Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis
Abstract
Background and Aims
Lysyl oxidase-like 4 (LOXL4) is an amine oxidase that is primarily involved in extracellular matrix remodeling and is highly expressed in HCC tissues, but its functional role in mediating liver carcinogenesis is poorly understood. Therefore, we aimed to investigate the role of LOXL4 in hepatocarcinogenesis.
Approach and Results
Here, we demonstrate that hepatic LOXL4 expression was increased during the liver carcinogenesis in mice concomitantly fed a choline-deficient, l-amino acid–defined diet. LOXL4 was secreted by the neoplastic cells and primarily localized within hepatic macrophages through exosome internalization. Supplementation of LOXL4 had minimal effect on neoplastic cells. In vitro exposure of macrophages to LOXL4 invoked an immunosuppressive phenotype and activated programmed death ligand 1 (PD-L1) expression, which further suppressed the function of CD8+ T cells. Injection of LOXL4 promoted macrophages infiltration into the liver and accelerated tumor growth, which was further abolished by adoptive T-cell transfer or PD-L1 neutralization. Label-free proteomics analysis revealed that the immunosuppressive function of LOXL4 on macrophages primarily relied on interferon (IFN)-mediated signal transducer and activator of transcription–dependent PD-L1 activation. Hydrogen peroxide scavenger or copper chelation on macrophages abolished the IFN-mediated PD-L1 presentation by LOXL4. In human HCC tissue, expression of LOXL4 in CD68+ cells was positively correlated with PD-L1 level. High expression of LOXL4 in CD68+ cells and low expression of CD8A in tumor tissue cooperatively predict poor survival of patients with HCC.
Conclusions
LOXL4 facilitates immune evasion by tumor cells and leads to hepatocarcinogenesis. Our study unveils the role of LOXL4 in fostering an immunosuppressive microenvironment during hepatocarcinogenesis.
Abbreviations
-
- AAV
-
- adenovirus
-
- BMDM
-
- bone marrow–derived macrophage
-
- CCl4
-
- carbon tetrachloride
-
- CDAA
-
- choline-deficient, l-amino acid–defined
-
- CFSE
-
- carboxyfluorescein succinimidyl ester
-
- CSAA
-
- choline-sufficient, l-amino acid–defined
-
- ECM
-
- extracellular matrix
-
- H2O2
-
- hydrogen peroxide
-
- IFN
-
- interferon
-
- iNOS
-
- inducible nitric oxide synthase
-
- LOX
-
- lysyl oxidase
-
- LOXL
-
- lysyl oxidase-like
-
- Ly6C
-
- lymphocyte antigen 6 complex locus C
-
- PD-L1
-
- programmed death ligand 1
-
- rLOXL4
-
- recombinant lysyl oxidase-like 4
-
- STAT
-
- signal transducer and activator of transcription
-
- TAM
-
- tumor-associated macrophage
-
- TTM
-
- tetrathiomolybdate
HCC, one of the most common human malignancies worldwide, has been well characterized as an inflammation-related cancer.(1) Approximately 90% of HCCs develop from underlying chronic liver inflammation, which is caused by various risk factors such as hepatitis viruses, chemicals, and metabolic stress.(2) Sustained inflammation in hepatocytes induces multiple alterations at both the cellular and molecular levels that lead to genetic instability and subsequently initiate a neoplastic transformation of parenchymal cells through the formation of preneoplastic niches in the liver.(3) Simultaneously, the innate and adaptive immune cells that reside in or have infiltrated the liver microenvironment, which are important for the detection and elimination of transformed cells, are compromised during tumorigenesis.(4) The mechanisms underlying the establishment of an immunosuppressive microenvironment in the inflamed liver are not fully understood. However, a recent study showed that inflammation may lead to the suppression of antitumor cytotoxic T cells through the promotion of programmed death ligand 1 (PD-L1)–expressing and IL-10–expressing IgA+ cells during hepatocarcinogenesis,(5) suggesting that the interactions between immune cells in the hepatic microenvironment blunt antitumor surveillance and thus facilitate tumorigenesis.
Five members of the lysyl oxidase (LOX) family, namely, LOX and LOX-like (LOXL) proteins 1-4, are copper-dependent monoamine oxidases in the extracellular matrix (ECM) that catalyze the crosslinking of collagens and elastin.(6) The correlation between LOX protein expression and tumor progression is evidenced by recent studies. The enzymatic function of LOX proteins in modifying the ECM facilitates the formation of cancer niches favoring tumor cell growth and metastasis.(7) In HCC, the expression of several LOX proteins is elevated, which is correlated with poor survival in patients.(8-10) Mechanistically, high expression of LOX proteins is associated with tumor progression in established HCC through the mediation of various components of the tumor microenvironment. For instance, LOX induces epithelial-to-mesenchymal transition, which further promotes the migration and invasion of HCC cells.(8) In addition, LOX-treated HCC cells induce VEGF secretion in tumor-initiating cells of HCC and thereby enhance the capillary formation and tumor angiogenesis.(11) High expression of LOXL2 in HCC cells mediates intrahepatic invasion of tumor cells by increasing tissue stiffness while facilitating extrahepatic metastasis by recruiting bone marrow–derived cells into the metastatic niche.(12) Moreover, LOXL2 expression is associated with vasculogenic mimicry formation and triggers HCC aggressiveness.(9) HCC cells secrete high levels of LOXL4 through exosomes, which in turn activates endothelial cells and promotes angiogenesis.(10) Epigenetic up-regulation of LOXL4 supports cancer growth(13); however, a contradicting study revealed that up-regulation of LOXL4 as a result of DNA damage promotes the binding of LOXL4 to p53 and further leads to DNA damage-induced cancer inhibition.(14) Therefore, the role of LOX proteins in hepatocarcinogenesis remains to be further explored.
In this study, we systematically characterized the role of LOX proteins in hepatocarcinogenesis. We found that one of the LOX proteins, LOXL4, was elevated in the tumorigenic stage of liver cancer. Contrary to the role of LOXL4 on neoplastic liver cells, LOXL4 was localized within the macrophages and in turn maintains the T-cell exhaustion to support tumor progression. Here, we show the crucial role of LOXL4 in establishing an immunosuppressive state during hepatocarcinogenesis. LOXL4 up-regulation presents a functional mediator of hepatocarcinogenesis and poses potential for clinical prediction of HCC occurrence in patients with chronic liver disease.
Materials and Methods
Human Samples
A tissue block containing 90 pairs of human HCC tissues and adjacent normal liver tissues was obtained from US Biomax, MD, USA (HLiv-HCC180Sur-03) along with survival data encompassing a follow-up time of 2.3-7 years. Tissue was collected from operations performed between January 2010 and September 2011. Patient informed consent and approval was obtained by US Biomax, Inc.
Animal Experiments
C57BL/6J mice were obtained from The Jackson Laboratory (USA). Four-week-old C57BL/6J mice were fed a choline-deficient, l-amino acid–defined (CDAA) diet (Dyets Inc., PA, USA) and given i.p. injections of carbon tetrachloride (CCl4; 0.2 μg/g) twice weekly. BALB/cAnN-nu (nude) mice obtained from Charles River Laboratories (MA, USA) were used to establish the orthotopic model of liver cancer, as described.(15) All mice were maintained on a 12-hour light and 12-hour dark cycle, and their body weight was measured weekly. All mice were housed in the pathogen-free laboratory animal unit of The University of Hong Kong and received humane care according to the criteria outlined in the NIH Guide for the Care and Use of Laboratory Animals. The animal procedures detailed here and in the Supporting Information were reviewed and approved by the Committee on the Use of Live Animals in Teaching and Research of The University of Hong Kong.
Statistical Analysis
All statistical analyses in this study were performed using GraphPad Prism 8 software (CA, USA). All data are presented as mean ± standard deviation. Two-group comparisons were performed using a two-tailed unpaired Student t test (data with normal distribution) and Mann-Whitney U test (data with non-normal distribution). For comparisons of more than two groups, both nonparametric test (Kruskal-Wallis test) and one-way ANOVA with Tukey’s multiple comparison test were performed. A P value of less than 0.05 was considered statistically significant.
Results
LOXL4 Is Overexpressed During Hepatocarcinogenesis
CDAA diet-induced hepatic tumors in rodents have been indicated to accurately mimic carcinogenesis in the human liver by our and other studies.(16, 17) CCl4 was suggested to promote hepatocarcinogenesis by accelerating liver inflammation, fibrosis, and hepatocellular ballooning.(18) In this study, we examined LOXL4 expression during hepatocarcinogenesis using a CDAA+CCl4–induced liver tumor model in C57BL/6J mice, whereas the choline-sufficient, l-amino acid–defined (CSAA) diet-fed rodents served as control. The livers of mice receiving CDAA+CCl4 exhibit extensive steatosis at 1-3 months and develop cirrhosis at 6 months and liver tumors at 6-9 months,(19) and this pattern was consistently observed in our study (Supporting Fig. S1A-D). Infiltration of different types of immune cells was then examined in mice fed a CDAA diet for 3, 6, and 9 months. Increased infiltration of CD45+ leukocytes was observed at the first 6 months—the nontumoral stage of hepatocarcinogenesis, reflecting the sustained chronic inflammation in the human liver at the early stage of cancer-related liver diseases. Interestingly, the leukocyte infiltration became less pronounced at the tumorigenesis stage, suggesting that the hepatic microenvironment underwent a switch that led to a change in the immune profile in the liver. However, the subpopulation of F4/80+ macrophages, CD11C+ dendritic cells and CD3+ T lymphocytes were consistently increased during hepatocarcinogenesis (Fig. 1A, Supporting Fig. S1E). However, the subpopulation of F4/80+ mouse T cell immunoglobulin and mucin domain 4 Tim-4+ Kupffer cells was reduced at 9 months (Supporting Fig. S1F), suggesting the depletion of resident macrophages on tumorigenesis. To identify the role of LOXL proteins during hepatocarcinogenesis, we first assessed the expression of LOXL proteins in HCC tissues and adjacent liver tissues. Significant increases in the expression of LOXL4 but not LOXL1-3 were observed in HCC tissues (Supporting Fig. S2A) and were correlated with the poor survival of patients with HCC (Supporting Fig. S2B). In the rodent model of hepatocarcinogenesis, the mRNA level of LOXL1 and LOXL3 was increased at the inflammation stage but decreased at the tumorigenesis stage, whereas that of LOXL4 was dramatically increased at the tumorigenesis stage (Fig. 1B). LOXL2 was not detected in the rodent model. In addition, no marked change over time in the expression of LOXL4 was found in the liver of mice receiving CSAA, suggesting that LOXL4 up-regulation is a pathogenesis-related phenomenon and may not be related to aging. The significant increase in LOXL4 protein expression in the liver of tumorigenic mice at 9 months compared with nontumorigenic mice at 6 months further supported the hypothesis that LOXL4 may participate in shaping the carcinogenic hepatic microenvironment (Fig. 1C). Immunofluorescence staining indicated massive colocalization of LOXL4 protein with F4/80, a specific marker for murine macrophages, but not with cellular surface markers representing other immune cells (Fig. 1D). These observations suggest that LOXL4 is involved in shaping macrophages during hepatocarcinogenesis.
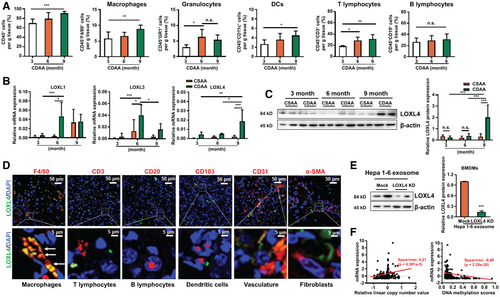
Several lines of evidence have shown that LOXL4 may play an oncogenic role in human HCC.(10, 20) To further identify its role and regulation, we compared the mRNA expression of LOXL4 in tumorigenic mice. In contrast to its protein localization, LOXL4 mRNA was not detectable in F4/80+ tumor-associated macrophages (TAMs) but was highly expressed in F4/80- parenchymal hepatic cells (Supporting Fig. S3A). On the other hand, the expression of LOXL4 in F4/80+ macrophages from tumor and nontumor region was minimally changed (Supporting Fig. S3B,C). This pattern was further proven by the observation that bone marrow–derived macrophages (BMDMs) expressed trace levels of LOXL4 mRNA, whereas immortalized murine hepatocytes and the hepatoma cell line Hepa 1-6 had higher levels of LOXL4 (Supporting Fig. S3D). Given the report of a study that liver cancer cells may secrete exosomes carrying LOXL4,(10) we hypothesized that internalization of neoplastic cell-derived exosomes by macrophages leads to an increase in the intracellular LOXL4 level. Indeed, LOXL4 protein but not mRNA was observed in exosomes derived from Hepa 1-6 cells (Supporting Fig. S3E) and could be absorbed by BMDMs (Supporting Fig. S3F). Internalization of Hepa 1-6 cell-derived exosomes increased the LOXL4 protein level in BMDMs, whereas internalization of exosomes from LOXL4-knockdown Hepa 1-6 cells resulted in low LOXL4 protein level in BMDMs (Fig. 1E). We then explored the possible mechanism leading to LOXL4 overexpression in HCC. By retrieving data from The Cancer Genome Atlas, we identified a positive correlation between the copy number and the mRNA expression of LOXL4 in HCC tissues. In addition, the mRNA level of LOXL4 was negatively correlated with its DNA methylation status (Fig. 1F). The DNA demethylating agent 5-azacytidine markedly induced LOXL4 mRNA expression in hepatoma cells (Supporting Fig. S4A). Collectively, these observations suggest that hepatocarcinogenesis involves LOXL4 overexpression through epigenetic regulation in neoplastic cells.
The Protumorigenic Role of LOXL4 in the Liver Requires Macrophages
To study the role of LOXL4, we used the adenovirus (AAV) gene transfer technologies to conditionally knock down the expression of hepatic LOXL4 in an orthotopic murine model of liver cancer. AAV8-delivered short hairpin (sh) RNA against LOXL4 target specifically silenced the expression of LOXL4 in the mice liver (Fig. 2A). Consistent with another study,(10) the hepatic LOXL4-ablated orthotopic mice showed decelerated weekly tumor growth, reduced end-point hepatic tumor volume, and microvascular density (Fig. 2A, Supporting Fig. S4B) compared with their littermates, the negative control AAV8-scrambled shRNA-treated mice. The hepatic infiltration of CD11b+ F4/80+ TAMs population was largely reduced in LOXL4 knockdown mice (Fig. 2B). LOXL4 silencing in HCC cells showed reduced in vitro survival and proliferation, whereas there was no growth difference in long-term culture (Supporting Fig. S4C). Nonetheless, supplementation of recombinant LOXL4 (rLOXL4) had minimal effect on the HCC cells proliferation and survival (Supporting Fig. S4D), suggesting that intracellular LOXL4 overexpression in HCC cells supports cellular proliferation, whereas secretory LOXL4 may act on tumor microenvironment.
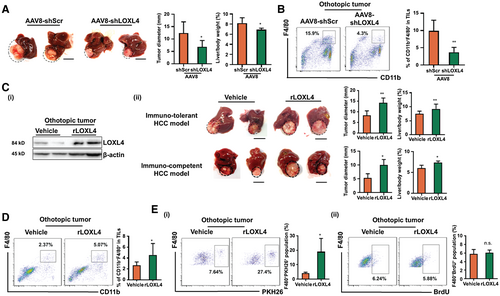
To confirm this, rLOXL4 was intraperitoneally injected into the mice orthotopic model of liver cancer (Supporting Fig. S5A). Injection of murine rLOXL4 increased the LOXL4 protein level in sorted TAMs and resulted in accelerated growth of orthotopic tumors in the livers (Fig. 2C), whereas the food intake and body weight of the mice remained unaltered (Supporting Fig. S5A). In addition, rLOXL4 injection significantly increased the population of CD11b+ F4/80+ TAMs in the hepatic microenvironment (Fig. 2D, Supporting Fig. S5B). Expansion of TAMs results from monocyte infiltration or local proliferation.(21) We intraperitoneally injected mice with fluorescent PKH26-stained bone marrow–derived monocytes and found that rLOXL4 injection potently increased the PKH26+ population in TAMs, whereas bromodeoxyuridine staining showed that minimal proliferation of resident macrophages was induced by LOXL4 (Fig. 2E). These data suggest that the LOXL4-induced increase in TAMs is primarily due to monocyte infiltration.
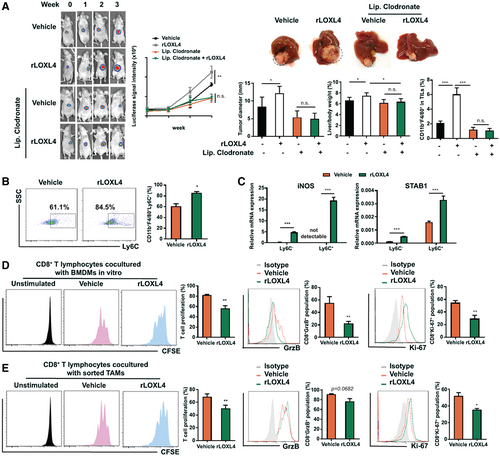
To further understand the role of LOXL4 in regulating macrophages, we supplemented BMDMs with rLOXL4 during in vitro maturation. rLOXL4 supplementation notably increased the F4/80+ population at the early stage of BMDM maturation; however, this effect became less pronounced at the later stage (Supporting Fig. S5C), suggesting that LOXL4 probably accelerated macrophage maturation. In addition, monocyte chemoattractant protein 1 expression was potently induced by rLOXL4 supplementation, which led to increased motility of BMDMs (Supporting Fig. S5D). To confirm the involvement of macrophages, liposomal clodronate was applied to deplete the TAMs from mice with orthotopic liver tumors. Injection of liposomal clodronate successfully depleted TAMs from mice harboring liver tumors regardless of rLOXL4 treatment, and the effect of rLOXL4 in promoting tumor growth became insignificant (Fig. 3A). As endogenous expression of LOXL4 in HCC cells directly induced cell proliferation, we performed the adoptive transfer of rLOXL4-treated BMDMs to LOXL4-ablated tumor-bearing mice to confirm the role of LOXL4-induced macrophages on HCC growth. Transfer of rLOXL4-treated BMDMs significantly accelerated growth of orthotopic tumors in the livers compared with vehicle-treated BMDMs (Supporting Fig. S5E), suggesting the predominant role of LOXL4 on macrophages in modulating HCC growth in vivo. These findings suggest that the tumorigenic action of LOXL4 in HCC requires the presence and modulation of macrophages.
LOXL4 Shapes the Immunosuppressive Phenotype of Macrophages
Macrophages are recruited to produce proinflammatory factors, which in turn also promote transformed epithelial cell growth.(22) A study postulated that these activated macrophages, which are supposed to kill the aberrant cells, are redifferentiated into cells with a tumor-promoting M2 phenotype in the tumor microenvironment.(23) However, we observed insignificant changes in the expression of the M2 macrophage marker CD206 in macrophages treated with rLOXL4 (Supporting Fig. S6A). This result was further supported by the unaltered expression of M2 cytokines, such as IL-10 and TGF-β (Supporting Fig. S6B). Interestingly, we then observed that rLOXL4 treatment substantially maintained the lymphocyte antigen 6 complex locus C (Ly6C) cell surface marker on differentiated BMDMs (Fig. 3B). Because a study suggested that macrophages expressing high levels of Ly6C exhibit immunosuppressive properties,(24) we sorted Ly6C+ and Ly6C- populations from rLOXL4-treated BMDMs. Treatment with rLOXL4 promoted the expression of immunosuppressive genes in both Ly6C- and Ly6C+ macrophages, and the effect was more prominent in Ly6C+ cells (Fig. 3C). The immunosuppressive property of rLOXL4-treated macrophages was further supported by the observation that coculture of rLOXL4-treated macrophages with CD8+ T cells significantly reduced T-cell proliferation. Intracellular staining of granzyme B and Ki-67, which is a feature of activated cytotoxic T cells, revealed that coculture with rLOXL4-treated macrophages blocked cytotoxic T-cell activation (Fig. 3D). Similarly, TAMs sorted from rLOXL4-treated mice with orthotopic liver tumors exhibited a significant inhibition on the T-cell proliferation and activation (Fig. 3E). rLOXL4 alone showed minimal direct activation on cytotoxic T cells (Supporting Fig. S6C).
Activation of T cells requires l-arginine, which is metabolized by arginase-1 and inducible nitric oxide synthase (iNOS) in macrophages.(25, 26) We observed that treatment with rLOXL4 had a minimal effect on arginase-1 expression but markedly elevated iNOS in macrophages (Supporting Fig. S6D). However, neither supplementation with l-arginine nor inhibition of iNOS restored T-cell proliferation (Supporting Fig. S6E) or the expressions of intracellular Ki-67 and granzyme B (Supporting Fig. S6F), suggesting that the regulatory mechanism of LOXL4 on immunosuppressive macrophages is independent of arginine.
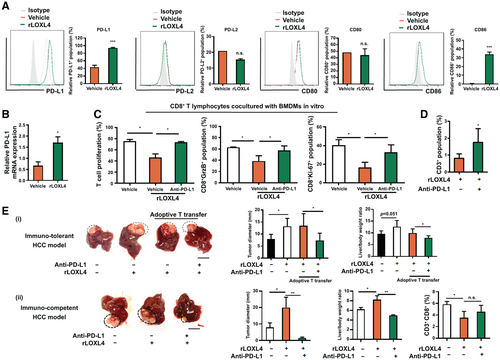
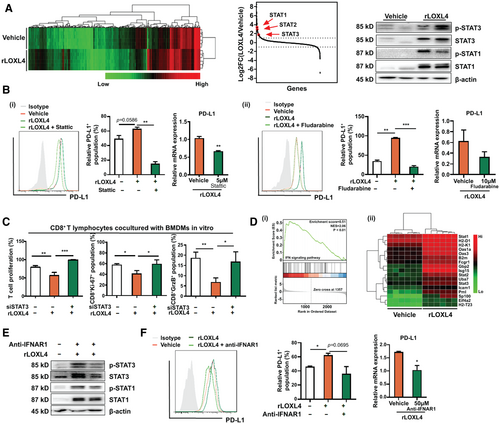
PD-L1 Mediates the Immunosuppression of Macrophages Induced by LOXL4
Macrophages promote immunosuppression in the tumor microenvironment through various mechanisms independent of l-arginine. Interaction of macrophages with tumoricidal T cells through cell antigens such as PD-L1/PD-L2 and CD86/CD80 impairs T-cell activation and induces functional exhaustion.(27, 28) We therefore examined whether LOXL4 is involved in the immune checkpoint regulation of macrophages during HCC growth. The expression of PD-L1 and CD86, instead of PD-L2 or CD80, was significantly induced in vitro in BMDMs treated with rLOXL4 (Fig. 4A). Moreover, induction of PD-L1 and CD86 by rLOXL4 was dose independent in macrophages (Supporting Fig. S7A). Interestingly, rLOXL4 supplementation or LOXL4 depletion failed to induce PD-L1 and CD86 expression in hepatic tumor cells (Supporting Fig. S7B), suggesting that the action of LOXL4 was specific in macrophages. Treatment of BMDMs with rLOXL4 significantly increased PD-L1 mRNA expression (Fig. 4B), suggesting the transcriptional activation of PD-L1 by LOXL4. Blockade of PD-L1 in rLOXL4-treated macrophages using a neutralizing antibody restored the proliferation and activation of cocultured T cells (Fig. 4C). To identify whether PD-L1 presentation mediates the in vivo immunosuppressive and tumorigenic effect, we used an anti-PD-L1 neutralizing antibody to block TAM-expressed PD-L1 in mice with orthotopic liver tumors. Adoptive transfer of splenic T cells was performed to reconstruct the immunogenic microenvironment in tumor-bearing nude mice, and we found that carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells homed to the orthotopic tumors 72 hours after adoptive transfer (Supporting Fig. S7C). rLOXL4 treatment reduced the CD3+ T-cell population in hepatic tumors of mice (Fig. 4D) and blunted the tumoricidal effect of the adoptive T-cell transfer. In addition, anti-PD-L1 neutralizing antibody significantly restored the T cell–associated tumor regression (Fig. 4E). Collectively, these findings demonstrate that the immunosuppressive action of LOXL4 on TAMs requires PD-L1 presentation.
To understand the mechanism underlying the regulation of LOXL4 on PD-L1 expression in macrophages, label-free proteomics analysis was performed to evaluate the intracellular protein expression changes in BMDMs exposed to rLOXL4. Analysis on protein expression revealed that expression of signal transducers and activators of transcription (STATs), including STAT1, STAT2, and STAT3, was up-regulated. Immunoblotting analysis, however, observed that rLOXL4 induced STAT1 and STAT3 activation, whereas STAT2 was barely detectable in BMDMs (Fig. 5A). A study observed that heterodimers of STAT1-STAT3 may act as transcription factors in facilitating expressions containing IRF responsive element, most of which are immunosuppressive.(29) Blockade of STAT3 or STAT1 by pharmacological inhibition (Supporting Fig. S7D) resulted in reduced expression and transcriptional activation of PD-L1 (Fig. 5B) in rLOXL4-treated BMDMs. In addition, inactivation of STATs-mediated PD-L1 expression in rLOXL4-treated macrophages abolished its suppressive effect on the proliferation and activation of cocultured cytotoxic T cells (Fig. 5C, Supporting Fig. S7D). Furthermore, gene set enrichment analysis revealed that genes associated with the interferon (IFN)-associated signaling pathway were positively regulated by LOXL4. Most of the genes related to IFN-associated signaling were significantly regulated by rLOXL4-exposed macrophages (Fig. 5D). Further validation confirmed the increase of type I IFN-stimulated genes, such as interferon-stimulated gene 15, chemokine (C-C motif) ligand 5, and intracellular adhesion molecule 1, in rLOXL4-treated BMDMs (Supporting Fig. S7E). Blockade of type I IFN-associated signaling by interferon alpha and beta receptor subunit 1 (IFNAR1) neutralizing antibody decreased the STAT3 and STAT1 activation induced by rLOXL4 (Fig. 5E) as well as the expressions of type I IFN-stimulated genes (Supporting Fig. S7E). Accordingly, the increased expression and transcriptional activation of PD-L1 induced by rLOXL4 were also attenuated by IFNAR1 neutralizing antibody (Fig. 5F).
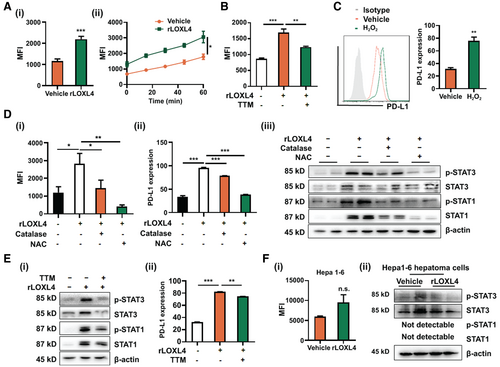
LOXL4 catalyzes the conversion of the amine group and generates hydrogen peroxide (H2O2) and ammonia as byproducts in a copper-dependent manner. LOXL4 intervention significantly increased the H2O2 level in BMDMs in a time-dependent manner (Fig. 6A). Treatment with the copper chelator tetrathiomolybdate (TTM) blocked H2O2 production in rLOXL4-treated BMDMs (Fig. 6B), suggesting that increase of H2O2 by LOXL4 requires copper-mediated enzymatic reaction. Acquisition of immunosuppressive phenotype may be induced by H2O2-associated redox imbalance in macrophages.(30) Direct incubation of BMDMs with H2O2 significantly induced the PD-L1 expression (Fig. 6C), whereas blockade of H2O2 in rLOXL4-treated BMDMs repressed the induction of PD-L1 (Fig. 6D). Activation of STATs by LOXL4 was also blocked on H2O2 scavenge (Fig. 6D), suggesting that LOXL4-induced H2O2 activates the IFN-associated STATs-mediated PD-L1. Consistently, copper chelation by TTM decreased the STAT3 and STAT1 activation as well as the expression of PD-L1 induced by rLOXL4 (Fig. 6E). Interestingly, we observed that LOXL4 increased the H2O2 level in HCC cells, but the increased H2O2 failed to alter STAT1/3 expression in HCC cells (Fig. 6F), suggesting that the H2O2-mediated PD-L1 expression by LOXL4 is cell type specific. These observations suggest that the copper-dependent H2O2 production was responsible for the activation of IFN-associated STATs-mediated PD-L1 expression in LOXL4-treated macrophages.
LOXL4 Accelerates Tumorigenesis in the Liver
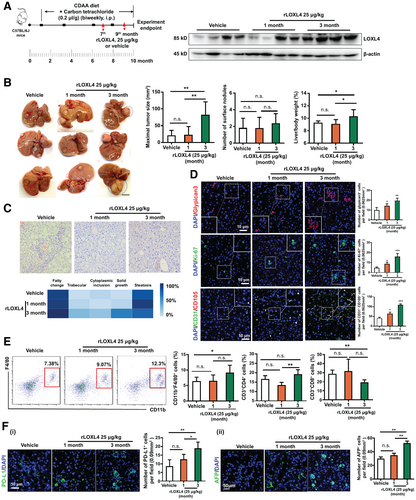
We reasoned that LOXL4 triggers the immunosuppressive function of macrophages through STAT1-STAT3-mediated PD-L1 activation and expected that this action promotes the liver carcinogenesis process. We then used CCl4 in the CDAA diet-fed rodent model of liver tumorigenesis. rLOXL4 supplementation was provided at the beginning of cirrhosis initiation (month 7) and after tumor formation (month 9). Treatment with rLOXL4 significantly increased the LOXL4 level in the liver (Fig. 7A). Notably, rLOXL4 supplementation accelerated tumor formation when it was continuously administered for 3 months beginning at month 7. This acceleration was also reflected by the increased maximal tumor size, tumor lesion frequency, and liver/body weight ratio (Fig. 7B). However, the effect of rLOXL4 was less pronounced when it was administered after significant tumor formation (month 9), although this pattern could not exclude the possibility of an effect from relatively short-term rLOXL4 treatment. Histological analysis showed that rLOXL4 treatment potently accelerated the neoplastic transformation of the liver, as evidenced by features including solid tumor formation, cytoplasmic inclusions, and a trabecular morphology (Fig. 7C) as well as increased carcinogenic glypican-3+, proliferating Ki-67+, and CD31+CD105+ microvascular cells (Fig. 7D). This transformation was accompanied by an increase in TAMs infiltration and a reduction in CD8+ cytotoxic T cells in the liver of rLOXL4-treated mice (Fig. 7E), whereas CD4+ T cells were not significantly changed. Similarly, we observed potent overexpression of PD-L1 and alpha-fetoprotein in the livers of rLOXL4-treated mice (Fig. 7F). These findings systematically reveal that LOXL4 is an important factor for hepatocarcinogenesis promotion.
Up-Regulation of LOXL4 Correlates With a Dismal Prognosis in HCC
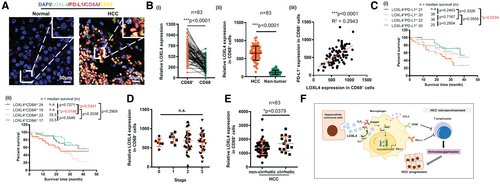
To determine whether LOXL4 correlates with the immune landscape and clinical outcome of HCC, we compared the expression of LOXL4 in liver cancer tissue and adjacent nontumoral tissue by assessing a tissue array containing sections from 90 patients (Supporting Fig. S8A, representative image in Fig. 8A, patient information in Supporting Table S1) and observed a significant increase in LOXL4 expression in tumors. Approximately 70% of LOXL4+ cells are CD68+ TAMs (67.87% ± 12.03%) (Supporting Fig. S8B), and LOXL4+CD68+ cells were significantly higher than LOXL4+CD68− cells in HCC tissues (Fig. 8B), suggesting that LOXL4 is primarily expressed in TAMs of HCC. Expression of TAMs-specific LOXL4 was positively correlated with PD-L1 level in CD68+ TAMs (Fig. 8B). To determine if LOXL4 correlates with the immune landscape and clinical outcome of HCC, we assessed the correlation of LOXL4 expression with patients’ survival. Combined LOXL4 and PD-L1 expression in CD68+ TAMs predicted the survival of patients with HCC. Patients with LOXL4lo PD-L1lo in CD68+ cells of HCC tumors had better overall survival than those bearing LOXL4hi PD-L1hi (Fig. 8C). Similarly, CD8Ahi and TAMs-specific LOXL4lo in CD68+ cells predicted better survival of patients with HCC than those of CD8Alo and TAMs-specific LOXL4hi (Fig. 8C). This indicates that LOXL4-mediated macrophages probably influence the function of CD8+ T cells and clinical outcomes of HCC. Although the expression of LOXL4 in CD68+ TAMs showed no significant difference among cohorts of patients with HCC at different stages (Fig. 8D), further subgroup analysis showed that patients with HCC and cirrhosis history expressed higher LOXL4 in CD68+ TAMs than participants without cirrhosis (Fig. 8E). LOX family proteins, including LOXL4, have been reported to catalyze the crosslinking of ECM components in a copper-dependent manner, which contributes to fibrogenesis through retarding matrix metalloproteinase–induced proteolytic degradation.(31) The difference in LOXL4 expression between patients with and without cirrhosis led us to identify the patient’s subgroup based on viral hepatitis status. LOXL4 was significantly overexpressed in HCC tumors of patients who were HBV positive (Supporting Fig. S8C). Furthermore, patients with HBV infection showed increased hepatic LOXL4 expression (Supporting Fig. S8D). Nonetheless, the viral load type had no significant effect on LOXL4 expression, given that patients with HCC and either HBV or HCV background both highly expressed LOXL4 (Supporting Fig. S8E). These data suggest that hepatic LOXL4 expression is a common feature in patients with chronic hepatitis and HCC. Therefore, high expression of LOXL4 in HCC may signify an immunosuppressive microenvironment as well as a dismal clinical outcome.
Discussion
This study indicates that LOXL4 and its downstream signaling cascade play an important role in fostering the immunosuppressive microenvironment that facilitates subsequent hepatic tumor progression. This role of LOXL4 may be accomplished through the shaping of macrophages in the liver—on the one hand, recruiting increased numbers of infiltrated macrophages, and on the other hand, modulating macrophage presentation of the inhibitory checkpoint molecule PD-L1, which in turn blunts cytotoxic T cell–mediated immune surveillance. LOXL4 was progressively increased during tumor development and primarily expressed in TAMs of HCC, but it was also localized in other stromal cells, such as endothelial cells.(10) Although we could not preclude the possibility that LOXL4 acts on endothelial cells, further analysis on human HCC tissues revealed that approximately 70% of LOXL4+ cells are CD68+ TAMs, suggesting the potential immunoregulatory function of LOXL4. The involvement of macrophages in extrinsic effect of LOXL4-mediated HCC growth was further ascertained by the observations that (1) macrophage depletion using liposomal clodronate led to nonsignificant growth promotion by LOXL4 and (2) adoptive transfer of rLOXL4-treated BMDMs to LOXL4-ablated tumor-bearing mice accelerated HCC growth. Although LOXL4 directly supports the intrinsic function of HCC cells, our current findings suggested that the extrinsic effect of LOXL4 through macrophage modulation is likely to predominate the HCC growth and progression.
Our study suggests that LOXL4 may participate in the carcinogenesis process and postulates that exosomal LOXL4 shapes immunosuppressive macrophages by inducing PD-L1 presentation. The LOXL4-induced PD-L1 expression in macrophages was STAT dependent (Fig. 8F). Activation of STAT1 by homodimerization is associated with the inflammatory phenotype of macrophages.(32) A study reported that evoked STAT3-mediated immunosuppression and impaired STAT1-driven immune surveillance facilitate immune evasion and regulate PD-L1 expression.(33) We observed that pharmacological inhibition of either STAT1 or STAT3 reduced the PD-L1 activation by LOXL4. It is likely that LOXL4 induced STAT1:STAT3 heterodimerization for the PD-L1 activation. Indeed, STAT3 is a well-reported transcription factor for PD-L1.(34) Epstein-Barr virus infection was shown to induce PD-L1 expression by increasing STAT3 activity in monocytes,(35) and blockade of STAT3 in myeloid cells enhanced CD8+ T activation during anti-PD-L1 treatment.(36) Our findings consistently observed that STAT3 inhibition restored the T cells’ function, and the effect is less prominent on STAT1 inhibition, despite both having reduced PD-L1 activation. How STAT1 and STAT3 proportionally mediate LOXL4-induced PD-L1 expression remains unclear, yet STAT3/STAT3 homodimers appeared to be more effective than STAT1:STAT3 heterodimers in activation of downstream effectors.(37) Additionally, STAT1 activity is prolonged in cells without STAT3,(38) suggesting that STAT3 promotes the balance of STATs through a negative regulation.
Blockade of type I IFN-associated signaling by neutralizing antibody abolished STATs activation and LOXL4-induced PD-L1 expression, suggesting that the interaction between type I IFN receptor and LOXL4 is necessary to activate the immunosuppressive signaling. Further analysis showed that LOXL4-induced H2O2 activates the IFN-associated STATs-mediated PD-L1; however, LOXL4-induced H2O2 showed differential actions on macrophages and HCC cells, indicating that the sensitivity of IFNAR signaling may play an important role in the cell type-specific induction of PD-L1 by LOXL4. H2O2 acts as messenger molecule to regulate kinase activity by catalyzing the post-translational modification of the cysteine residue of related proteins and contributes to the Janus kinase activation.(39) Impaired IFN signaling has been reported in the hepatic tissues of patients with advanced liver disease and HCC.(40) Patients with malignant tumor sections demonstrated inhibition in IFN signaling compared with the benign counterpart,(41) suggesting that IFN signaling is selectively shut down during malignancy. A recent study reported patients with HCC carrying at least one promoter polymorphism in IFNAR-1 transcript that is HCC specific,(42) resulting in a variable response of HCC cells to IFN-α/β. This echoed our observation that secretory LOXL4 had minimal action on HCC cell growth. Although further investigation may be needed to confirm the modification status of type I IFN signaling proteins, our findings supported the claims that copper-dependent production of H2O2 by LOXL4 serves as a key activator of type I IFN signaling-mediated PD-L1 presentation in macrophages.
Analysis of the expression of LOXL4 between patients with and without cirrhosis revealed that LOXL4 was overexpressed in patients with chronic hepatitis and HCC. Although studies reporting the role of LOXL4 in fibrosis are fewer compared with other LOX proteins, LOXL4 was remarkably increased in liver fibrotic tissues caused by different pathological factors,(43) which is in line with our observations. The exact mechanism underlying the LOXL4 induction during hepatic fibrosis has not yet been fully understood; however, profibrogenic factors may initiate the transcription of LOXL4. Supplementation of TGF-β1, a cytokine that is overexpressed in hepatic fibrogenesis, induced LOXL4 expression in fibroblast through activation of mothers against decapentaplegic homolog and activator protein 1 transcription factors,(44) and LOXL4 expression was responsible for TGF-β1–induced fibrogenesis.(45) The inflammatory factor IL-1β was able to induce LOXL4 expression in vitro.(46) Different profibrogenic factors as well as their interaction during fibrogenesis may jointly contribute to the increased LOXL4 expression in cirrhotic liver of patients with HCC.
In summary, we identified a role for the LOX protein LOXL4 in the hepatocarcinogenesis. Our study asserts that LOXL4 plays an important role in fostering an immunosuppressive microenvironment during hepatocarcinogenesis.
Acknowledgment
We express our appreciation to Mr. Keith Wong, Ms. Cindy Lee, Mr. Alex Shek, and the Centre for PanorOmic Sciences: Imaging and Flow Cytometry Core and Proteomics and Metabolomics Core of Li Ka Shing Faculty of Medicine, The University of Hong Kong, for their technical support.
Author Contributions
Y.F. and N.W. conceived and designed the study; H.-Y.T., Y.-T.C., and C.Z. did the experiments. H.-Y.T., N.W., and Y.F. wrote the manuscript. N.W. and Y.F. interpreted the data. M.-F.Y. revised the manuscript. All authors approved the final manuscript.




