMHN over the entire study period (Supporting Fig. S4). Overall, 97.4% of modeled HCV incidence estimates (by PWID status and overall) across the four prisons lay within the 95% CI of the corresponding incidence data from the pretreatment scale-up phase of SToP-C.
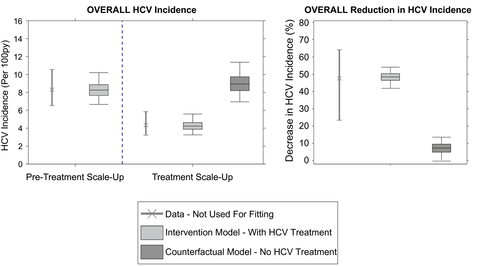
Validation of Model With Trial Outcomes on HCV Incidence
The model was not calibrated to the overall or prison-specific decreases in HCV incidence observed during the treatment scale-up phase. Despite this, when the effects of HCV treatment were included, the model accurately predicted the incidence decrease observed in the trial, both overall (Fig. 2) and by IDU status (Supporting Fig. S5). Over the treatment scale-up phase, the model projected that the overall HCV incidence across the four prisons decreased by 48.5% (95% UI, 41.9-54.1), from 8.3 (95% UI, 6.7-10.2) to 4.2 (95% UI, 3.3-5.6), with this closely agreeing with the observed decrease in HCV incidence (47.6%; 95% CI, 23.4-64.2) from the SToP-C study. This goodness-of-fit was emphasized by 96.8% of modeled estimates for the overall HCV incidence for the treatment scale-up phase lying within the 95% CI of the empirical data.
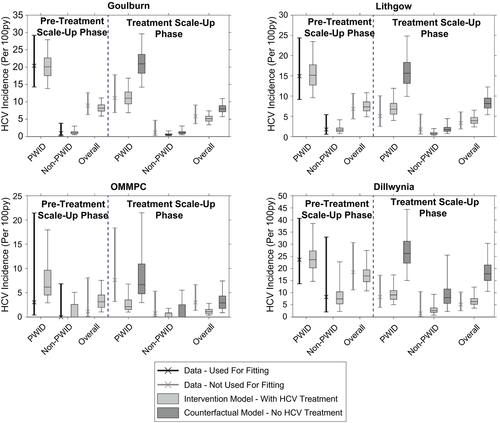
The model also agreed well with empirical incidence data in the treatment scale-up phase for three of the SToP-C prisons, although the fit was not as good for OMMPC (Fig. 3; Supporting Tables S6 and S7). Excluding OMMPC, >92% of modeled incidence estimates lay within 95% CI of the empirical data for the treatment scale-up phase (Fig. 4), with this decreasing to 79.0% across all prisons and 34.4% for OMMPC. The model projected the overall HCV incidence during the treatment scale-up phase to be 5.1 (95% UI, 3.4-7.4) per 100 py in GLB, 3.9 (95% UI, 2.4-6.5) per 100 py in LGW, and 6.3 (95% UI, 3.7-12.3) per 100 py in DIL, with these median estimates deviating by <25% from the empirical estimates (Fig. 3). In contrast, modeled HCV incidence in the treatment scale-up phase for OMMPC differed by two thirds from the empirical estimate (Fig. 3). The reduced goodness-of-fit for OMMPC was attributable to considerable uncertainty in the empirical incidence estimates for OMMPC (only seven incident infections were recorded, with only one occurring in the pretreatment scale-up phase), paired with incidence increasing in the treatment scale-up phase. This caused considerable uncertainty in the initial fitting of the model to pretreatment scale-up incidence, whereas the modeled prevention effects of HCV treatment conflicted with the uncertain, but increasing, empirical incidence data observed in that prison.
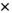 ” indicates that none of the counterfactual model projections for overall HCV incidence in the treatment scale-up phase lay within 95% CI of the empirical estimate.
” indicates that none of the counterfactual model projections for overall HCV incidence in the treatment scale-up phase lay within 95% CI of the empirical estimate.In comparison to the model including treatment, when all HCV treatments were removed (SToP-C and JH&FMHN), this counterfactual model predicted much smaller decreases in HCV incidence (Figs. 2 and 3). None of the counterfactual model projections for the overall decrease in incidence lay within 95% CI of the empirical data, whereas only 42.3% of the projections for each prison lay within 95% CI of the empirical data for those prisons (Fig. 4). Median modeled HCV incidence estimates for each prison then deviated from empirical estimates by one third to 2.5 times, except in OMMPC, which was the only prison where the counterfactual model fitted the data better (Fig. 3).
Impact of HCV Treatment
The scale-up in HCV treatment decreased incidence by 41.4% (95% UI, 34.0-47.8) or 85.1% (95% UI, 72.6-100.6) of the overall decrease in incidence; 43.0% (95% UI, 37.8-49.8) attributable to treatments from SToP-C and 41.9% (95% UI, 32.1-54.9) attributable to JH&FMHN treatments (Fig. 5 and Supporting Fig. S8). This comparable impact reflects the similar numbers of people treated by SToP-C (n = 324) and JH&FMHN (n = 301) over the study. In contrast, other effects, mainly the observed decline in HCV prevalence among prison entrants, resulted in a 7.2% (95% UI, −0.3 to 13.6) decrease in incidence or 14.9% (95% UI, −0.6 to 27.4) of the overall decrease in HCV incidence.

Impact varied across the four SToP-C prisons (Fig. 5), with the overall decrease in incidence in OMMPC and DIL being ~50% greater than in GLB and LGW. This is attributable to declining HCV prevalence and other effects adding to the decrease in incidence achieved by HCV treatment in OMMPC and DIL, but less so for GLB and LGW. The impact of HCV treatment varied less (Fig. 5), from a 31.0% (95% UI, 22.2-40.1) decrease in incidence attributable to treatment in GLB, to a 41.3% (95% UI, 34.2-51.4) decrease in OMMPC (conflicting with the observed but uncertain increase in incidence), and 41.9% (95% UI, 34.3-48.9) decrease in LGW. The differential impact of treatment in each prison was not associated with the overall proportion of infections treated, but rather on the proportion treated following treatment scale-up (Supporting Fig. S10).
Sensitivity Analysis
Our sensitivity analyses showed that including observed reductions in OAT coverage had minimal effect on the modeled reduction in HCV incidence achieved in the trial (0.2% change from baseline). The modeled HCV incidence reduction was also robust to different assumptions about the prevalence of IDU and HCV among prisoners with unknown status (e.g., those never enrolled in SToP-C), with the overall reduction in HCV incidence varying by <5% from the baseline scenario (Supporting Fig. S11).
Discussion
In this analysis, we used dynamic HCV transmission modeling to show that the decrease in HCV incidence observed during the SToP-C intervention study was largely attributable to the treatment-as-prevention effect of the scale-up of HCV treatment occurring in the four study prisons. Including achieved levels of HCV treatment, the model replicated the near halving of HCV incidence observed in the study,(15) with the model suggesting that 85.1% of the observed reduction was attributable to HCV treatment and 14.9% was attributable to other effects, including temporal declines in HCV prevalence among prison entrants (likely attributable to the concurrent scale-up in treatment in the community in NSW). Our findings show that, despite limited provision of prevention interventions, high levels of HCV treatment can yield substantial benefits to halting HCV transmission, providing strong evidence to support the expansion of HCV treatment in any HCV prevention strategy.
Importantly, our results are based on data from four NSW prisons that exhibit diverse characteristics, with two being maximum-security male prisons (for more-severe crimes) and two being medium-security prisons (less-severe crimes), one for males and one for females. The prisons show variability in the distribution of prisoners’ average lengths of stay, with 3 times as many prisoners with shorter lengths of stay (≤1 month) in GLB (46.4%) than in LGW (15.9%). Through undertaking the trial and this modeling in a diverse range of prisons, we are able to show that HCV treatment-as-prevention is likely to have an impact on reducing HCV incidence in prison settings with long and short lengths of stay as well as among male and female prisoners, and so our main findings are likely generalizable to other prison settings in Australia and elsewhere. Although other countries and their prisons may have different characteristics, which could affect the impact of HCV treatment-as-prevention, this study shows that if treatment is possible within the prison setting, then prevention impacts should be achieved.
Strengths and limitations
This modeling study evaluated the prevention impact of HCV treatment in a real-world setting. In such scenarios where no control group is present, modeling is an important tool for helping to determine the impact that an intervention has had, in comparison to other effects that may have reduced incidence. In this study, these effects included decreases in HCV prevalence over time among prison entrants and changes in the coverage of OAT. No other changes were modeled, as suggested by empirical data. We showed that, despite only being calibrated to HCV incidence during the pretreatment scale-up phase, our model replicated the empirically measured reduction in HCV incidence during the treatment scale-up phase, providing a real-world validation of the modeled hypothesis that HCV treatment can have a large prevention benefit. This enabled us to quantify the direct contribution of HCV treatment-as-prevention to an observed decrease in HCV incidence. Our modeling analyses were further strengthened through extensive incorporation of real-world individual-level data on the complex dynamics of incarceration, patterns of injecting drug use within prisons, and ongoing DAA treatment provision within the prison setting.
However, there were limitations. First, although our model could accurately replicate the observed declines in HCV incidence in three SToP-C prisons and overall, it had difficulty in capturing the changes in OMMPC. This was mainly attributable to the considerable uncertainty in the data from OMMPC, with only seven incident infections being recorded over the study period. Indeed, although empirical data suggested an increase in HCV incidence in OMMPC following the scale-up in HCV treatment, this was only based on six incident infections during this period.
Second, we did not have data on the IDU risk behavior or HCV prevalence of prison entrants who were not enrolled in SToP-C. We therefore assumed that nonenrolled prisoners had the same characteristics as enrolled prisoners, with our sensitivity analyses suggesting that varying this assumption does not affect our findings. Additionally, IDU was probably under-reported in the SToP-C study, as shown by the high incidence among non-PWID in DIL; we did not associate uncertainty to this parameter because we needed to match what was done in the trial analysis.
Third, we did not incorporate the impact of OAT in our baseline model, which is available in NSW prisons but has uncertain efficacy.(4, 22) Access to OAT decreased over time among PWID in the SToP-C prisons from 42.4% in 2014-2015 to 14.5% in 2019. Even if we assumed the same efficacy (~50%) as for community OAT,(15) our sensitivity analyses suggest that the resulting increase in HCV transmission risk over time would not have affected our findings. We also did not investigate the impact of scaling up other interventions, such as needle and syringe programs (NSPs), because they were not part of the trial, which we were trying to evaluate. Other theoretical modeling work done by us among PWID in this prison system suggests that combining NSPs and OAT with antiviral therapy can improve the prevention impact achieved.(23)
Comparison with other studies
Our analysis builds on previous modeling studies that evaluate the potential impact of prison-based interventions on HCV transmission, and is consistent with model findings that suggest a prevention benefit of HCV treatment in prison settings(23-25) and the community.(26, 27) However, none of these earlier HCV treatment modeling studies were linked to a real-world HCV treatment-as-prevention study and so were not validated against empirical data. Our modeling makes an important step in using real-world data from the SToP-C trial to evaluate the true impact of an actual HCV treatment-as-prevention intervention in a prison setting. Our study shows how modeling can be used to disentangle the contributions of HCV treatment from other effects to estimate the impact of HCV treatment-as-prevention. Similar modeling methods have been used previously for improving the evidence for an intervention having impact (or not) on HIV or HCV transmission in settings where other factors play a role,(12, 28-31) but never for confirming the prevention benefits of HCV treatment.
Our model-based evaluation demonstrates that HCV treatment in prisons can have substantial prevention benefits (i.e., treatment-as-prevention) which, because of the dynamic nature of incarceration, could also reduce transmission in the community.(25, 32) This is particularly important given the overconcentration of PWID in prisons globally(2) and the substantial contribution of IDU to HCV transmission.(33) However, it is important to consider that people in prison may be reticent to come forward for HCV treatment when primary prevention services are absent or limited (e.g., OAT and NSPs),(34-36) which therefore gives impetus to improving these services for scaling up treatment.
Previous studies have shown that, despite being effective,(21) primary prevention interventions are important but insufficient to reduce HCV incidence to low levels.(37) The hypothesis that HCV treatment scale-up in PWID and other high-risk groups could have a prevention benefit has led to the World Health Organization developing targets to eliminate HCV as a major public health problem by 2030(38)—with treatment being a key component. Although mathematical modeling has underpinned the development of this hypothesis and targets, our study provides the best evidence that HCV treatment-as-prevention is feasible and worthwhile, adding further optimism that HCV elimination can be achieved. However, few countries are on track to meet the HCV elimination targets, with further treatment and prevention intervention scale-up being urgently needed. Given that empirical evidence of the preventative benefits of HIV treatment has led to a global scale-up of ART, our findings should encourage further scale-up of HCV treatment in the prison setting and community.
Acknowledgment
The SToP-C study was funded by an Australian National Health and Medical Research Council (NHMRC) Partnership Project Grant (1092547), including support from Gilead Sciences. A.G.L., J.S., and P.V. acknowledge support from the NIHR Health Protection Research Unit in Behavioural Science and Evaluation at the University of Bristol. P.V. also acknowledges support from the NIHR-funded EPIToPe project and the NIHR HTA project (NIHR128513). J.G. is supported by an NHMRC Investigator Grant (1176131). N.K.M. is supported by NIAID and NIDA (R01AI147490) and the University of San Diego Center for AIDS Research (CFAR), an NIH-funded program (P30 AI036214). J.G., G.J.D., and A.R.L. were supported by Fellowships from NHMRC (Nos. 1176131, 1118864, and 1137587). This work was carried out using the computational facilities of the Advanced Computing Research Centre, University of Bristol (http://www.bristol.ac.uk/acrc/). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. The SToP-C study is a partnership project involving the Kirby Institute, Justice Health & Forensic Mental Health Network, Corrective Services NSW, NSW Ministry of Health, NSW Users and AIDS Association, Hepatitis NSW, and Gilead Sciences. The SToP-C study group includes members of the Protocol Steering Committee; Coordinating Centre, The Kirby Institute, UNSW Sydney; and Study Site Coordinators. The views expressed in this publication are those of the authors and do not necessarily represent the position of the Australian Government or Gilead Sciences. We also acknowledge the contributions of members of the SToP-C Study Group: Protocol Steering Committee: Stuart Loveday (Chair, Hepatitis NSW); Gregory Dore, Andrew Lloyd, Jason Grebely, Tony Butler, Georgina Chambers, Carla Treloar, and Marianne Byrne (UNSW Sydney); Roy Donnelly, Colette McGrath, Julia Bowman, Lee Trevethan, and Katerina Lagios (Justice Health & Forensic Mental Health Network); Luke Grant and Terry Murrell (Corrective Services NSW); Nicky Bath, Victor Tawil, Annabelle Stevens, and Libby Topp (NSW Health); Alison Churchill and Kate Pinnock (Community Restorative Centre); Natasha Martin (University of California San Diego); Steven Drew (Hepatitis NSW); and Mary Harrod (NSW Users and AIDS Association). Coordinating Centre (The Kirby Institute, UNSW Sydney): Gregory Dore, Andrew Lloyd, Behzad Hajarizadeh, Tony Butler, Pip Marks, Mahshid Tamaddoni, Stephanie Obeid, Gerard Estivill Mercade, Maria Martinez, and Marianne Byrne. Laboratory Services (NSW Health Pathology): William Rawlinson, Malinna Yeang, Matthew Wynn, and Christiana Willenborg. Site Research Coordinators: Angela Smith, Ronella Williams, Brigid Cooper, Kelly Somes, Carina Burns, Camilla Lobo, Karen Conroy, Luke McCredie, Carolyn Café, and Jodie Anlezark.
Author Contributions
P.V. and N.K.M. conceived the modeling study with G.J.D., A.R.L., and J.G. A.G.L., J.S., and P.V. designed the study. A.G.L. developed the model and performed all model analyses. A.G.L., J.S., and B.H. undertook additional data analyses for parameterizing and calibrating the model. P.V. supervised the modeling and data analyses, with support from J.S. A.G.L. wrote the initial draft of the manuscript. All authors contributed to guiding the overall analysis plan, interpreting interim and final results, and critically reviewing the final version of the manuscript.




