Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study
Abstract
Background and Aims
Data regarding outcome of COVID-19 in patients with autoimmune hepatitis (AIH) are lacking.
Approach and Results
We performed a retrospective study on patients with AIH and COVID-19 from 34 centers in Europe and the Americas. We analyzed factors associated with severe COVID-19 outcomes, defined as the need for mechanical ventilation, intensive care admission, and/or death. The outcomes of patients with AIH were compared to a propensity score–matched cohort of patients without AIH but with chronic liver diseases (CLD) and COVID-19. The frequency and clinical significance of new-onset liver injury (alanine aminotransferase > 2 × the upper limit of normal) during COVID-19 was also evaluated. We included 110 patients with AIH (80% female) with a median age of 49 (range, 18-85) years at COVID-19 diagnosis. New-onset liver injury was observed in 37.1% (33/89) of the patients. Use of antivirals was associated with liver injury (P = 0.041; OR, 3.36; 95% CI, 1.05-10.78), while continued immunosuppression during COVID-19 was associated with a lower rate of liver injury (P = 0.009; OR, 0.26; 95% CI, 0.09-0.71). The rates of severe COVID-19 (15.5% versus 20.2%, P = 0.231) and all-cause mortality (10% versus 11.5%, P = 0.852) were not different between AIH and non-AIH CLD. Cirrhosis was an independent predictor of severe COVID-19 in patients with AIH (P < 0.001; OR, 17.46; 95% CI, 4.22-72.13). Continuation of immunosuppression or presence of liver injury during COVID-19 was not associated with severe COVID-19.
Conclusions
This international, multicenter study reveals that patients with AIH were not at risk for worse outcomes with COVID-19 than other causes of CLD. Cirrhosis was the strongest predictor for severe COVID-19 in patients with AIH. Maintenance of immunosuppression during COVID-19 was not associated with increased risk for severe COVID-19 but did lower the risk for new-onset liver injury during COVID-19.
Abbreviations
-
- AIH
-
- autoimmune hepatitis
-
- ALT
-
- alanine aminotransferase
-
- BMI
-
- body mass index
-
- CLD
-
- chronic liver disease
-
- IgG
-
- immunoglobulin G
-
- MMF
-
- mycophenolate mofetil
-
- 6-MP
-
- 6-mercaptopurine
-
- PBC
-
- primary biliary cholangitis
-
- PSC
-
- primary sclerosing cholangitis
-
- ULN
-
- upper limit of normal
COVID-19, caused by the severe acute respiratory syndrome coronavirus 2, was first described in December 2019 in Wuhan, China.(1) Following this first report, it rapidly spread worldwide and caused an international pandemic. Most COVID-19 cases have mild symptoms, but the disease can result in hospitalization, progression to respiratory failure, and death.(2-4) Older age, cardiovascular diseases, chronic lung diseases, active cancer, obesity, and diabetes mellitus are risk factors for severe COVID-19 outcomes.(5) COVID-19 often affects the liver, and individuals with underlying chronic liver diseases (CLD) have high rates of hospitalization and mortality.(6, 7)
Autoimmune hepatitis (AIH) is a chronic immune-mediated liver disease.(8) Corticosteroids alone or in combination with azathioprine is the standard therapy in AIH. Several alternative immunosuppressive drugs, including tacrolimus, mycophenolate mofetil (MMF), methotrexate, 6-mercaptopurine (6-MP), rituximab, and infliximab, are used in patients who do not respond or are intolerant to standard therapy.(8, 9) The majority of patients with AIH require lifelong immunosuppressive therapy, which may increase the risk of bacterial and viral infections.(8) Existing data regarding the clinical presentation and outcome of COVID-19 in patients with AIH are limited to small case series and expert opinions.(10, 11) The stage of liver disease has been shown to be a risk factor, but it is unclear whether the type of underlying liver disease is a major contributing factor to poor outcomes with COVID-19. Specifically for AIH, immunosuppressive therapy enhances the risk of severe COVID-19. On the other hand, it may be argued that immunosuppression protects against the inappropriate immune response, or cytokine storm, which is a characteristic of severe COVID-19.(12)
The aims of this international multicenter study were to assess the clinical characteristics and outcomes of patients with AIH infected with COVID-19 and to explore the frequency and factors associated with new-onset liver injury and severe COVID-19 outcome in these patients.
Materials and Methods
Study Design
We retrospectively evaluated data of patients with AIH who were diagnosed with COVID-19 between March 11 and November 12, 2020, from 34 centers in 10 countries. All participants independently identified patients and collected data from electronic health records and patients’ follow-up charts. Patients with AIH who were older than 16 years at the time of COVID-19 with a diagnosis confirmed by a PCR-based test were included in the study. Patients with typical radiological findings but with negative PCR tests were not included. All patients with AIH, or overlap with primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC), were diagnosed and treated according to international guidelines.(8, 13)
The presence of cirrhosis was diagnosed based on standard imaging studies (elastography, ultrasound, CT, or MRI), liver tissue examination, or clinical findings of portal hypertension or its complications. Complete biochemical response was defined as normal alanine aminotransferase (ALT), aspartate aminotransferase (AST) and immunoglobulin G (IgG) levels. Partial response was defined as a decrease of ALT or AST to below 2 times the upper limit of normal (ULN), and nonresponse was defined as persistently elevated transaminase levels more than 2 times the ULN despite appropriate immunosuppressive therapy.(8, 13) High alcohol consumption was defined as more than 2 drinks/day for men and more than 1 drink/day for women.(14)
New-onset liver injury was defined as rising ALT values during COVID-19 and categorized as no or mild (< 2 × ULN), moderate (2-5 × ULN), or severe (>5 × ULN). New-onset ALT > 2 × ULN was used as the definition of liver injury for the study. Patients who were nonresponders to AIH therapy were excluded from new-onset liver injury analysis as they already had ALT > 2 × ULN levels before the COVID-19 diagnosis. The cutoffs for normal values of ALT were considered 25 U/L for women and 35 U/L for men.(15) Severe COVID-19 infection was defined as a composite of intensive care unit (ICU) admission, ventilator use, and/or death, in line with previously reported COVID-19 data.(3) The Harran University Hospital of Şanlıurfa was the coordinating center (HRE ID 2020-00682), and the local ethical review boards of the participating centers approved the study.
Data Collection
Collected patient data included general information on patients, autoimmune liver serology, types and doses of immunosuppression, the patient’s AIH response status at last follow-up before COVID-19, and presence of cirrhosis. At the time of COVID-19 diagnosis, body mass index (BMI), clinical features, and comorbid conditions were recorded. Laboratory values at COVID-19 diagnosis and at peak/worst values any time in the first month of COVID-19 were used to evaluate liver injury. Any modification in the dose or type of immunosuppressive drugs during COVID-19, highest care level, hospitalization time, specific COVID-19 therapies, and patient outcomes, providing a minimum of 4 weeks after COVID-19, were recorded.
In order to determine how AIH impacts clinical outcomes with COVID-19, we compared AIH patients to a control group of patients with non-AIH CLD and COVID-19. To identify a control group, we used data from a multicenter, observational study of adult patients with CLD and PCR-confirmed COVID-19. More details regarding the inclusion and exclusion criteria of this cohort have been published.(7) Only patients without AIH from this cohort were included in the control group. None of the patients in the control group were on immunosuppression.
Statistical Analysis
Continuous variables are presented as mean with standard deviation or as median with range. The Student t test or the Mann-Whitney U test was used for comparisons, as appropriate depending on the distribution. Categorical variables are presented as numbers and percentages, and the chi-squared test was used for comparisons. Multivariable logistic regression analysis models were performed to predict the two major outcomes of the study (dependent variables): significant new-onset liver injury and severe COVID-19. According to the predefined statistical plan, we evaluated associations between each independent variable and outcome after having analyzed the number of outcome events. In addition to predefined sex and age in terms of their clinical relevance for the outcomes, independent variables with a statistically significant relationship (P < 0.1) with dependent (outcome) variables in the univariate analysis were then included in the multivariable models. Hosmer-Lemeshow goodness-of-fit statistics were used to assess model fit. P < 0.05 was considered to represent statistical significance.
We compared clinical outcomes of COVID-19 in patients with AIH and non-AIH CLD. We used propensity score analysis to identify a cohort of patients with non-AIH CLD and COVID-19 who were statistically matched on a 1:2 basis using the nearest neighbor approach. We matched AIH and non-AIH for crucial variables which are known to impact clinical outcomes with COVID-19 including age, gender, presence of cirrhosis, diabetes mellitus, hypertension, and heart diseases.
Results
General Characteristics of the Study Population
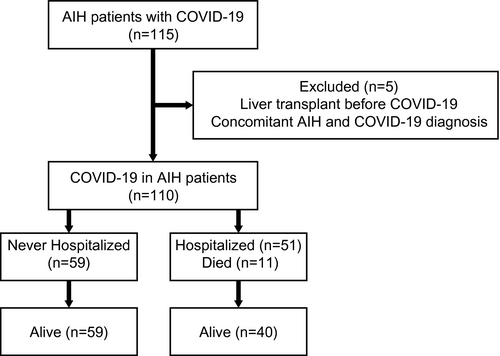
Medical data of 115 patients with AIH who acquired COVID-19 were analyzed. Five patients were excluded: 3 patients had previously undergone liver transplantation, and 2 patients were concomitantly diagnosed with AIH and COVID-19 (Fig. 1). The final study group included 110 patients with AIH (80% female) with a median age of 49 (range, 18–85) years at COVID-19 infection. Four of these patients had been included in a previous study.(11) The general characteristics, clinical features, and outcomes of the patients are presented in Table 1. Twelve (10.9%) patients had overlap with PBC and 4 (3.6%), with PSC. Coexistence of other immune-mediated disorders was noted in 28 (25.5%) patients, including autoimmune thyroid diseases in 17 (15.4%) patients, inflammatory bowel diseases in 3 (2.7%), Sjögren syndrome in 2 (1.8%), systemic sclerosis in 2 (1.8%), rheumatoid arthritis in 2 (1.8%), systemic lupus erythematosus in 1 (0.9%), celiac disease in 1 (0.9%), ankylosing spondylitis in 1 (0.9%), and antiphospholipid syndrome in 1 (0.9%).
| Region | |
| North America, n (%) | 34 (30.9) |
| South America, n (%) | 22 (20) |
| Europe, n (%) | 54 (49.1) |
| Sex (female), n (%) | 88 (80) |
| Overlap syndromes (PBC/PSC), n (%) | 12/4 (10.9)/(3.6) |
| Concomitant autoimmune diseases, n (%) | 28 (25.5) |
| ANA, n (%)* | 77 (76.5) |
| SMA, n (%)† | 46 (45.1) |
| LKM-1, n (%)‡ | 3 (3.8) |
| LC-1, n (%)§ | 3 (6.2) |
| SLA, n (%)|| | 2 (4.1) |
| AIH activity at last visit before COVID-19 | |
| Complete response, n (%) | 88 (80) |
| Partial response, n (%) | 19 (17.3) |
| Nonresponse, n (%) | 3 (2.7) |
| Presence of cirrhosis, n (%) | 32 (29.1) |
| Features of patients with AIH at COVID-19 infection | |
| Age (years), mean (SD) | 47.9 (15.8) |
| Time from AIH diagnosis, median (months) | 60 (1-480) |
| BMI, kg/m2, mean (SD) | 26.5 (5.3) |
| Comorbidities, (%) | 53 (48.2) |
| Smoking | 7 (6.4) |
| Alcohol | 2 (1.8) |
| Hypertension | 26 (23.6) |
| Diabetes | 27 (24.5) |
| Coronary artery disease | 5 (4.5) |
| Atrial fibrillation | 2 (1.8) |
| Heart failure | 2 (1.8) |
| Respiratory disease | 9 (8.3) |
| Cancer history | 5 (4.5) |
| Kidney insufficiency | 1 (0.9) |
| Immunosuppressive therapy before COVID, n (%) | 102 (92.7) |
| Azathioprine/6-MP | 27 (24.5) |
| Azathioprine/6-MP + prednisolone/budesonide | 39 (35.4) |
| Azathioprine/6-MP + tacrolimus | 1 (0.9) |
| Azathioprine/6-MP + prednisolone/budesonide + tacrolimus | 1 (0.9) |
| Azathioprine/6-MP + prednisolone/budesonide + infliximab | 1 (0.9) |
| Azathioprine/6-MP + prednisolone/budesonide + vedolizumab | 1 (0.9) |
| Prednisolone/budesonide | 20 (18.1) |
| Prednisolone/budesonide + tacrolimus | 2 (1.8) |
| Prednisolone/budesonide + MMF | 4 (3.6) |
| Prednisolone/budesonide + methotrexate | 1 (0.9) |
| Prednisolone/budesonide + tacrolimus +MMF | 1 (0.9) |
| MMF | 3 (2.7) |
| Sirolimus | 1 (0.9) |
| Symptoms at presentation, n (%) | 96 (87.3) |
| Fever | 65 (59.1) |
| Cough | 68 (61.8) |
| Dyspnea | 39 (35.5) |
| Headache | 22 (20) |
| Fatigue and/or myalgia | 65 (59.1) |
| Anosmia | 19 (17.3) |
| Gastrointestinal symptoms, n (%) | 30 (27.2) |
| Abdominal pain | 11 (10) |
| Diarrhea | 13 (11.8) |
| Nausea | 7 (6.4) |
| Vomiting | 11 (10) |
| Continued immunosuppression during COVID-19, n (%) | 69 (62.7) |
| Antibiotic therapy during COVID-19, n (%) | 40 (36.4) |
| Medical therapies for COVID-19, n (%) | 67 (60.9) |
| Hydroxychloroquine | 29 (26.4) |
| Antivirals | 22 (20) |
| Favipiravir | 17 (15.5) |
| Remdesivir | 1 (0.9) |
| Lopinavir/ritonavir | 4 (3.6) |
| High-dose steroids | 15 (13.6) |
| Rituximab | 1 (0.9) |
| Tocilizumab | 1 (0.9) |
| Low–molecular weight heparin | 26 (23.6) |
| Plasma exchange | 3 (2.7) |
| Oxygen therapy, n (%) | 42 (38.2) |
| Nasal cannula | 25 (22.7) |
| Noninvasive ventilation/mechanical ventilation | 9 (8.2)/8 (7.3) |
| New-onset liver injury during COVID-19, n (%)¶ | 33 (37.1) |
| Outcome of study population | |
| Hospitalized, n (%) | 51 (46.4) |
| Intensive care admission, n (%) | 15 (13.6) |
| Death, n (%) | 11 (10) |
- * ANA was available in 101 patients; BMI was available in 83.
- † SMA was available in 101 patients.
- ‡ LKM-1 was available in 80 patients.
- § LC1-1 was available in 48 patients.
- || SLA was available in 48 patients.
- ¶ Liver injury was evaluated in 89 patients.
- Abbreviations: ANA, antinuclear antibody; LC-1, liver-cytosol type 1; LKM-1, liver kidney microsome type 1; SLA, soluble liver antigen; SMA, smooth muscle antibody.
AIH Characteristics Before COVID-19
At last follow-up before COVID-19 diagnosis, 88 (80%) patients were complete responders, 19 (17.3%) were partial responders, and 3 (2.7%) were nonresponders. Thirty-two (29.1%) patients with AIH had features of cirrhosis. A total of 53 (48.2%) patients had comorbid conditions; diabetes mellitus (n = 27, 24.5%) and hypertension (n = 26, 23.6%) were the most common. Seven (6.4%) patients reported active smoking, and 2 (1.8%) had high alcohol consumption. Five (4.5%) patients had a history of malignancy, two cases of which were active during COVID-19.
Most patients (92.7%) were on immunosuppressive therapy prior to the COVID-19 diagnosis. Eight patients (7.3%) were not on immunosuppressive therapy; 6 patients had withdrawn therapy (3 by themselves and 3 by a physician), 1 patient was a nonresponder to available therapies, and 1 had inactive (“burned-out”) cirrhosis. At the time of COVID-19 diagnosis, 65 (59.1%) patients were on prednisone therapy (alone or in combination with other immunosuppressants) with a median dose of 5 (range, 2.5-60) mg/day. Among them, 25 (22.7%) patients were on prednisone ≥ 10 mg/day. The patients’ immunosuppressive therapies are presented in Table 1.
Management of AIH During COVID-19
The majority of patients (n = 96, 87.3%) were symptomatic at the time of COVID-19 diagnosis; cough (n = 68, 61.8%) and fever (n = 65, 59.1%) were the most commonly reported symptoms. Gastrointestinal symptoms (abdominal pain, diarrhea, nausea, and vomiting) were noted in 30 (27.2%) patients.
The dose or type of immunosuppression was modified in 33 (30%) patients during COVID-19 but remained unchanged in 69 (62.7%). General characteristics of the patients according to immunosuppressive therapy status are presented in Table 2. Among patients on azathioprine/6-MP monotherapy (n = 10), doses were reduced in 3 and discontinued in 7. Among patients on steroid monotherapy (n = 2), the dose was reduced in 1 and discontinued in 1. In patients treated with a combination of azathioprine/6-MP and steroids (n = 14), the dose of only azathioprine/6-MP was reduced in 1 and discontinued in 5, the dose of only steroids was reduced in 5, while the doses of both azathioprine/6-MP and steroids were reduced in 1 and discontinued in 2. Azathioprine was discontinued and tacrolimus reduced in a patient who was treated with both drugs. In 1 patient who was on triple immunosuppression, azathioprine was discontinued, and both the steroid and tacrolimus doses were reduced. Among patients treated with MMF and steroids (n = 4), only MMF was discontinued in 3, and both drugs were reduced in 1. The dose was reduced in a patient on MMF monotherapy.
| Immunosuppression Unchanged (n = 69, %) | Immunosuppression Reduced/Stopped (n = 41, %) | P | |
|---|---|---|---|
| Age > 65 (years) | 13 (18.8) | 4 (9.8) | 0.278 |
| Gender (female) | 57 (82.6) | 31 (75.6) | 0.461 |
| BMI > 30 | 8 (16.3) | 8 (23.5) | 0.572 |
| Comorbidity | 37 (53.6) | 18 (43.9) | 0.430 |
| Cirrhosis | 21 (30.4) | 11 (26.8) | 0.829 |
| Remission before COVID-19 | 60 (87) | 28 (68.3) | 0.026 |
| Symptoms at presentation | 59 (85.5) | 37 (90.2) | 0.564 |
| Treatment for COVID-19 | 43 (62.3) | 24 (58.5) | 0.840 |
| New-onset liver injury* | 12 (22.6) | 21 (58.3) | 0.001 |
| Severe COVID-19 | 10 (14.5) | 7 (17.1) | 0.788 |
| All-cause mortality | 4 (5.8) | 7 (17.7) | 0.096 |
- * New-onset liver injury was evaluated in 89 patients.
Antibiotics were given to 40 (36.4%) patients during COVID-19 infection. Sixty-seven (60.9%) patients received specific therapy for COVID-19. Hydroxychloroquine (n = 29, 26.4%) and antivirals (n = 22, 20%) were the most commonly used therapies. High-dose steroids were given to 15 (13.6%) patients, and plasma exchange was performed in 3 (2.7%) patients. Tocilizumab and rituximab were used in 1 (0.9%) patient each. The details of COVID-19 therapies are presented in Table 1.
Liver Injury During COVID-19
New-onset liver injury was reported in 37.1% (33/89) of the patients: 16 (18%) had moderate and 17 (19.1%) had severe liver injury. New-onset liver injury was observed in 85.7% (6/7) of patients who were not on immunosuppression, in 51.7% (15/29) of patients whose immunosuppression was modified, and in 22.6% (12/53) of patients whose immunosuppression was unchanged during COVID-19. Predictors of liver injury are presented in Table 3. Multivariate regression analysis revealed that antivirals (defined in Table 1) were associated with new-onset liver injury (P = 0.041; OR, 3.36; 95% CI, 1.05-10.78) and that continued immunosuppression during COVID-19 reduced the probability of liver injury (P = 0.009; OR, 0.26; 05% CI, 0.09-0.71).
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Sex (female) | 1.21 | 0.37-3.93 | 0.742 | 1.85 | 0.42-8.05 | 0.407 |
| Age (>65 years) | 0.56 | 0.16-1.94 | 0.364 | 0.76 | 0.18-3.10 | 0.704 |
| BMI (>30) | 2.05 | 0.62-6.75 | 0.235 | |||
| Complete response to therapy | 0.27 | 0.09-0.85 | 0.025 | 0.27 | 0.06-1.15 | 0.077 |
| Cirrhosis | 1.05 | 0.42-2.63 | 0.908 | |||
| Comorbidities | 1.06 | 0.44-2.51 | 0.890 | |||
| Immunosuppression maintenance | 0.20 | 0.08-0.52 | 0.001 | 0.26 | 0.09-0.71 | 0.009 |
| Antibiotics | 1.64 | 0.68-3.91 | 0.263 | |||
| Antivirals | 3.00 | 1.06-8.49 | 0.039 | 3.36 | 1.05-10.78 | 0.041 |
| Hydroxychloroquine | 0.86 | 0.33-2.23 | 0.757 | |||
| High-dose steroid | 0.82 | 0.25-2.65 | 0.742 | |||
| O2 therapy (any) | 2.56 | 1.06-6.20 | 0.037 | 2.23 | 0.81-6.13 | 0.118 |
- Comorbidities and antivirals are presented in Table 1.
Outcomes of COVID-19 in Patients With AIH Compared to Non-AIH CLD
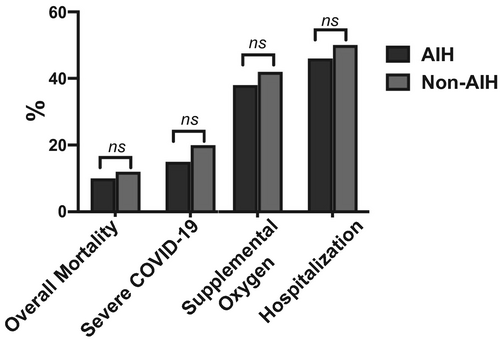
When the outcomes of patients with AIH (n = 110) were compared to a cohort of non-AIH patients with CLD (n = 220) and COVID-19 (Supporting Table S1), no statistically significant differences were noted regarding the rates of all-cause mortality (10% versus 11.5%; P = 0.852), severe COVID-19 (15.5% versus 20.2%; P = 0.231), need for supplemental oxygen (38.2% versus 42.2%; P = 0.553), and hospitalization (46.4% versus 50.0%; P = 0.560) (Fig. 2).
Seventeen AIH patients had severe COVID-19, and 11 patients died due to COVID-19. Predictors of severe outcomes in patients with AIH are presented in Table 4. After adjusting for age, gender, coexistence of PBC/PSC or extrahepatic autoimmune diseases, and AIH therapy response, the presence of cirrhosis was independently associated with severe COVID-19 (P < 0.001; OR, 17.46; 95% CI, 4.22-72.13). Patients with AIH and comorbid conditions (defined in Table 1) tended to have severe COVID-19 infection (P = 0.06; OR, 4.16; 95% CI, 0.94-18.36). Presence of liver injury and continued immunosuppression during COVID-19 were not associated with severe COVID-19.
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Sex (female) | 1.19 | 0.31-4.59 | 0.792 | 2.20 | 0.44-10.82 | 0.330 |
| Age (>65 years) | 2.81 | 0.84-9.40 | 0.093 | 1.03 | 0.23-4.48 | 0.964 |
| Overlap of PBC/PSC | 1.31 | 0.33-5.23 | 0.694 | |||
| Extrahepatic autoimmune diseases | 1.26 | 0.40-3.98 | 0.684 | |||
| BMI (>30) | 1.94 | 0.44-8.54 | 0.378 | |||
| Comorbidities | 6.46 | 1.73-24.02 | 0.005 | 4.16 | 0.94-18.36 | 0.060 |
| Prednisolone (≥10 mg) | 2.12 | 0.70-6.48 | 0.192 | |||
| Complete response to therapy | 0.53 | 0.16-1.72 | 0.297 | |||
| Cirrhosis | 19.44 | 5.04-74.92 | <0.001 | 17.46 | 4.22-72.13 | <0.001 |
| Immunosuppression maintenance | 0.82 | 0.28-2.36 | 0.718 | |||
| Acute liver injury | 1.67 | 0.57-4.86 | 0.346 | |||
Discussion
In this international multicenter study, we evaluated the clinical presentation, laboratory features, and outcomes of COVID-19 in patients with AIH. In our study population, 37.1% of the patients developed new-onset liver injury during COVID-19. Among 110 patients with AIH, 15.5% had severe COVID-19, and 10% died. Our data show that (1) the overall outcome of COVID-19 was favorable in patients without cirrhosis, (2) ongoing immunosuppression was not associated with increased risk of severe COVID-19, and (3) maintenance of immunosuppression was associated with a lower risk for new-onset liver injury.
We found similar rates of severe COVID-19 outcome and mortality in patients with AIH and a matched control group of non-AIH CLD and COVID-19. Among patients with AIH, those with cirrhosis had higher risk for severe COVID-19 outcomes (43.8% versus 3.9%). Overall mortality was also significantly higher in patients with cirrhosis than those without (31.3% versus 1.3%). In line with our results, other studies have reported 30%-34% mortality rates in patients with cirrhosis.(16, 17) These results suggest that, regardless of etiology, cirrhosis is a strong predictor of worse prognosis in patients with COVID-19. Consistent with previous reports,(5) patients with AIH and comorbid conditions in our study tended to have severe COVID-19.
New-onset liver injury was observed in 37.1% during COVID-19. Other studies have reported the rate to be 27.4% in a general population,(18) 34.6% in liver transplant recipients, and 47.5% in patients with CLD.(19) Liver injury was found to be a predictor of poor outcome in these studies.(7, 18, 19) In our AIH cohort, liver injury was not associated with adverse COVID-19 outcomes. There are several possible causes of liver injury in COVID-19, and all causes probably do not carry the same mortality risk.(18, 20) Patients who develop hepatocyte necrosis induced by systemic inflammatory response syndrome or ischemic injury due to circulatory or respiratory failure may have a higher mortality risk compared to those who have a drug-induced liver injury or develop an AIH relapse. The similar outcomes of patients with and without liver injury in our study might be explained by the likely incidence of AIH relapse or drug-induced liver injury, both manageable causes of liver injury.
Relapse of AIH following infection is a well-known phenomenon.(14) COVID-19 can trigger hyperstimulation of the immune system, an event associated with high serum levels of inflammatory cytokines and chemokines.(12) Four patients with AIH and nonsevere COVID-19 had new-onset liver injury along with high IgG levels, thus suggesting that they experienced AIH relapse rather than COVID-19-induced liver injury (Table 5). Development of various inflammatory or autoimmune conditions has been reported during or shortly after COVID-19.(12, 21) Some case reports have suggested that AIH presents for the first time during COVID-19.(21, 22) In fact, 2 patients in our cohort (both excluded) did receive their initial diagnosis of AIH during COVID-19. Overall, our results suggest that COVID-19 may be associated with new onset or flare of AIH, especially in patients whose immunosuppression is reduced.
| Case | Sex | Age at COVID-19 | Diagnosis | Response at Last Visit | Therapy During COVID-19 | Care for COVID-19 | ALT/IgG (×ULN) | Management/Response |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 60 | AIH | Complete | Azathioprine, 50 mg/day, Prednisolone, 5 mg/day | Home | 6.9/1.1 | Azathioprine and prednisolone increased/Yes |
| 2 | F | 52 | AIH | Complete | MMF, 1,000 mg/day, Prednisolone, 10 mg/day | Hospital, 5 days, no O2 therapy | 6.8/1.7 | MMF switched to tacrolimus/Yes |
| 3 | F | 44 | AIH | Complete | In remission without therapy | Home | 4.3/1.2 | Azathioprine and prednisolone added/Yes |
| 4 | F | 51 | AIH/PBC | Complete | Prednisolone, 5 mg/day, UDCA, 750 mg/day | Hospital, 6 days, nasal O2 | 4.8/1.2 | Prednisolone increased/Yes |
- Abbreviation: UDCA, ursodeoxycholic acid.
A lack of clear data has made it difficult to make definitive recommendations for management of immunosuppression during COVID-19. In our study, the doses and types of immunosuppression were continued unchanged in 62.7% of the patients when they acquired COVID-19, and this was not associated with adverse COVID-19 outcomes. Two previous studies(19, 23) also reported that continued immunosuppression did not increase the risk of severe COVID-19 in liver transplant recipients; only MMF usage was linked to severe COVID-19 outcome.(23) In our study, MMF was discontinued or reduced in 62.5% (5/8) of the patients who were on this drug. Also, therapy with prednisone (≥ 10 mg/day) was associated with higher hospitalization rates in a rheumatology setting,(24) and similar results were also reported in patients with inflammatory bowel disease.(25) However, the rates of severe COVID-19 were not different in patients with AIH who were on prednisone ≥10 mg/day compared to patients on <10 mg/day (24 versus 12.9%, P = 0.211). Due to the low number of patients who were on ≥10 mg/day prednisone or MMF therapy, we cannot draw definitive conclusions about the associated risks. Of note, patients whose immunosuppressive therapy was modified during COVID-19 experienced higher rates of liver injury or AIH relapse than those in whom immunosuppressive therapy was not modified. Relapse of AIH due to reduction or withdrawal of immunosuppression during COVID-19 represents diagnostic and therapeutic challenges. Elevated aminotransferases may be misinterpreted as a marker for severe COVID-19 and lead to unnecessary hospitalizations. Induction therapy to achieve AIH remission requires higher doses of immunosuppression, which may adversely affect the outcome of COVID-19. Our results support maintaining immunosuppressive therapy during COVID-19.
We acknowledge that the retrospective nature of our study is a main limitation. However, given the unprecedented nature of the pandemic and the low prevalence of AIH, we believe that our study offers valuable insights into the management of these patients. Another limitation is the possibility of selection bias because asymptomatic patients and those with mild disease were likely not included in our study. We have, however, included both outpatients and inpatients, and > 50% our study population were outpatients. We also observed that some of the hospitalized patients had a short hospital stay. Because patients with AIH were immunosuppressed or had concomitant comorbid conditions and because of a lack of information about appropriate use of immunosuppressive therapy during COVID-19, it is possible that patients were admitted out of caution and that hospitalization may not necessarily reflect the severity of COVID-19. We also found that management and therapeutic strategies for COVID-19 vary significantly between centers and countries. To overcome these limitations, we used widely accepted clinical endpoints of severe COVID-19 infection like ICU admission, need for mechanical ventilation, and/or death.(3, 23, 25)
Our study also has several strengths. We derived data from many hepatology centers across Europe and the Americas, and this represents the largest cohort describing COVID-19 in AIH to date. Moreover, we collected detailed information on immunosuppression changes during the course of COVID-19, allowing us to discern the effects of dose reductions and modifications on clinical outcomes. Lastly, we compared the AIH cohort with a matched non-AIH cohort, which allowed us to examine the impact of AIH itself on clinical outcomes of COVID-19.
In conclusion, this large multicenter study of patients with AIH and COVID-19 found overall mortality and severe COVID-19 rates of 10% and 15.5%, respectively. Patients with AIH, despite being immunosuppressed, did not experience worse clinical outcomes with COVID-19 than patients with non-AIH CLD. Presence of cirrhosis was the strongest predictor of severe COVID-19, and the overall outcome of COVID-19 was favorable in patients with noncirrhotic AIH. Maintenance of immunosuppression was associated with lower risk of new-onset liver injury. We recommend that immunosuppression not be decreased or discontinued upon diagnosis of COVID-19.
Author Contributions
C.E., T.D.S., S.W., R.D., and E.R. were responsible for the study concept. C.E., M.T., R.D., B.E., A.R.Ç., S.W., and E.R. were responsible for data analysis. M.T. was responsible for statistical analysis. C.E., T.D.S., R.D., E.R., P.I., and S.W. were responsible for data interpretation and preparing the manuscript for final submission. All authors contributed data and approved final manuscript.




