Analysis and Validation of Human Targets and Treatments Using a Hepatocellular Carcinoma–Immune Humanized Mouse Model
Abstract
Background and Aims
Recent development of multiple treatments for human hepatocellular carcinoma (HCC) has allowed for the selection of combination therapy to enhance the effectiveness of monotherapy. Optimal selection of therapies is based on both HCC and its microenvironment. Therefore, it is critical to develop and validate preclinical animal models for testing clinical therapeutic solutions.
Approach and Results
We established cell line–based or patient-derived xenograft–based humanized-immune-system mouse models with subcutaneous and orthotopic HCC. Mice were injected with human-specific antibodies (Abs) to deplete human immune cells. We analyzed the transcription profiles of HCC cells and human immune cells by using real-time PCR and RNA sequencing. The protein level of HCC tumor cells/tissues or human immune cells was determined by using flow cytometry, western blotting, and immunohistochemistry. The HCC tumor size was measured after single, dual-combination, and triple-combination treatment using N-(1ʹ,2-Dihydroxy-1,2ʹ-binaphthalen-4ʹ-yl)-4-methoxybenzenesulfonamide (C188-9), bevacizumab, and pembrolizumab. In this study, human immune cells in the tumor microenvironment were strongly selected and modulated by HCC, which promoted the activation of the IL-6/Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway in tumor cells and led to augmented HCC proliferation and angiogenesis by releasing angiogenic cytokines in humanized-immune-system mice with HCC. In particular, intratumor human cluster of differentiation–positive (hCD14+) cells could produce IL-33 through damage-associated molecular pattern/Toll-like receptor 4/activator protein 1, which up-regulated IL-6 in other intratumor immune cells and activated the JAK2/STAT3 pathway in HCC. Specific knockdown of the CD14 gene in human monocytes could impair IL-33 production induced by cell lysates. Subsequently, we evaluated the in vivo anti-HCC effect of C188-9, bevacizumab, and pembrolizumab. The results showed that the anti-HCC effect of triple-combination therapy was superior to that of single or dual treatments.
Conclusions
Humanized-immune-system HCC mouse models are suitable for identifying targets from cancer and immune components and for testing combinational therapies.
Abbreviations
-
- Ab
-
- antibody
-
- AKT
-
- protein kinase B
-
- AP-1
-
- activator protein 1
-
- BM
-
- bone marrow
-
- CCL
-
- C-C motif chemokine ligand
-
- CXCL
-
- C-X-C motif chemokine ligand
-
- DAMP
-
- damage-associated molecular pattern
-
- DE
-
- differentially expressed
-
- EDU
-
- 5-ethynyl-2′-deoxyuridine
-
- HCC
-
- hepatocellular carcinoma
-
- hCD
-
- human cluster of differentiation
-
- HLA
-
- human leukocyte antigen
-
- HMGB1
-
- high mobility group box 1
-
- HSC
-
- hematopoietic stem cell
-
- IFN-gamma
-
- interferon-gamma
-
- ILC
-
- innate lymphoid cell
-
- JAK2
-
- Janus kinase 2
-
- LPS
-
- lipopolysaccharide
-
- MAPK
-
- mitogen-activated protein kinase
-
- MDSC
-
- myeloid-derived suppressor cell
-
- M-MDSC
-
- mononuclear MDSC
-
- MMP
-
- matrix metalloproteinase
-
- MØ1
-
- type 1 macrophage
-
- MØ2
-
- type 2 macrophage
-
- MVD
-
- microvessel density
-
- NK
-
- natural killer
-
- NSG
-
- NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ
-
- p-
-
- phospho-
-
- PCA
-
- principal component analysis
-
- PD-1
-
- programmed death 1
-
- PDGF
-
- platelet-derived growth factor
-
- PD-L1
-
- programmed death ligand 1
-
- PDX
-
- patient-derived xenograft
-
- shRNA
-
- short-hairpin RNA
-
- STAT
-
- signal transducer and activator of transcription
-
- TAM
-
- tumor-associated macrophage
-
- Tc
-
- T cytotoxic
-
- TcCM
-
- central memory Tc cell
-
- TcEM
-
- effector memory Tc cell
-
- Th
-
- T-helper
-
- ThCM
-
- central memory Th cell
-
- ThEM
-
- effector memory Th cell
-
- TIL
-
- tumor-infiltrating leukocyte
-
- TLR
-
- Toll-like receptor
-
- VCSA
-
- microvessel cross-sectional area
-
- VD3
-
- 1,25-dihydroxyvitamin D3
Hepatocellular carcinoma (HCC) remains one of the most common causes of cancer-related deaths and has a high recurrence rate.(1) HCC tumorigenesis is impacted by chronic inflammation induced by liver injury,(2-4) which promotes HCC progression and development.(5-8) However, the mechanisms that underlie the crosstalk between human HCC and the human immune system remain poorly understood. Therefore, examination of the interactions between the human immune system and HCC, which may improve existing therapy and help to identify strategies for HCC treatment, is warranted.
HCC cells attract various immune cells into the tumor microenvironment by releasing multiple chemokines, including C-X-C motif chemokine ligand 12 (CXCL12), C-C motif chemokine ligand 1 (CCL1), CCL22, CXCL16, CCL5, CXCL10, and CXCL11.(9-13) Furthermore, HCC cells can modulate the activities and functions of immune cells and drive the acquisition of tumor-associated phenotypes in these cells.(14-17) Tumor-associated immune cells, including T-helper (Th) type 2 (Th2) cells, type 2 innate lymphoid cells (ILC2s), tumor-associated regulatory T cells, tumor-associated macrophages (TAMs), tumor-associated neutrophils, and myeloid-derived suppressor cells (MDSCs) are responsible for producing various cytokines (IL-1, IL-6, IL-8, and IL-10), growth factors (VEGF, platelet-derived growth factor [PDGF], EGF, HGF, FGF, and insulin-like growth factor), matrix metalloproteinase 2 (MMP-2) and MMP-9, and tissue inhibitor of metalloproteinase 1 (TIMP-1) and TIMP-2 to create an optimal environment for the proliferation, survival, and metastasis of tumor cells.(18-21) Previous studies have demonstrated that numerous signaling pathways dysregulated in HCC were associated with these HCC tumor–associated immune cells.(22-24) For example, IL-8 released from TAMs may enhance the epithelial–mesenchymal transition (EMT) in HCC through the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3)/Snail pathway.(22) Similarly, activation of the JAK2/STAT3 pathway, induced by IL-6 from TAMs, also played a critical role in HCC stem-cell expansion.(23) In addition, increased functional activity in the rat sarcoma/rapidly accelerated fibrosarcoma/mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin and JAK/STAT pathways, caused by multiple growth factors released from MDSCs, were also observed in human HCC.(24) Although this evidence demonstrated the interactions between the human immune system and HCC, countless other mechanisms remain unclear, particularly because of the lack of a robust in vivo human cancer–immune system model. The absence of sufficient models also limits the development of therapeutics targeting tumor-associated immune cells and their modulation pathways.
Over time, various monotherapies that target HCC proliferation, angiogenesis, and immune resistance have been developed.(25, 26) These include, for example, treatments counteracting elevated IL-6 signaling. Serum IL-6 levels are elevated in patients with HCC, compared with healthy individuals, which might enhance HCC proliferation and angiogenesis through activation of the JAK2/STAT3 signaling pathway.(27, 28) Hence, tocilizumab (IL-6 receptor blockade) and N-(1ʹ,2-Dihydroxy-1,2ʹ-binaphthalen-4ʹ-yl)-4-methoxybenzenesulfonamide (C188-9) (STAT3 inhibitor) were developed and achieved optimistic results in reducing HCC growth in vivo by impairing IL-6 signaling from TAMs to HCC.(6, 29)
VEGF is essential for tumor growth and angiogenesis. Bevacizumab, an anti-VEGF monoclonal antibody (Ab), has been applied to patients with HCC and has proven to be effective in clinical trials.(30, 31) Similarly, immune checkpoint blockade therapies, such as nivolumab and pembrolizumab (programmed death 1 [PD-1] blockade) and atezolizumab (programmed death ligand 1 [PD-L1] blockade) also exerted certain levels of anti-HCC effects by protecting and recovering T-cell function in animal models and patients.(32) Despite the development of different monotherapies, patients with HCC only exhibit partial responses to these treatments.(33) In recent years, combination therapy (polytherapy) has been considered for synergistic efficacy, which may improve the overall survival of patients with HCC by reducing their resistance to monotherapy.(33) Previous studies have demonstrated that the combination of anti–IL-6 and anti–PD-L1 treatment could ameliorate the anti-HCC efficacy in vivo.(34) In clinical settings, a combination of bevacizumab and atezolizumab treatment led to effective antitumor immune response in patients with HCC.(35) Furthermore, combined blockade of IL-6 and VEGF receptor also enhanced antitumor activity, as was reported in another study.(36) Therefore, it is anticipated that the combined therapy of anti–IL-6–or IL-6–related signaling pathways, anti-VEGF, and immune checkpoint blockade may be more efficacious.
We have shown that an HCC mouse model can be generated through the engraftment of human HCC cell lines or patient-derived xenografts (PDXs) into immunodeficient mice bearing human leukocyte antigen (HLA)-matched human immune systems (humanized mice).(37) Intriguingly, some of the HCC-PDX tumors regressed in the humanized mice following pembrolizumab and ipilimumab treatment, suggesting that the HCC humanized mice may represent an emerging platform for the evaluation of cancer monotherapy or combination therapy.(37) In the present study, we demonstrate that the human immune system in humanized mice may enhance HCC proliferation and angiogenesis through a network involving damage-associated molecular patterns (DAMPs), Toll-like receptors (TLRs), cytokines, and intracellular protein phosphorylation. More importantly, triple-combination therapy targeting STAT3-induced HCC proliferation, VEGF-related angiogenesis, and the PD-1 immune checkpoint resulted in a combinational anti-HCC effect in our humanized mouse model.
Materials and Methods
MICE
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (The Jackson Laboratory) and humanized mice were bred and generated, respectively, as described earlier.(37, 38) All manipulations with mice were approved by Institutional Animal Care and Use Committee in Agency for Science, Technology and Research. All animal experimental procedures were conducted in accordance to the approved protocols and Guide for the Care and Use of Laboratory Animals. All human fetal liver and HCC Patient tumor tissues with written consent were obtained from guardians of donors, and in accordance with the ethical guidelines of Kandang Kerbau (KK) Women’s and Children’s Hospital (Singapore) and National University Hospital (Singapore). HCC humanized mice were generated by engrafting different HCC cell lines or PDXs subcutaneously or orthotopically (intrasplenic injection with 2 × 106 purified and live human tumor cells in 50 µL of saline) according to HLA-A (HCC-PDX tumor 1# [A*02 and A*11] matched with hematopoietic stem cell [HSC] 1# [A*02 and A*11]; HCC-PDX tumor 2# [A*02 and A*11] matched with HSC 1# [A*02 and A*11]; HCC-PDX tumor 3# [A*02 and A*01] matched with HSC 2# [A*02 and A*01]; HCC-PDX tumor 4# [A*02 and A*11] matched with HSC 1# [A*02 and A*11]; and HepG2 and LM3 cell lines [A*02 and A*24] matched with HSC 3# [A*02 and A*24]) (Supporting Table S1). To deplete specific immune-cell subsets in immune humanized mice, anti–human cluster of differentiation 45 (anti-hCD45) (a human leukocyte marker), anti-hCD4 (a human Th cell marker), anti-hCD8 (a human Tc cell maker), anti-hCD14 (a human monocyte marker), anti-hCD19 (a human B-cell marker), and anti-hCD56 (a human natural killer [NK] cell marker) (50 µg/mouse) were given i.v. weekly. For the evaluation of drug efficacy, small molecules or Abs were injected into the mice either alone or in combination. Briefly, C188-9 dissolved in DMSO was injected intraperitoneally (75 mg/kg) on a daily basis, whereas anti–human IL-33 Ab (50 µg/mouse), bevacizumab, and pembrolizumab (5 mg/kg) were injected i.v. on a weekly basis. Further methodological details can be found in the Supporting Information.
Results
The Human Immune System Promotes HCC Proliferation and Angiogenesis
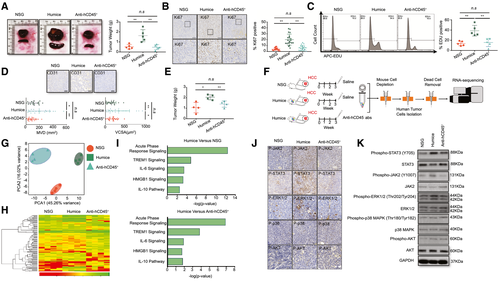
To explore the role of the humanized immune system with respect to HCC, we engrafted HCC-PDX tumor tissue into NSG humanized mice and hCD45-depleted humanized mice that were treated with anti-hCD45 Ab to remove the humanized immune system 2 weeks after engraftment. Interestingly, the size and weight of the HCC tumors and spleens from humanized mice increased compared with those from the NSG and hCD45-depleted humanized mice (Fig. 1A). Similar results were also observed in HCC-PDX tumors from different donors, in both subcutaneous and orthotopic models (Supporting Figs. S1A-S6A). The expression of MKI67 (Ki67) on the HCC-PDX tumor was quantified by using immunohistochemical analysis, which indicated a significant increase in the number of Ki67+ cells in the HCC tumor from humanized mice (Fig. 1B and Supporting Figs. S1B-S6B). Moreover, the 5-ethynyl-2′-deoxyuridine (EDU) levels in isolated and purified HCC-PDX tumor cells from the three groups were analyzed using flow cytometry. Similarly, we observed that the HCC-PDX tumor cells isolated from humanized mice had the highest EDU level among these groups (Fig. 1C). Furthermore, angiogenesis was quantified in the vascular endothelium of the HCC-PDX tumor by counting the vessels with a lumen (CD31 immunohistochemical staining that was both negative and positive) (Fig. 1D and Supporting Figs. S1C-S6C). Both the microvessel density (MVD) and the microvessel cross-sectional area (VCSA) were calculated (Fig. 1D and Supporting Figs. S1D-S6D). More vascular invasion was observed in HCC tumors in humanized mice than in both NSG and hCD45-depleted humanized mice, suggesting that the humanized immune system could augment HCC tumor proliferation and angiogenesis. To dissect the underlying molecular mechanisms that regulated the proliferation and angiogenesis affected by the humanized immune system, the LM3 (HCC) cell line was selected because of its sharing the HLA-A type with the HCC-PDX tumor (all are HLA-A type 2). In concordance with the results from HCC-PDX tumors, LM3 tumors in humanized mice were larger than those in NSG and humanized mice treated with anti-hCD45 Abs (Fig. 1E). After 3 weeks of engraftment, the LM3 tumors were harvested, and the purified LM3 HCC cells were subjected to RNA sequencing (Fig. 1F). Principal component analysis (PCA) of all expressed genes (Fig. 1G) and heatmap analysis of differentially expressed (DE) genes (Fig. 1H and Supporting Table S2) revealed that the transcriptomic profiles among these groups were distinct and well separated, indicating that LM3 cells were strongly influenced by the immune system. Results from the hCD45-depleted group indicated that the immune system had already exerted some impact on the tumors even though it had been removed 2 weeks after tumor implantation. Ingenuity pathway analysis revealed that the acute-phase-response signaling pathways were highly modulated in LM3 cells from humanized mice, including triggering receptor expressed on myeloid cells 1, IL-6, high mobility group box 1 (HMGB1), and IL-10 signaling pathways, compared with NSG and hCD45-depleted humanized mice (Fig. 1I). Immunohistochemistry staining of HCC-PDX tumors and western blot analysis of isolated HCC-PDX tumor cells indicated that the JAK2/STAT3 of the tumor was highly phosphorylated in humanized mice (Fig. 1J,K). Hence, we anticipated that the humanized immune system in our model may enhance HCC-PDX proliferation through the activation of the JAK2/STAT3 intracellular signaling pathways in the HCC cells.
Intratumor hCD14 Cells Contribute to Promoting HCC Proliferation and Angiogenesis
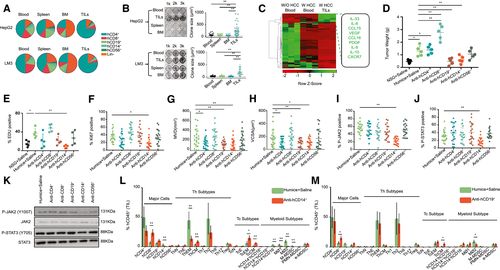
To validate the functions of human immune cells from different organs on HCC in vitro, hCD45+ cells were isolated from blood, spleen, bone marrow (BM), and tumor-infiltrating leukocytes (TILs) of humanized mice bearing HepG2 cells and LM3 cells (with HLA-A2, similar to HCC-PDX tumors 1#-4#), and the average composition of immune-cell subsets was analyzed (Fig. 2A). A colony formation assay was performed by two-dimensional co-culturing of hCD45+ cells and HepG2 cells or LM3 cells for 10 days. The results showed that only TILs could strongly stimulate HepG2 cell and LM3 cells proliferation when they were compared with immune cells from other origins (Fig. 2B). Subsequently, hCD45+ cells were isolated from blood and TILs from humanized mice bearing HCC-PDX tumor 1#. The mRNA expression levels of various chemokines, cytokines, and growth factors were analyzed by RT–quantitative real-time PCR; IL-33, IL-6, CCL15, VEGF, CCL16, PDGF, IL-8, IL-10, and CXCR7 were highly expressed in hCD45+ TILs (Fig. 2C and Supporting Table S3). To further identify the subtypes of hCD45+ TILs that influenced HCC proliferation and angiogenesis, different immune-cell subsets were depleted in vivo using humanized mice bearing HCC-PDX tumor 1# (Supporting Fig. S7). The weight of each HCC-PDX tumor 1# was measured 4 weeks after treatment, and the results revealed that hCD14+ or CD19+ cell depletion could reduce HCC tumor weight. In contrast, hCD8+ cell depletion increased tumor weight (Fig. 2D). Immunohistochemical staining of Ki67 and EDU flow cytometry assays showed a decrease in HCC proliferation after hCD14+ cell depletion (Fig. 2E,F and Supporting Fig. S8). Moreover, angiogenesis in the HCC tumor, indicated by the MVD and VCSA, was impaired by hCD14+ or hCD19+ cell depletion (Fig. 2G,H and Supporting Fig. S8). The presence and protein expression levels of phospho-JAK2 (p-JAK2) and p-STAT3 in the HCC tumor and the purified HCC cells were examined by using immunohistochemistry (Fig. 2I,J and Supporting Fig. S8) and western blotting (Fig. 2K), respectively. Our results showed that only hCD14+ cell depletion could significantly diminish the expression of JAK2 and STAT3 in the tumor. In addition, hCD14+ or CD19+ cell depletion also altered the composition of human immune-cell subsets of TILs (Fig. 2L,M). After the depletion of hCD14+ cells, the proportion of total CD8+ cells, central memory Th cells (ThCMs), effector memory cytotoxic T (Tc) cells (TcEMs), and central memory Tc cells (TcCMs) increased, whereas the proportion of effector memory Th cells (ThEMs), type 1 macrophages (MØ1s) or type 2 macrophages (MØ2s), and mononuclear MDSCs (M-MDSCs) decreased (Fig. 2L). The total number of CD8+ cells, CD19+ cells, ThCMs, TcEMs, and TcCMs increased per gram of tumor tissue, whereas effector memory Th cells (ThEMs), MØ1s or MØ2s, and M-MDSCs decreased in hCD14+ cell–depleted tumor tissues (Supporting Fig. S9A). Similarly, a depletion in hCD19+ cells also led to an increase in the percentage of total CD8+ cells and TcCMs and a decrease in the proportion of MØ2s (Fig. 2M). In terms of the cell number per gram of tumor tissue, total CD8+ cells, ThCMs, TcEMs, TcCMs, hCD14+hCD16− cells, and early-stage MDSCs increased after hCD19+-cell depletion (Supporting Fig. S9B). Taken together, the intratumor hCD14+ cells could stimulate HCC tumor proliferation and angiogenesis by modulating the profile of the immune cells in the tumor and plausibly through the activation of the IL6/JAK2/STAT3 signaling pathway.
Intratumor Human Immune Cells are Strongly Modulated by HCC Tumors
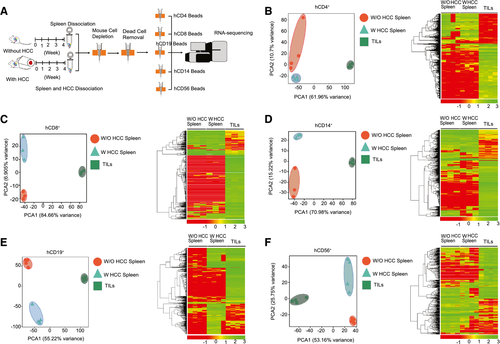
To characterize the phenotypes and functions of the humanized immune system in the absence or presence of an HCC tumor burden, we isolated multiple immune-cell subsets, including CD4+, CD8+, CD14+, CD19+, and CD56+ cells, from the spleen and from among the TILs of humanized mice bearing an HCC-PDX tumor and analyzed their RNA profiles (Fig. 3A). Through PCA and heatmap analysis, it was found that the profiles of CD4+ (Fig. 3B), CD8+ (Fig. 3C), CD14+ (Fig. 3D), CD19+ (Fig. 3E), and CD56+ (Fig. 3F) cells among the groups were distinct and well separated. The list of DE genes was detected and calculated for CD4+, CD8+, CD19+, CD14+, and CD56+ cells (Supporting Table S2). Despite minor differences between splenic immune cells from humanized mice with and without HCC, the intratumor human immune cells were significantly different, including in terms of the expression of surface markers, chemokines, cytokines, and functional proteins. For example, cell migration (CCL13 and CCL18), angiogenesis and invasion (CCL2, CCL7, CCL9, CCL10, IL-8, IL-10, PDGFA, VEGFA, EGF, MMP7, MMP9, and MMP19), and MØ2 type markers (CD163 and peroxisome proliferator–activated receptor gamma genes) were found to be up-regulated in hCD14+ TILs, whereas MØ1 type maker (CD86) and phagocytosis markers (neutrophil cytosol factor 1 [NCF-1], phosphatidylinositol 4-phosphate 5-kinase type-1 gamma [PIP5K1C], vasodilator-stimulated phosphoprotein [VASP], hematopoietic cell kinase [HCK], phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit gamma [PIK3CG], spleen tyrosine kinase [SYK], actin related protein 2/3 complex subunit 4 [ARPC4], AKT1, WASP family member 2 [WASF2], vav guanine nucleotide exchange factor 1 [VAV1], actin related protein 2/3 complex subunit 1B [ARPC1B], P21 (RAC1) activated kinase 1 [PAK1], cofilin 1 [CFL1] and AKT serine/threonine kinase 2 [AKT2]) were down-regulated. These results suggested that HCC tumors could alter human immune cells in humanized mice, particularly on intratumor human immune cells.
IL-33 Secreted From Intratumor hCD14+ Cells Up-Regulates IL-6 Expression in Multiple Intratumor Immune Cells and Enhances HCC Proliferation
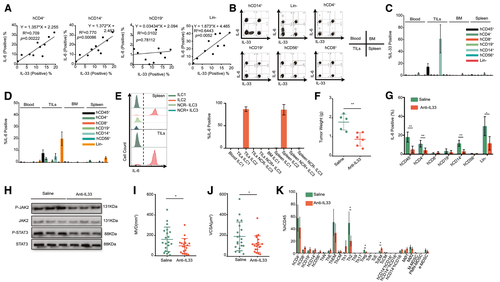
Previous studies have shown that serum IL-33 levels are higher in patients with HCC than in healthy donors.(39) Consistent with these clinical findings, our results also verified the elevation of IL-6 and IL-33 expression in intratumor hCD45+ cells (Fig. 2C). In our HCC-PDX humanized mouse model, IL-33+ cells were only present in intratumor hCD14+ cells, and these HCC cells did not express IL-33 (data not shown), whereas IL-6+ cells were detected among the CD4+, CD14+, and lineage (Lin)− TILs (Fig. 4A-C). Therefore, the correlation between the IL-6 level in the captioned immune cells and the IL-33 level in intratumor hCD14+ cells was analyzed in HCC samples from 10 patients. The results showed that the IL-33 level in intratumor hCD14+ cells was closely correlated with the IL-6 level in intratumor hCD4+ and hCD14+ cells but was weakly correlated with the intratumor hCD19+ cells and Lin− cells (Fig. 4A). Interestingly, ILC groups from Lin− cells were found in our model, particularly in BM and among TILs (Supporting Fig. S10A). The major ILC subtype in the BM and among TILs were ILC1 and ILC2, respectively (Supporting Fig. S10B). Meanwhile, the majority of the ILC2s could express IL-6, whereas other ILC subsets, such as ILC1, NCR+ILC3, and NCR−ILC3, did not express IL-6 (Fig. 4E). Interleukin 1 receptor-like 1 (ST2) is a member of the IL-1 receptor family, which is the receptor of IL-33. We found that ST2 was highly expressed in intratumor CD4+, CD14+, and Lin− cells (Supporting Fig. S11A). Furthermore, the proportion of ST2+ IL-6+ cells was much higher than that of ST2− IL-6+ cells in CD4+, CD14+, and Lin− cells (Supporting Fig. S11B), suggesting that IL-33 could modulate IL-6 expression through the ST2 receptor. To confirm the regulatory effect of IL-33 on HCC in vivo, anti–human IL-33 Ab was injected into HCC-PDX humanized mice to neutralize IL-33. After 4 weeks of treatment, the tumor weight for these mice was drastically reduced compared with that of their saline-injected counterparts (Fig. 4F). Meanwhile, human IL-33 neutralization led to a decrease in the IL-6 level in intratumor CD4+, CD14+, and Lin− cells (Fig. 4G) and led to lower activation of JAK2 and STAT3 in HCC cells (Fig. 4H). Furthermore, the MVD and VCSA suggested that IL-33 was also involved in angiogenesis (Fig. 4I,J). After human IL-33 neutralization, the proportions of intratumor Th2 and regulatory T cells decreased, whereas the proportion of TcEMs increased, suggesting that CD14+ monocyte–derived IL-33 may also be involved in regulating other immune-cell types in the tumor environment (Fig. 4K). These results suggested that IL-33 from intratumor hCD14+ cells could up-regulate IL-6 expression in multiple cell types, thereby enhancing HCC proliferation.
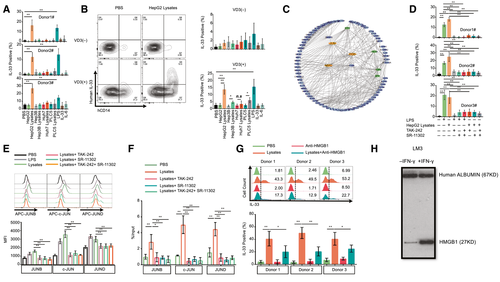
Co-receptor CD14 Is Essential for IL-33 Production Through Damp (HMGB1)/TLR4/Activator Protein 1 Pathway
To clarify the mechanism of IL-33 production from intratumor hCD14+ cells only, we induced IL-33 expression using primary human immune cells and U937 cells in vitro. We attempted to detect IL-33 expression induced by using different reagents, including co-culture with HepG2, Hep3B, Huh7, and PLC5 cell lines and stimulation by lipopolysaccharide (LPS), IL-33, IL-6, HepG2 lysates, Hep3B lysates, Huh7 lysates, and PLC5 lysates. We observed that only HepG2 lysates and LPS could induce high levels of IL-33 in primary human immune cells (Fig. 5A). Meanwhile, we stimulated U937 cells using HepG2 lysates. However, no IL-33 expression caused by the direct addition of HepG2 lysates to U937 cells was observed (Fig. 5B). U937 is a monocytic cell line, and low levels of CD14 and 1,25-dihydroxyvitamin D3 (VD3) may induce CD14 expression in U937; these are results we validated (Supporting Fig. S12). We observed that VD3 could induce CD14 expression and that HepG2 lysates stimulated the induction of IL-33 in VD3-treated U937 cells (Fig. 5B). The flow cytometry plot also indicated that only CD14+ cells expressed IL-33 (Fig. 5B). In VD3-treated U937 cells, HepG2 lysates, Hep3B lysates, PLC5 lysates, and LPS could induce high levels of IL-33. We also performed specific targeted hCD14 gene knockdown in U937 cells using five short-hairpin RNAs (shRNAs) (Supporting Fig. S13). TRC1.5 vector controls and shRNAs expressing lentiviral particles were generated using third-generation lentivirus packaging systems. The results indicated that hCD14 shRNAs could significantly impair CD14 expression induced by VD3 in U937 cells (Supporting Fig. S13B). As a result, the intracellular level of IL-33 induced by VD3 and HepG2 lysates was down-regulated in hCD14-silenced U937 cells (Supporting Fig. S13C). In conclusion, CD14 is an essential molecule for IL-33 production. By analyzing the network of highly expressed genes in intratumor CD14+ cells, we found that activator protein 1 (AP-1) was as a key transcription factor because of its regulation of many of the up-regulated genes. Similar results, including activation of the AP-1 pathway, can also be observed in the RT–quantitative real-time PCR heatmap of CD45+ TILs (Supporting Fig. S14A), U937 cells treated with VD3, HepG2 lysates (Supporting Fig. S14B), and healthy donor CD45+ cells treated with HepG2 lysates (Supporting Fig. S14C). To further validate the role of AP-1 signaling in IL-33 expression, we treated primary hCD45+ cells with LPS, HCC lysates (HepG2), (R)-Ethyl 6-(N-(2-chloro-4 fluorophenyl) sulfamoyl) cyclohex-1-enecarboxylate (TAK-242) (inhibitor of TLR4) and (E,E,Z,E)-3-Methyl-7-(4-methylphenyl)-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4,6,8-nonatetraenoic acid (SR-11302) (inhibitor of AP-1). The results showed that either blockage of TLR4 or inhibition of AP-1 can suppress LPS- or HCC lysate–induced IL-33 expression (Fig. 5D). Additionally, we found that LPS and HCC lysates could increase Jun family (JUNB, c-JUN, and JUND) protein levels in hCD14+ cells. However, TAK-242 and SR-11302 can mitigate the elevation of Jun Proto-Oncogene (JUN)-family proteins (Fig. 5E). We also confirmed that AP-1 can bind multiple motifs in the promoter region of IL-33 in U397 cells (Supporting Fig. S15A,B). HCC lysates can enhance the binding ability of AP-1 (JUN family) to the promoter of IL-33. TAK-242 and SR-11302 can mitigate the effect of HCC lysates (Fig. 5F). Moreover, HMGB1 protein is a type of DAMP that is typically located in the nucleus as a nuclear factor. However, it can also be translocated to the cytoplasm and released into the extracellular matrix, linking inflammation and HCC. HMGB1 may activate several intracellular signaling pathways (NF-κB or AP-1) that regulate monocyte-induced cytokine synthesis by binding TLRs 2, 4, 5, and 9. Earlier studies have also demonstrated that HMGB1 is highly expressed in HCC tissues compared with paratumor and normal tissues and that it also promotes HCC proliferation and angiogenesis. To determine the role of HMGB1 in IL-33 synthesis, we treated primary hCD45+ cells with HCC lysates and anti-HMGB1 neutralizing Abs. The results indicated that anti-HMGB1 neutralizing Abs could attenuate HCC lysate–induced IL-33 levels in CD14+ monocytes (Fig. 5G). Furthermore, increased supernatant HMGB1 levels were observed in interferon-gamma (IFN-gamma)–treated HCC cells (Fig. 5H). Overall, IL-33 could be synthesized by CD14+ cells through the DAMP(HMGB1)/TLR4/AP-1 pathway.
hCD14+ Monocytes Among TILs are Crucial for Combinatorial Immunotherapy on HCC
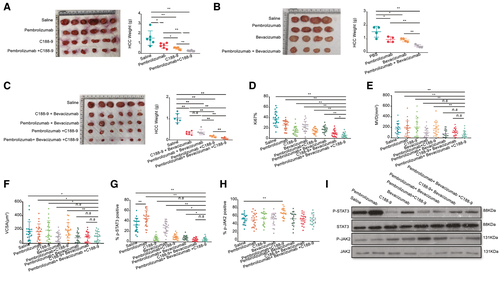
The above data proved that the presence of intratumor CD14+ cells enhances HCC proliferation and angiogenesis by activating the JAK2/STAT3 pathway or releasing angiogenic cytokines (VEGF and so on). HCC-PDX humanized mice were treated with C188-9 (STAT3 inhibitor) or bevacizumab (VEGF inhibitor) alone or in combination with pembrolizumab, and the additive effects were observed following treatment (Fig. 6A,B). Next, the efficacy and safety of triple-combination therapy using C188-9, bevacizumab, and pembrolizumab were evaluated and showed a more significant anti-HCC effect (Fig. 6C). Similar results were consistently observed from subcutaneous (Supporting Figs. S1G, S2G and S3G) and orthotopic mouse models reconstituted with PDXs from different donors (Supporting Figs. S4D, S5D and S6D). The Ki-67 immunohistochemical staining revealed that HCC tumors treated with triple-combination therapy had the lowest level of proliferation (Fig. 6D and Supporting Fig. S15). Besides this, MVD and VCSA results also indicated that triple-combination therapy could reduce angiogenesis (Fig. 6E,F and Supporting Fig. S15). Interestingly, the phosphorylation of STAT3 in HCC tumors was reduced after triple-combination therapy, albeit with an increase in the tumor size following pembrolizumab treatment (Fig 6G,I and Supporting Fig. S16). Meanwhile, the expression of JAK2 was unchanged after triple-combination therapy (Fig. 6H,I and Supporting Fig. S16). To further investigate the role of individual human immune-cell types in this triple-combination therapy, we compared the anti-HCC effect of triple-combination therapy when hCD14 and hCD8 cells were depleted separately in both subcutaneous (HCC-PDX tumor 1#) (Supporting Fig. S17) and orthotopic models (HCC-PDX tumors 1# and 2#) (Supporting Figs. S18 and S19). The results revealed that the anti-HCC effect of triple-combination therapy were greatly hampered without hCD8 or hCD14 cells in HCC humanized mice. It confirmed that these two human immune-cell types were both critical in this combination therapy. Taken together, the results showed that triple-combination therapy using C188-9, bevacizumab, and pembrolizumab could significantly increase the efficacy of the anti-HCC response in vivo compared with monotherapy or dual-therapy.
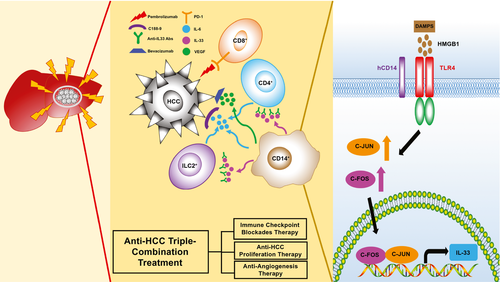
In summary, our humanized-immune-system HCC mouse model provides a useful in vivo platform for testing HCC combinational immunotherapy on the basis of the dissection of molecular features of the human immune microenvironment and human tumors. A general picture of tumor–immune-cell interaction is proposed in our study (Fig. 7). Initially, when the human immune system is exposed to an HCC tumor, it becomes activated and releases proinflammatory cytokines, such as IFN-gamma. The HCC tumor senses the immune responses and generates resistant reactions by releasing DAMPs, such as HMGB1, to immunomodulate CD14+ monocytes. For example, this occurs through the binding of DAMPs to TLR4 and the subsequent synthesis of IL-33 through the TLR4/AP-1 signaling pathway and VEGF, and so on. IL-33 stimulates CD4+ cells, CD14+ cells, and ILC2s to release IL-6, stimulating HCC proliferation through the JAK2/STAT3 pathway and VEGF-related angiogenesis to overcome the killing activity of the immune system. In this context, we demonstrated that a triple therapy comprising C188-9 to inhibit STAT3-related HCC proliferation induced by IL-6, bevacizumab to block VEGF-induced angiogenesis from intratumor monocytes, and pembrolizumab to block PD-1–triggered T-cell exhaustion induced by PD-L1/2 has a combinational effect.
Discussion
The human immune system is instrumental in modulating HCC progression. On the one hand, cytotoxic effector cells eliminate HCC cells, whereas tumor-associated immune cells stimulate the proliferation, angiogenesis, EMT, invasion, and metastasis of the HCC.(40-42) In addition, several inflammatory signaling pathways in HCC, namely JAK/STAT, MAPK, PI3K/AKT, and extracellular signal-regulated kinases1/2 pathways are induced by the immune cells.(22-24, 43, 44) In our humanized-immune-system HCC mouse model, increased HCC proliferation was associated with activation of the JAK/STAT pathway in the tumor cells, and VEGF-induced angiogenesis was also triggered by the human immune system. These results suggest that the human immune system could alter HCC progression by changing the signaling pathways in our humanized-immune-system HCC mouse model, in a way similar to that observed in patients with HCC.
Different human immune-cell subsets, such as T cells, B cells, monocytes, NK cells, and NK T cells, were present in the HCC tumor microenvironment.(45) These human immune cells may promote or inhibit HCC growth, depending on the immune responses elicited. For example, depletion of CD4+ T cells and CD8+ T cells could accelerate HCC progression,(46, 47) which was also seen in our model. By contrast, we also demonstrated that depletion of B cells, monocytes, and macrophages could attenuate the growth and angiogenesis of HCC, consistent with findings in relation to other models.(23, 48) In our study, intratumor hCD14+ cells triggered HCC proliferation through IL-33 and triggered angiogenesis through VEGF. The angiogenesis seen in this model is likely of mouse origin; however, human VEGF is highly conserved in mice and could thus stimulate mouse endothelial cells and be validated in our model.(49) CD14 is a co-receptor of TLR4 and the TLR4/AP-1 pathway, which has been reported to up-regulate IL-33 expression.(50, 51) Consistent with findings from earlier studies, we found that CD14+ cells could up-regulate IL-33 after stimulation with HCC lysates in vitro, and we further proved that intratumor CD14+ cells are the only immune-cell type responsible for IL-33–induced HCC proliferation in vivo. The AP-1 (JUN and FOS [Fos proto-oncogene]) signaling pathway was activated in intratumor CD14+ cells and HCC lysate–treated primary CD14+ cells, in which the expression of IL-33 was reduced after TLR4 and AP-1 inhibition. Another study demonstrated that HMGB1 could induce IL-33 expression by binding with TLR4.(52) Our results indicated that the increased IL-33 expression level induced by HCC lysates was hampered after HMGB1 neutralization. On the basis of these results, we demonstrated that the DAMP (HMGB1)/TLR4/AP-1/IL-33 pathway was critical for HCC survival and proliferation. It also appeared that intratumor hCD14+ cells and their associated pathways could serve as targets for anticancer treatment. Besides CD14+ cells, other immune-cell types in the tumor microenvironment all exhibited significant differential RNA-sequencing profiles compared with their counterparts in peripheral organs, such as blood and spleen, suggesting that HCC tumors have robust selection and learning capabilities regarding immune cells, enabling them to escape immune surveillance, which is an interesting avenue for further study of the individual functions of various immune subsets in tumors. However, HCC intracellular signaling pathways were also significantly impacted by the immune system, as evidenced by the comparison of tumors from mice with and without the human immune system. These changes may contribute to the evolution of the tumor, selection of mutations, and development of drug resistance. Study of the pool of molecular changes in this model, whereby different conditions could be created by manipulating immune components, could facilitate enhanced understanding of the complexity of heterogeneity, mutation burden, and drug resistance and may be further developed into potential therapeutic targets. When combining the discoveries from the angles of the tumor and immune system, our model may subsequently be used to validate the targets and the interfering strategies developed against these targets in single or combined approaches.
In addition to target identification, this model also demonstrated its usefulness in testing existing anticancer and repurposed drugs without putting patients at risk, particularly in combination-therapy settings. A combination therapy that targets HCC and the tumor microenvironment showed a synergistic effect and has been identified as a promising treatment regimen for patients with HCC.(25, 26, 33) Recently, the results of a phase III IMbrave150 trial (a combination of atezolizumab [Tecentriq] and bevacizumab [Avastin]) indicated that the therapy could markedly decrease the risk of progression or death in patients with HCC, compared with treatment with sorafenib (Nexavar).(26, 35) Hence, there is an urgent need to validate existing combination therapies and to explore therapies with the aid of preclinical animal models in HCC-PDX and HCC microenvironments that are similar to those encountered in patients with HCC. However, the risk and cost of combining different drugs that possess various toxicity levels have increased significantly. As such, it is difficult and costly to conduct combinational clinical trials. Our results indicate that monotherapy, dual therapy, and triple therapy could be used to validate the anti-HCC effects and side effects in humanized mice. Monotherapy, such as the depletion of hCD14+ cells, neutralization of human IL-33, and the use of C188-9, bevacizumab, and pembrolizumab, resulted in differential anti-HCC effects. Dual therapy using the combination of C188-9 and pembrolizumab and the combination of bevacizumab and pembrolizumab in humanized mice showed the additive effects compared with the aforementioned monotherapy. Furthermore, triple therapy using pembrolizumab, bevacizumab, and C188-9 was evaluated in our HCC, immune system–humanized mouse model and resulted in an improved combinational effect compared with the monotherapy and dual therapy. This suggested that triple-targeting of HCC proliferation, angiogenesis, and the immune checkpoint together may offer a combination therapy scheme for HCC treatment. In conclusion, our HCC humanized-immune-system mouse model provides a platform for studying the interaction between the HCC tumor and human immune cells and for testing combination therapy that targets the molecular features of HCC and the HCC immune-system microenvironment.
Acknowledgment
We thank the KK Women’s and Children’s Hospital and National University Hospital for provision of human fetal liver/cord blood and HCC tumor samples.
Authors Contributions
Y.Z. designed and performed the experiments, analyzed and interpreted data, and prepared the manuscript. J.W., S.Y.F., S.Y.T., J.Y.C., W.W.S.T., and M.L. performed the experiments and analyzed data; W.N.L., T.W.H.S., S.H., J.K.Y.C., C.E.C., G.H.L., H.C.T., S.G.L., and Y.W. contributed research tools and reagents and prepared the manuscript. Q.C. conceived the study, designed the experiments, supervised the project, and prepared the manuscript.




