Contrast-Enhanced Ultrasonography–Based Hepatic Perfusion for Early Prediction of Prognosis in Acute Liver Failure
Abstract
Background and Aims
Acute liver failure (ALF) is a rare but dramatic clinical syndrome characterized by massive hepatic necrosis leading to multiorgan failure. It is difficult to predict the outcomes in patients with ALF using existing prognostic models. We aimed to analyze hepatic perfusion using contrast-enhanced ultrasound and Doppler ultrasound in patients with ALF and investigate its utility as a prognostic biomarker.
Approach and Results
In this prospective observational study, 208 patients with acute liver injury/ALF were enrolled from 2015 to 2019. We evaluated 50 consecutive patients with ALF with Doppler ultrasound and contrast-enhanced ultrasound performed on admission. The cases were divided into the following two groups: survivors (recovered without surgical intervention) and nonsurvivors (died of ALF or underwent liver transplantation). The time to peak and peak intensity of hepatic artery, portal vein, hepatic vein, and liver parenchyma were calculated using the time-intensity curve analysis. The hepatic artery (HA) resistive index was calculated using the fast Fourier transform analysis of Doppler ultrasound. The time interval (TI) between the time to peak of HA and liver parenchyma (LP) was significantly shorter in the nonsurvivors than in the survivors (P < 0.0001). The area under the receiver operating curve values for TI (HA, LP), Japanese scoring system, HE prediction model, Model for End-Stage Liver Disease score, and King’s College Hospital criteria for the prediction of poor prognosis were 0.953, 0.914, 0.861, 0.816, and 0.731, respectively. The most appropriate cutoff value of TI (HA, LP) was 6.897 seconds; the sensitivity, specificity, positive and negative predictive values were 94.4%, 90.6%, 85.0%, and 96.7%, respectively.
Conclusions
TI (HA, LP) accurately predicts the outcome in patients with ALF and may be useful in clinical decision making.
Abbreviations
-
- ALF
-
- acute liver failure
-
- ALI
-
- acute liver injury
-
- AUROC
-
- area under the receiver operating curve
-
- AVI
-
- Audio Video Interleave
-
- CEUS
-
- contrast-enhanced ultrasound
-
- CTLV
-
- CT-derived liver volume
-
- FFT
-
- fast Fourier transform
-
- HA-HVTT
-
- hepatic artery to hepatic vein transit time
-
- HA
-
- hepatic artery
-
- HARI
-
- hepatic artery resistive index
-
- ICC
-
- intraclass correlation coefficient
-
- INR
-
- international normalized ratio
-
- JSS
-
- Japanese scoring system
-
- KCHC
-
- King’s College Hospital criteria
-
- LP
-
- liver parenchyma
-
- LT
-
- liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NPV
-
- negative predictive value
-
- PI
-
- peak intensity
-
- PPV
-
- positive predictive value
-
- PT
-
- prothrombin time
-
- PV
-
- portal vein
-
- ROC
-
- receiver operating characteristic
-
- ROI
-
- region of interest
-
- SLV
-
- standardized liver volume
-
- TI
-
- time interval
-
- TIC
-
- time-intensity curve
-
- TTP
-
- time to peak
-
- Vmax
-
- peak systolic flow velocity
-
- Vmin
-
- end diastolic flow velocity
Acute liver failure (ALF) is a clinical syndrome with a variety of causes resulting in rapid loss of hepatocyte function. It is typically associated with coagulopathy and encephalopathy and causes significant morbidity and mortality.(1, 2) The clinical treatment of ALF involves intensive therapy including artificial liver support consisting of plasma exchange and continuous hemodiafiltration; however, the morbidity and mortality remain high.(3, 4) In contrast, liver transplantation (LT) has been an innovative treatment option for ALF and has led to the reduction in the deaths of thousands of high-risk patients.(5-7) However, it is expensive, necessitates life-long immunosuppression, and is limited by the global shortage of available organs.(8) It is known that the early prediction of the development of HE and early initiation of intensive therapy can improve the prognosis of patients with severe acute liver injury (ALI).(9) Thus, it is necessary to predict the outcome early in patients with ALF who are at risk of death if LT is not performed, as well as in patients who are expected to survive with intensive care. Therefore, there is an urgent need to develop early and noninvasive techniques that can predict massive hepatic necrosis resulting from ALF and also help in evaluating the need for LT. The severity of liver-tissue damage and the immediate reconstruction of liver tissue in ALF are critical factors that affect the prognosis.(10, 11) Histological examination of liver biopsy specimens obtained through transvenous biopsies can be used to assess liver damage and reconstruction. However, liver biopsy can be dangerous and impractical in seriously ill patients; thus, clinicians are forced to rely on clinical findings.(12) In contrast, prognostic models like the Model for End-Stage Liver Disease (MELD) score, the King’s College Hospital criteria (KCHC), the HE prediction model, and the Japanese scoring system (JSS), used to predict the mortality in patients with fulminant hepatitis and late-onset hepatic failure are useful for assessing the risk of poor outcome in patients with ALF.(9, 13-15) However, MELD and its variations have been criticized for including laboratory measurements in the model, which vary from one laboratory to another, and therefore are difficult to standardize and replicate worldwide.(16)
Hepatic perfusion is a major determining factor in hepatic repair and the regenerative capability.(17, 18) Previous studies using multidetector CT in patients with ALF have shown abnormal hepatic perfusion.(19-21) Tanaka et al. showed that increased hepatic arterial blood flow during the acute phase may act as a marker for the early recovery from hepatitis-induced damage.(22) Contrast-enhanced ultrasound (CEUS) perfusion imaging permits tissue hemodynamic imaging and is advantageous, as it is noninvasive and can be performed at the bedside of a seriously ill patient.(23) Miyazaki et al. showed that CEUS is a useful method to estimate the changes in hepatic hemodynamics in patients with ALI.(24) CEUS-derived time-intensity curves (TICs) reflect the hemodynamics of the tissue of interest by measuring the changes in the intensity of the harmonic frequencies versus time.(25, 26) As a result, CEUS with TIC analysis enables the quantitative evaluation of the hepatic perfusion. However, so far, no studies have been designed to assess the usefulness of CEUS in estimating liver tissue damage, such as massive necrosis, and for the early prediction of the prognosis in patients with ALF.
Therefore, the primary goal of our prospective study was to measure hepatic perfusion using CEUS with TIC analysis in patients with ALF and to investigate the usefulness of CEUS for the early prediction of the prognosis in patients with ALF.
Materials and Methods
Patients
This prospective observational study enrolled 208 patients with ALI and ALF between October 2015 and November 2019 (Fig. 1). The inclusion criteria for registration were as follows: no diagnosis of either chronic hepatitis or cirrhosis, ALI (aspartate aminotransferase > 200 IU/L and/or alanine aminotransferase > 300 IU/L) and prothrombin time (PT) to international normalized ratio (PT-INR) > 1.2, or PT activity < 80%. After registering, 53 patients met the criteria for ALF. ALF was defined as the presence of coagulopathy (PT-INR > 1.5 or PT activity < 40%) occurring within 8 weeks of the first onset of symptoms in patients without underlying liver disease.(27) All patients received intensive therapy including artificial liver support consisting of plasma exchange and/or continuous hemodiafiltration that were administered since the diagnosis of ALF with HE. LT was performed according to the JSS, to predict the prognosis of ALF.(14) Of the 53 patients with ALF enrolled in this prospective study aimed to assess the potential of CEUS findings as an early imaging biomarker for the prognosis in patients with ALF, 3 were excluded because their CEUS images could not be obtained due to the disturbance of consciousness in the patients related to hepatic coma.
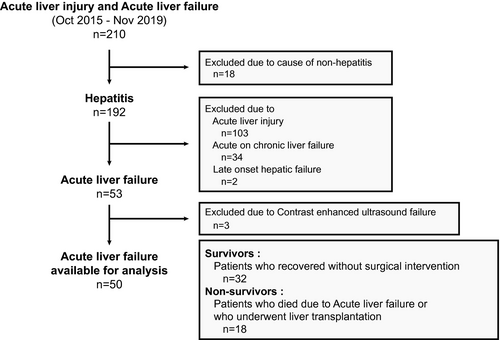
All of the remaining patients (n = 50) were divided into “survivors” (n = 32) and “nonsurvivors” (n = 18) groups. The survivors were patients who recovered following intensive therapies including artificial liver support, whereas the nonsurvivors were patients who either died of ALF or underwent LT because there was no response to intensive therapies. The control group consisted of 10 subjects matched according to the mean age and sex ratio of the patients with ALF, and all had normal liver enzyme levels. All of the protocols followed in this study were approved by the institutional review board of Iwate Medical University (approval number H20-36). All of the patients provided written, informed consent before the study, in accordance with the principles of the Declaration of Helsinki (revision of Fortaleza, 2013).
CEUS Imaging
CEUS was performed at baseline before the initiation of treatment, and after 7 days. Ultrasonography was performed using Aplio500 (Canon Medical Systems, Ohtawara, Japan) and 3.5-MHz convex transducer ultrasound probe. All ultrasound images were analyzed by two radiologists (H.K. and A.T. who have 20 years of experience in performing abdominal ultrasound examinations) who were blinded to the treatment information. CEUS imaging was recorded for 60 seconds immediately after the injection of a bolus (0.0075 mL/kg) of Sonazoid (perfluorobutane microbubbles; GE Healthcare, Oslo, Norway). The acoustic power of the contrast harmonic sonography was set to the default setting with a mechanical index of 0.25, a rate of 12 frames per second, dynamic range of 70 dB, gain of 70, depth of 11-13 cm, and focus of 8.0 cm, following the methods recommended by the guidelines.(28) In each patient, the right hepatic artery (HA), right portal vein (PV), hepatic vein (HV), and liver parenchyma (LP) were simultaneously scanned using the right intercostal view. Following the injection of Sonazoid, the patients were asked to hold their breath for as long as possible (at least 20 seconds); the cine sequences were saved in a digital imaging and communication in medicine file format for subsequent analyses.
TIC Analysis
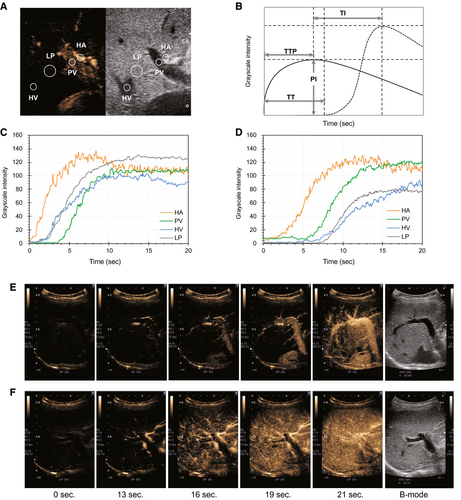
The TIC analysis was performed using an off-line personal computer with an image analysis software program (ImageJ; National Institutes of Health, Bethesda, MD). First, we decompressed the cine saved in Audio Video Interleave (AVI) format into uncompressed AVI files. In the uncompressed AVI file, the interval of each frame was 1/15th of a second. A total of 15 frames of the grayscale images were processed per second using the ImageJ software program. We observed the cine image frame by frame and set the time of the first echogenic microbubble observed in the HA at baseline. A circular region of interest (ROI) was established within the HA, PV, HV, and LP, and the intensity values were measured automatically using the ImageJ software program. The ROI with a 5-mm diameter was set on the HV 3-5 cm from the inferior vena cava and on the first branch of the HA or PV. Then, the ROI with a 10-mm diameter was set on the LP area, avoiding vessels, and at the same depth as the other ROIs (Fig. 2A). The intensity value of each pixel was expressed as 0 at minimum and 255 at maximum. After measuring the intensity values in the ROIs, we created a TIC of the arterial phase for 20 seconds from the time the contrast agent arrived at HA, using the Excel software program (Microsoft, Redmond, WA). The time to peak (TTP) and peak intensity (PI) were evaluated according to the TIC. We then calculated the time intervals (TIs) between TTP of HA and PV (TI [HA, PV]), HA and LP (TI [HA, LP]), and PV and LP (TI [PV, LP]). Furthermore, we calculated and recorded the arrival times of HA and HV, and then HA to HV transit time (HA-HVTT), with reference to previous reports(29) (Fig. 2B-F). All TIC parameters were measured until recovery or death, from hospital admission, at 7-day intervals. The investigators (H.K. and T.A.) were blinded to all patient data. All TIC parameters were measured three times for each patient, to investigate the intra-observer variability. Furthermore, the TI (HA, LP) of all patients was successively evaluated by two investigators to determine interobserver variability.
Fast Fourier Transform Analysis of Doppler Ultrasound Signals
We evaluated the peak systolic maximum velocity (Vmax) of the right HA, right PV, and HA resistive index (HARI) for the controls and all patients using the FFT analysis of Doppler ultrasound signals just before CEUS. The FFT analysis of the Doppler ultrasound signals was performed as described in previous reports, and the procedure is summarized as follows: The Aplio500 ultrasound scanner (Canon Medical Systems) was used with a 3.5-MHz convex transducer ultrasound probe. The ultrasound scanning was performed with the patient in the supine position. The Doppler ultrasonography was used initially for the detection of the right HA and right PV, and a Doppler waveform was obtained (Supporting Fig. S1A,B). The Vmax and the end-diastolic flow velocity (Vmin) were obtained after correcting for the angle of insonation, and then HARI ([Vmax Vmin]/Vmax) was calculated.
Vmin]/Vmax) was calculated.
Prognostic Models for ALF
In all patients, biochemical tests, CT imaging, and CEUS were performed on the first day at the hospital. The CT-derived liver volume (CTLV)/standardized liver volume (SLV) was calculated using the formula by Urata et al.(30) Liver atrophy was assessed by the CTLV/SLV ratio. In the present study, we performed a comprehensive evaluation to identify the independent prognostic factors from among the parameters recorded at admission, including results of biochemical tests, CTLV/SLV ratio, and the TIC parameter, using univariate and multivariate regression analysis.
The MELD score, KCHC, HE-prediction model, and JSS for ALF were calculated for each patient based on the results of the hematological examination and background information of the patient on admission. The early predictive performance of the TIC parameter, MELD score, KCHC, HE-prediction model, and JSS for ALF to predict the prognosis of ALF was assessed by receiver operating characteristic (ROC) analysis. The cutoff values for the prognostic prediction were estimated using the area under the ROC (AUROC).
Statistical Analysis
Statistical analyses were performed using the SPSS software program for Windows, version 23 (IBM, Armonk, NY). The values are presented as the mean ± SD or median (25th-75th percentiles) according to the distribution of the variables. A statistical analysis of the differences in TIC or FFT parameters among control, survivors, and nonsurvivors was performed using the Tukey–Kramer method. Interobserver and intra-observer agreement were evaluated using the intraclass correlation coefficient (ICC) for TI (HA, LP). Logistic regression analysis was used to determine the factors associated with nonsurvivors. ROC curves were constructed, and AUROC was calculated by the trapezoidal rule. Optimal cutoff values for the prediction of nonsurvivors were identified from the highest Youden index and were selected to maximize the sensitivity and specificity. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using cutoffs obtained from the ROC curves. Repeated-measures ANOVA was used to assess changes in TI (HA, LP) over the course of 7 days. P values less than 0.05 were considered statistically significant.
Results
Baseline Characteristics of the Patients
A total of 53 patients were enrolled in the study. The success rate for CEUS was 94.3% (50 of 53), since 3 patients were excluded due to their inability to perform the breath-holding procedure optimally. Therefore, 50 (94.3%) patients were included in the statistical analysis. The background characteristics of the 10 control subjects, the patients in the two groups, and their laboratory data on admission are summarized in Table 1. There were significant differences in the PT-INR, HGF, coma grade ≥ II (%), CTLV/SLV ratio, MELD score, KCHC (%), HE-prediction model, and JSS for ALF between the survivors and nonsurvivors. Pathological findings of the hepatic surgical specimen or autopsy tissue in nonsurvivors revealed massive or submassive hepatic necrosis in all of the cases (18 of 18: 100%).
| Characteristics | Control | Survivors | Nonsurvivors | Overall | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | 10 | 32 | 18 | 50 | ||||
| Sex (male/female) | 4/6 | 13/19 | 8/10 | 21/29 | ||||
| Mean age, years (range) | 58.0 (28-65) | 58.5 (20-79) | 58.3 (27-76) | 58.5 (20-79) | ||||
| Etiology (n) | ||||||||
| HAV | 1 | 1 | 2 | |||||
| HBV | 9 | 4 | 13 | |||||
| Drug | 6 | 3 | 9 | |||||
| AIH | 8 | 4 | 12 | |||||
| Unknown | 8 | 6 | 12 | |||||
| T.Bil (mg/dL) | 0.7 | (0.5-0.9) | 9.3 | (4.4-15.4)* | 11.3 | (7.4-20.5)* | 9.6 | (5.3-17.4)* |
| AST (U/L) | 24.0 | (18.0-27.0) | 675.5 | (345.0-1,386.0)* | 406.2 | (161.0-1,287.0)* | 595.5 | (394.0-1,214.5)* |
| ALT (U/L) | 23.0 | (17.3-28.0) | 777.2 | (253.7-1,531.7)* | 769.5 | (116.0-1,940.1)* | 677.5 | (368.7-1,728.8)* |
| CRNN (mg/dL) | 0.6 | (0.5-0.7) | 0.7 | (0.5-0.8) | 0.8 | (0.6-1.0) | 0.7 | (0.6-0.9) |
| PT-INR | 0.99 | (0.98-1.03) | 1.8 | (1.7-2.1)* | 2.3 | (1.9-3.2)*,† | 2.0 | (1.7-2.3)* |
| HGF (ng/mL) | 0.19 | (0.14-0.29) | 1.2 | (0.8-1.5)* | 2.4 | (1.8-4.6)*,† | 1.4 | (1.0-2.0)* |
| Plt (×104/mm3) | 20.8 | (19.8-22.6) | 14.3 | (9.5-17.7)* | 10.9 | (8.3-14.8)* | 12.1 | (9.4-16.8)* |
| Coma grade ≥ II (%) | 2/32 | (6.3%) | 14/18 | (77.8%)† | 16/50 | (32.0%) | ||
| CTLV/SLV ratio | 0.9 | (0.8-1.2) | 0.8 | (0.7-0.9)‡ | 0.9 | (0.8-1.1) | ||
| MELD score | 19.0 | (15.7-23.0) | 26.0 | (22.5-29.3)† | 22.0 | (17.2-25.7) | ||
| KCHC met (%) | 3/32 (9.4) | 10/18 (55.6)† | 13/50 (26.0) | |||||
| HE-prediction model | 39.6 | (21.3-49.9) | 66.9 | (53.0-76.4)† | 48.6 | (28.3-66.7) | ||
| JSS for ALF | ||||||||
| (1/2/3/4/5/6/7) | 5/16/9/2/0/0/0 | 0/0/7/4/5/1/1† | 5/16/16/6/5/1/1 | |||||
- Note: The values represent the mean (range), median (25th-75th percentile), or number of patients.
- * P < 0.01 (compared with control).
- † P < 0.01 (compared with survivors).
- ‡ P < 0.05 (compared with survivors).
- Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRNN, creatinine; HAV, hepatitis A virus; HBV, hepatitis B virus; Plt, platelet; T.Bil, total bilirubin.
TIC and FFT Parameters in the Controls, Survivors, and Nonsurvivors
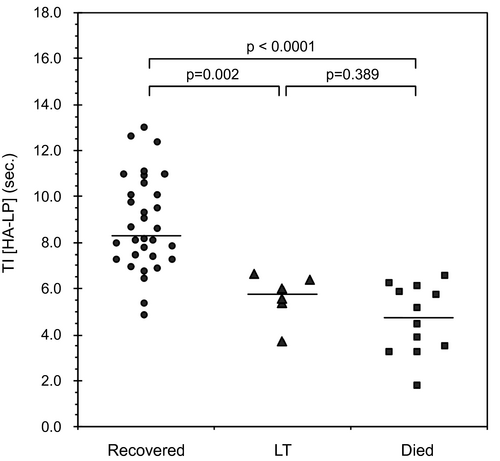
Table 2 lists the comparison of the TIC and FFT parameters among the controls, survivors, and nonsurvivors. The nonsurvivors showed significantly shorter TTP (HA) (P = 0.035), TTP (LP) (P = 0.005), and TI (HA, LP) (P < 0.0001) than the survivors. PI (PV) and PI (LP) were significantly lower in the nonsurvivors than in the survivors (P = 0.012, P = 0.026). There were no statistically significant differences in the HA-HVTT and FFT parameters between the survivors and nonsurvivors. Each TI (HA, LP) was plotted into three categories: patients who recovered following intensive therapies including artificial liver support (n = 32), patients who received LT (n = 6), and patients who died (n = 12) (Fig. 3). The median TIs (HA, LP) in patients who survived, received LT, or died from liver failure were 8.31, 5.77, and 4.73 seconds, respectively. A significant difference was noted between survivors and LT patients, and between survivors and those who died. The ICC for intra-observer agreement on TI (HA, LP) measurements was 0.820 (95% CI: 0.745-0.882). Furthermore, the reproducibility of TI (HA, LP) between observers (for all patients) yielded an ICC of 0.791 (95% CI: 0.708-862).
| Parameters | Control | Survivors | Nonsurvivors | P Value | |||
|---|---|---|---|---|---|---|---|
| TTP (HA) | 8.60 | (7.55-11.15) | 6.19 | (4.93-7.85)‡ | 4.84 | (3.21-6.12)‡ | 0.035† |
| TTP (PV) | 19.80 | (19.50-19.95) | 9.62 | (7.45-11.28)‡ | 8.15 | (7.35-11.45)‡ | 0.731 |
| TTP (LP) | 20.00 | (19.95-20.00) | 13.10 | (10.91-17.69)‡ | 7.80 | (6.57-9.39)‡ | 0.005† |
| PI (HA) | 119.32 | (115.96-129.63) | 117.43 | (115.21-125.53) | 115.63 | (110.24-129.25) | 0.333 |
| PI (PV) | 111.87 | (109.67-121.01) | 108.81 | (104.24-132.75) | 90.53 | (65.98-112.27)‡ | 0.012† |
| PI (LP) | 89.40 | (82.61-95.69) | 86.12 | (79.75-101.23) | 70.83 | (53.76-105.53)* | 0.026† |
| TI (HA, PV) | 10.90 | (8.80-12.15) | 5.45 | (3.59-7.52)‡ | 6.47 | (4.84-7.96)‡ | 0.728 |
| TI (HA, LP) | 10.80 | (8.65-12.20) | 8.32 | (7.20-10.12)‡ | 5.43 | (3.73-6.15)‡ | <0.0001† |
| TI (PV, LP) | 0.40 | (0.10-0.60) | 4.16 | (1.60-6.66)‡ | 1.45 | (0.76-1.98)‡ | 0.056 |
| HA-HVTT | 8.28 | (7.12-10.68) | 1.85 | (1.05-2.28)‡ | 1.75 | (1.23-2.00)‡ | 0.445 |
| Vmax (HA) | 40.0 | (35.0-41.0) | 79.0 | (59.0-89.0)‡ | 86.0 | (62.8-97.7)‡ | 0.346 |
| Vmax (PV) | 21.0 | (19.0-22.0) | 15.7 | (12.2-20.1)‡ | 13.0 | (6.7-16.5)‡ | 0.205 |
| HARI | 0.66 | (0.59-0.72) | 0.75 | (0.67-0.80)‡ | 0.81 | (0.75-0.83)‡ | 0.148 |
- Note: The values represent the median (25th-75th percentile).
- * P < 0.05 (compared with control).
- † There was a statistically significant difference between non-survivors and survivors.
- ‡ P < 0.01 (compared with control).
Predictive Factors Associated With Nonsurvivors by Univariate and Multivariate Regression Models
We analyzed the predictive factors associated with nonsurvivors from the baseline parameters at admission. Univariate regression analysis revealed that etiology (P = 0.045), PT-INR (P = 0.019), HGF (P = 0.005), coma grade ≥ II (P = 0.019), CTLV/SLV ratio (P = 0.014), and TI (HA, LP) (P = 0.002) were significant parameters for predicting poor prognosis. Furthermore, these factors were analyzed using multiple regression analysis, which revealed that TI (HA, LP) (P = 0.016) was the only independent factor for predicting poor prognosis (Table 3).
| Parameter | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Sex (male) | 1.169 | (0.363-3.756) | 0.793 | |||
| Age | 0.995 | (0.959-1.041) | 0.982 | |||
| Etiology (viral/others)* | 3.571 | (1.025-12.434) | 0.045 | 8.785 | (0.820-94.071) | 0.172 |
| T.Bil | 1.041 | (0.973-1.113) | 0.239 | |||
| AST | 0.985 | (0.999-1.212) | 0.804 | |||
| ALT | 1.022 | (0.983-1.002) | 0.493 | |||
| CRNN | 3.507 | (0.707-28.071) | 0.111 | |||
| PT-INR* | 4.715 | (1.283-17.322) | 0.019 | 0.918 | (0.122-6.206) | 0.579 |
| HGF* | 5.120 | (1.412-6.983) | 0.005 | 2.216 | (0.873-5.623) | 0.094 |
| Plt | 0.899 | (0.794-1.078) | 0.092 | |||
| Coma grade ≥ II (present/absent)* | 22.552 | (8.574-321.457) | 0.019 | 3.933 | (0.032-51.056) | 0.895 |
| CTLV/SLV ratio* | 0.099 | (0.022-0.894) | 0.014 | 0.129 | (0.052-1.516) | 0.135 |
| TI (HA, LP)† | 0.183 | (0.062-0.532) | 0.002 | 1.354 | (1.036-1.769) | 0.016 |
- * Significant factor by univariate analysis.
- † Significant factor by both univariate and multivariate analyses.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRNN, creatinine; Plt, platelet; T.Bil, total bilirubin.
Performance Characteristics of TI (HA, LP) and Other Scoring Models for Predicting Poor Prognosis
The AUROC for the early prediction of poor prognosis was 0.953, 0.914, 0.861, 0.816, 0.731 for TI (HA, LP), JSS for ALF, HE-prediction model, MELD score, and KCHC, respectively (Table 4 and Supporting Fig. S2). The differences between TI (HA, LP) and the JSS for ALF or the HE-prediction model did not reach statistical significance (P = 0.373 and P = 0.119, respectively). In contrast, the differences between TI (HA, LP) and the MELD score or the KCHC were statistically significant (P = 0.033 and P = 0.001, respectively). The most appropriate cutoff value of TI (HA, LP) in predicting poor prognosis was 6.897 seconds, and the sensitivity, specificity, PPV, and NPV were 94.4%, 90.6%, 85.0%, and 96.7%, respectively.
| TI (HA, LP) | JSS for ALF | HE-Prediction Model | MELD Score | KCHC | |
|---|---|---|---|---|---|
| AUROC (95% CI) | 0.953*,† | 0.914* | 0.861‡ | 0.816 | 0.731 |
| (0.895-0.998) | (0.849-0.979) | (0.757-0.965) | (0.693-0.939) | (0.602-0.860) | |
| Cutoff value | 6.897 | 3.0 | 48.9 | 20.0 | Any 3 CPIs |
| Sensitivity | 0.944 | 0.667 | 0.889 | 0.833 | 0.556 |
| (0.713-0.997) | (0.435-0.837) | (0.657-0.979) | (0.598-0.948) | (0.337-0.754) | |
| Specificity | 0.906 | 0.938 | 0.750 | 0.563 | 0.906 |
| (0.748-0.974) | (0.786-0.992) | (0.576-0.869) | (0.335-0.699) | (0.704-0.974) | |
| PPV | 0.850 | 0.846 | 0.667 | 0.517 | 0.769 |
| (0.707-1.000) | (0.674-1.000) | (0.478-0.855) | (0.332-0.768) | (0.540-0.998) | |
| NPV | 0.967 | 0.811 | 0.923 | 0.857 | 0.784 |
| (0.902-1.000) | (0.712-0.955) | (0.821-1.000) | (0.699-0.997) | (0.651-0.916) |
- * P < 0.01 (compared with KCHC).
- † P < 0.05 (compared with MELD score).
- ‡ P < 0.05 (compared with KCHC).
- Abbreviations: AUROC, area under receiver the operating curve; CPI, clinical prognostic indicator.
Serial Changes in TI (HA, LP) of the Survivors and Nonsurvivors
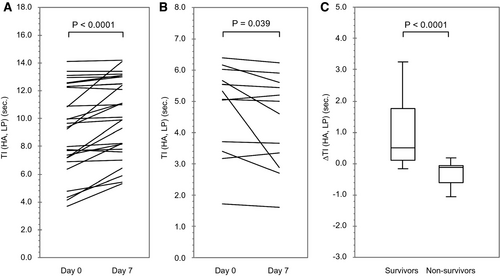
Figure 4 shows the serial changes in TI (HA, LP) and the amount of change ΔTI (HA, LP) in 40 cases (28 survivors and 12 nonsurvivors) after 7 days. The causes of failure following CEUS were recovery (n = 4), LT (n = 2), death (n = 4), and deterioration of the general condition (n = 2). In the survivors, the median TI (HA, LP) extended from 9.35 seconds (7.17, 12.27) to 10.01 seconds (8.07, 12.62) on day 7 (P < 0.0001). In contrast, in nonsurvivors, it was reduced from 5.19 seconds (3.63, 5.75) to 4.80 seconds (3.23-5.48) on day 7 (P = 0.039). The ΔTI (HA, LP) was +0.51 (0.12, 1.75) in survivors and −0.12 (−0.59, −0.06) in nonsurvivors, with a significant difference between the two groups (P < 0.0001).
Discussion
In this prospective study, we evaluated 50 patients with ALF, measured the hepatic perfusion using CEUS with TIC analysis, and investigated its utility as a prognostic indicator. The results of the study confirm that in patients with ALF and massive hepatic necrosis, peculiar changes are seen on CEUS-based hepatic perfusion. Moreover, TI (HA, LP), a real-time, noninvasive prognostic indicator, reveals tissue damage in these patients. These results show the potential of CEUS for making early predictions of the prognosis of patients with ALF and massive hepatic necrosis.
Sinusoids are low-pressure vascular channels that receive the HA and PV at the periphery of the lobules and deliver them into the central veins. Microbubble ultrasound contrast agents, such as Sonazoid, possess hemodynamic characteristics similar to those of the red blood cells and can reach the microvascular organs, allowing images to be captured. The changes observed in the hemodynamics of these organs lead to an understanding of the organ function.(23) The hepatic microcirculatory milieu, composed primarily of liver sinusoidal endothelial cells, hepatic stellate cells, and hepatic macrophages, have an essential role in liver homeostasis, including the preservation of the hepatocyte function and in control of inflammation.(31)
In our study, the Vmax (HA) in patients with ALF was significantly increased compared with that in controls. Moreover, the TTP (HA) in patients with ALF was significantly shorter than that in controls. Several previous reports using dynamic CT or Doppler ultrasound have shown similar results of increased HA blood flow during the acute phase in patients with ALF.(20, 22) In contrast, TI (HA, LP) was statistically significantly shortened in nonsurvivors compared with that in controls and survivors. We concluded that the shortened TI (HA, LP) may have been caused by a hepatic microcirculation disorder and the subsequent changes in the hepatic blood flow with reference to changes in blood flow in ALF and ALI (Supporting Fig. S3). In ALF, hypercytokinemia(32-34) and endotoxemia(35, 36) are caused by severe inflammation and apoptosis. As a result, hypercoagulopathy occurs in the hepatic sinusoids and induces hepatic microcirculation disorders, such as an increase in sinusoidal pressure and a decrease in sinusoidal blood flow.(36-38) Hepatic microcirculation disorders and destruction of the sinusoidal structure due to massive hepatic necrosis may decrease the low-pressure portal venous blood flow. Moreover, fibrin deposition occurs in the hepatic sinusoids when Kupffer cells or hepatic macrophages are activated to increase the activity of the tissue factor, which is an initiator of the blood coagulation cascade.(39) Consequently, the slow transit rate of blood in the hepatic sinusoids, expanded interstitial space, and changes in the diffusion rate between the interstitial space and vascular space may result in peculiar changes in the hepatic perfusion.(21, 40) On the other hand, as observed, the increased hepatic arterial blood flow is due to an inflammatory response,(41) hepatic arterial buffer response,(42, 43) or destruction of the peribiliary capillary plexus.(44) Our study demonstrates that in ALF, when an increase in the sinusoidal pressure decreases the portal venous blood flow, the reduction in the hepatic parenchymal perfusion was immediately counterbalanced by the HA. Limited reports have focused on blood flow changes in ALF and ALI. Tanaka et al. showed that the Vmax of the HA in acute hepatitis was significantly greater than that in controls.(22) Miyazaki et al. reported that in patients with ALI, the arrival time of HV was similar to that of PV and indicated the formation of intrahepatic shunts as a result of hepatic microcirculatory disturbances.(24) Feng et al. showed, in a rat model of acute radiation‑induced liver injury, that HA-HVTT was shorter in the severe liver injury group than in the mild and moderate groups.(29) Adrian et al. reported that the HVTT decreased with the development of hepatic fibrosis, and it may be useful for the assessment of liver disease.(45, 46) In this study, ALF significantly increased Vmax and HARI in the HA compared with those in controls. In the acute phase of ALF, massive necrosis, inflammation, and the expansion of extracellular matrix and fibrosis increase the elasticity of liver tissue, as measured using ultrasound elastography.(47, 48) We speculated that the increase in HARI is due to the pressure of tissue on the bloodstream. In our study, it was confirmed that HA-HVTT in ALF was shorter than that in controls. However, HA-HVTT and some FFT parameters showed no statistically significant differences between survivors and nonsurvivors. The possible reason for this result is that HA-HVTT and some FFT parameters reveal the measurements in the hepatic in–out flow, and indirectly infer the degree of the hepatic microcirculation, whereas TI (HA, LP) is a parameter that more directly reflects the hepatic microcirculation disorder. Therefore, TI (HA, LP), as a sensitive indicator of both massive hepatic necrosis and the hepatic microcirculation disorder, is important in early diagnosis of ALF with poor prognosis.
We also observed that TI (HA, LP) showed the highest AUROC compared with the existing prognostic models for ALF, such as the JSS for ALF, the HE-prediction model, the MELD score, and the KCHC. Remarkably, TI (HA, LP) shows statistically superior results to the MELD score and the KCHC, which are used commonly worldwide. Moreover, it must be considered that CEUS is a minimally invasive technique that can be repeated easily. Thus, TI (HA, LP) may be used as an early, noninvasive predictor for the prognosis for ALF in evaluating the need for LT. In our study, the serial changes in TI (HA, LP) and the amount of change, ΔTI (HA, LP), were observed after 7 days. In the survivors, the median TI (HA, LP) was significantly higher, and it was significantly lower in nonsurvivors. Significant differences in ΔTI (HA, LP) were noted between the two groups. The evaluation of hepatic perfusion by CEUS is a simple and noninvasive test that can be performed in real time and is repeatable. Continuous TI (HA, LP) measurements may complement the assessment of the baseline value and may also be a useful parameter to reflect the need for LT, because the medical condition of patients with ALF can change rapidly.
This study has several limitations. First, the sample size is small. Larger-scale prospective clinical studies are needed to confirm these findings. Second, compared with dynamic CT, which is used commonly, CEUS is an operator-dependent examination. CEUS evaluates only a single scanning plane, which prevents us from understanding the global image of ALF. CEUS and TIC analysis applications are not available on all ultrasound instruments. Moreover, the results of our study obtained using the Aplio500 ultrasound scanner with Sonazoid may not translate directly to those obtained with other ultrasound machines and other microbubble ultrasound contrast agents. Third, acetaminophen overdose is the leading cause of ALF in the developed world; however, there was only 1 patient in this study with acetaminophen as the etiology of ALF. Finally, the influence of selection bias cannot be denied.
In conclusion, the findings of this study suggest that TI (HA, LP) measured by CEUS with TIC analysis reflects the severity of liver damage in patients with ALF. It may be used as an early, precise prognostic biomarker for ALF, making it useful in clinical decision making.
Acknowledgment
The authors thank Ms. Yuriko Mikami and Ms. Koko Motodate for their excellent technical assistance.
Author Contributions
H.K. and Y.T. were responsible for the study concept and design. T.N., Y.F., and T.A. were responsible for the data acquisition. H.K. and T.A. were responsible for the data analysis and interpretation. H.K. and T.A. were responsible for the manuscript draft. H.K., N.T., Y.S., K.K., and Y.T. were responsible for critical revision of the manuscript.




