Activating Adenosine Monophosphate–Activated Protein Kinase Mediates Fibroblast Growth Factor 1 Protection From Nonalcoholic Fatty Liver Disease in Mice
Abstract
Background and Aims
Fibroblast growth factor (FGF) 1 demonstrated protection against nonalcoholic fatty liver disease (NAFLD) in type 2 diabetic and obese mice by an uncertain mechanism. This study investigated the therapeutic activity and mechanism of a nonmitogenic FGF1 variant carrying 3 substitutions of heparin-binding sites (FGF1△HBS) against NAFLD.
Approach and Results
FGF1△HBS administration was effective in 9-month-old diabetic mice carrying a homozygous mutation in the leptin receptor gene (db/db) with NAFLD; liver weight, lipid deposition, and inflammation declined and liver injury decreased. FGF1△HBS reduced oxidative stress by stimulating nuclear translocation of nuclear erythroid 2 p45-related factor 2 (Nrf2) and elevation of antioxidant protein expression. FGF1△HBS also inhibited activity and/or expression of lipogenic genes, coincident with phosphorylation of adenosine monophosphate–activated protein kinase (AMPK) and its substrates. Mechanistic studies on palmitate exposed hepatic cells demonstrated that NAFLD-like oxidative damage and lipid accumulation could be reversed by FGF1△HBS. In palmitate-treated hepatic cells, small interfering RNA (siRNA) knockdown of Nrf2 abolished only FGF1△HBS antioxidative actions but not improvement of lipid metabolism. In contrast, AMPK inhibition by pharmacological agent or siRNA abolished FGF1△HBS benefits on both oxidative stress and lipid metabolism that were FGF receptor (FGFR) 4 dependent. Further support of these in vitro findings is that liver-specific AMPK knockout abolished therapeutic effects of FGF1△HBS against high-fat/high-sucrose diet–induced hepatic steatosis. Moreover, FGF1△HBS improved high-fat/high-cholesterol diet–induced steatohepatitis and fibrosis in apolipoprotein E knockout mice.
Conclusions
These findings indicate that FGF1△HBS is effective for preventing and reversing liver steatosis and steatohepatitis and acts by activation of AMPK through hepatocyte FGFR4.
Abbreviations
-
- ACC
-
- acetyl-coenzyme A carboxylase
-
- ALT
-
- alanine aminotransferase
-
- AMPK
-
- adenosine monophosphate–activated protein kinase
-
- AMPK-LKO
-
- liver-specific adenosine monophosphate–activated protein kinase knockout
-
- apoE-KO
-
- apolipoprotein E knockout
-
- AST
-
- aspartate aminotransferase
-
- FAS
-
- fatty acid synthase
-
- FGF
-
- fibroblast growth factor
-
- FGF1wt
-
- wild-type fibroblast growth factor 1
-
- FGF1△HBS
-
- fibroblast growth factor 1 variant carrying 3 substitutions of heparin-binding sites
-
- FGFR
-
- fibroblast growth factor receptor
-
- HFD
-
- high-fat diet
-
- HFHC
-
- high-fat/high-cholesterol
-
- HFHS
-
- high-fat/high-sucrose
-
- MDA
-
- malondialdehyde
-
- NADPH
-
- nicotinamide adenine dinucleotide phosphate, reduced form
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NAS
-
- nonalcoholic fatty liver disease activity score
-
- NASH
-
- nonalcoholic steatohepatitis
-
- Nrf2
-
- nuclear erythroid 2 p45-related factor 2
-
- PBS
-
- phosphate-buffered saline
-
- SCD-1
-
- stearoyl-coenzyme A desaturase 1
-
- siRNA
-
- small interfering RNA
-
- SREBP-1
-
- sterol regulatory element binding protein 1
-
- T2D
-
- type 2 diabetes
-
- db/db
-
- diabetic mice carrying a homozygous mutation in the leptin receptor gene
-
- WT
-
- wild type
Liver steatosis and nonalcoholic fatty liver disease (NAFLD), characterized by hepatic fat accumulation, inflammation, and oxidative stress, are strongly linked with obesity, insulin resistance, and dyslipidemia (metabolic syndrome) and type 2 diabetes (T2D).(1) NAFLD is especially serious in T2D because diabetic hyperglycemia provides excess glucose to the liver, leading to more hepatic oxidative stress and greater imbalance of hepatic metabolism. Because the prevalence of NAFLD among patients with T2D is up to 75%(2, 3) and the consequences are especially serious in patients with diabetes, NAFLD is recognized as a major health burden to the T2D population. Currently, there are no effective therapies for T2D-associated NAFLD. Therefore, there is an urgent need to develop new therapeutic approaches to reduce the metabolic pathology of T2D responsible for the high prevalence of NAFLD.
Fibroblast growth factor (FGF) 1, a well-characterized mitogen belonging to the 18-member FGF family, is widely studied for its therapeutic benefits in cardiovascular disorders, nerve injury, and wound healing.(4, 5) Recently, FGF1 was shown to exert an unexpected metabolic activity by regulating lipid metabolism and glucose homeostasis in obesity and diabetes.(6, 7) Furthermore, our recent study indicated that FGF1 prevents diabetic nephropathy in both type 1 diabetes and T2D animal models.(8) However, because native FGF1 induces hyperproliferation, its use can potentially increase tumorigenic risk,(9) which limits its widespread application, especially in cancer-prone diseases, including diabetes.
If the mitogenic and metabolic functions of FGF1 can be dissociated, then it may provide a safe and potent agent for prevention and/or therapy of diabetes and diabetic complications while minimizing risk of cancer. Thus, we engineered an FGF1 partial agonist with 3 substitutions of heparin-binding sites (i.e., FGF1ΔHBS) that dramatically diminished its ability to induce heparan sulfate-assisted FGF receptor (FGFR) dimerization and activation.(10) Moreover, FGF1ΔHBS has reduced proliferative potential yet maintains full metabolic activity of wild-type (WT) FGF1 (FGF1WT) in vitro and in vivo.(10) Notably, FGF1ΔHBS 28-day treatment protects the liver from fatty acid accumulation in diabetic mice carrying a homozygous mutation in the leptin receptor gene (db/db),(10) which is consistent with FGF1WT action to inhibit hepatic steatosis in high-fat diet (HFD)–induced obesity(6) and in leptin-deficient ob/ob mice.(7, 11) These exciting findings support the idea that FGF1ΔHBS is a potential safe and effective therapeutic approach for treatment of NAFLD, especially in the setting of T2D. Nonetheless, the mechanism(s) underlying the beneficial activity of either FGF1WT or FGF1ΔHBS in NAFLD remains to be determined. In addition, the clinically important capacity of FGF1 to reverse established NAFLD or to prevent nonalcoholic steatohepatitis (NASH) has not been tested.
In the present study, we demonstrate the potent beneficial effects of FGF1△HBS on established NAFLD in T2D and on NASH in an apolipoprotein E knockout (apoE-KO) mouse model. Importantly, we identify a likely mechanism required for FGF1△HBS actions on hepatic oxidative stress and lipid metabolism. Our data demonstrate that FGF1△HBS reverses NAFLD in late-stage T2D by inhibition of hepatic inflammation, oxidative stress, and lipid dysregulation. Significantly, our data identify a mechanism of FGF1△HBS protection against NAFLD through activation of hepatic adenosine monophosphate–activated protein kinase (AMPK). Furthermore, our in vivo and in vitro studies demonstrate that FGF1ΔHBS activation of nuclear erythroid 2 p45-related factor 2 (Nrf2) is essential to prevent oxidative stress, but Nrf2 activation alone is insufficient for FGF1 prevention of hepatic lipid metabolic dysregulation. Moreover, activation of AMPK is essential to the ability of FGF1ΔHBS, both to protect from dysregulated hepatic lipid metabolism and to inhibit oxidative stress.
Materials and Methods
db/db (BKS.Cg-Dock7m+/+ Leprdb/J) and apoE-KO (B6.129P2-Apoetm1Unc/J) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Nine-month-old male db/db mice were administered FGF1△HBS (0.5 mg/kg body weight) or vehicle (phosphate-buffered saline [PBS]) through intraperitoneal injection every other day for 3 months in the therapeutic study. Liver-specific AMPK knockout (AMPK-LKO) mice were generated by crossing albumin-cyclization recombination (Cre) recombinase transgenic mice with floxed AMPKα1/α2 mice, as reported.(12) WT littermates (albumin-Cre recombinase-negative, AMPKα1/α2flox/flox) were used as the control. At the age of 2 months, male mice were fed a high-fat/high-sucrose (HFHS) diet (Catalog No. D12327, Research Diets) for 5 months and then supplemented with FGF1△HBS (0.5mg/kg body weight) or PBS every other day for 1 month. Eight-week-old male apoE-KO mice were fed either a normal chow or a high-fat/high-cholesterol (HFHC) diet (Catalog No. TD88137, Envigo) to induce the development of NASH. At the same time, these mice were administered FGF1△HBS (0.5 mg/kg body weight) or PBS through intraperitoneal injection every other day for 3 months in the preventive study. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Louisville or the Shanghai Institute of Nutrition and Health of the Chinese Academy of Sciences. The human hepatocellular carcinoma (HepG2) cell line was obtained from the American Type Culture Collection. Mouse primary hepatocytes were isolated as described.(13) Mechanistic studies were performed under the optimized experimental conditions established in pilot studies (Supporting Figs. S1 and S2). Details of methods used in this study are described in the Supporting Materials and Methods.
Results
FGF1△HBS Reverses NAFLD in Late-Stage T2D Mice
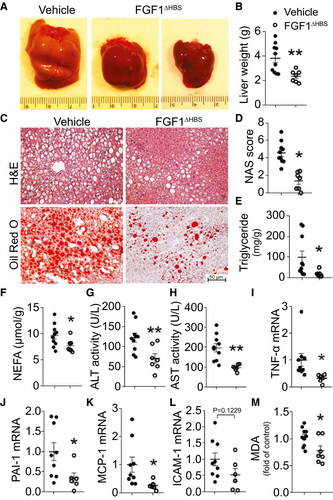
To extend our observation(10) that either FGF1WT or FGF1△HBS treatment reduces early-stage NAFLD in 2-month-old db/db mice, in the current study, we tested if FGF1△HBS could reverse more severe liver injury in late-stage T2D mice. FGF1△HBS treatment was initiated at 9 months and continued through 12 months of age in db/db mice. Nine-month-old db/db mice had obvious T2D, as indicated by high blood glucose (Supporting Fig. S3A) and body weight (Supporting Fig. S3B). Prolonged diabetes coincided with development of liver pathological characteristic of NAFLD. Parameters of NAFLD found in db/db mice included dramatically elevated liver weight (Supporting Fig. S3C), abnormal morphology quantified as NAFLD activity score (NAS) (Supporting Fig. S3D,E), and hepatic lipid accumulation indicated both by greater Oil Red O staining (Supporting Fig. S3D) and higher hepatic triglyceride contents (Supporting Fig. S3F). In addition, 9-month-old db/db mice exhibited significantly higher plasma markers of liver injury: alanine aminotransferase (ALT; Supporting Fig. S3G) and aspartate aminotransferase (AST; Supporting Fig. S3H). However, there was no obvious hepatic fibrosis in this db/db NAFLD model (data not shown), which is consistent with reports that db/db mice rarely show any features of hepatic fibrosis when fed a normal chow diet.(14, 15)
Three months of treatment with FGF1△HBS decreased diabetes, as indicated by significantly lower blood glucose (Supporting Fig. S4A), improved glucose tolerance (Supporting Fig. S4B,C), and slightly reduced body weight (Supporting Fig. S4D). Most importantly, FGF1△HBS reduced most characteristics of NAFLD: liver size and weight were much lower (Fig. 1A,B). FGF1△HBS also improved hepatic histopathology (Fig. 1C, upper panel), which was evident as reduced NAS (Fig. 1D) and decreased lipid droplet deposition (Fig. 1C, lower panel). FGF1△HBS treatment reduced hepatic triglycerides (Fig. 1E) and nonesterified fatty acid (Fig. 1F) content. In addition, FGF1△HBS reduced the plasma markers of liver injury, ALT (Fig. 1G) and AST (Fig. 1H); decreased hepatic mRNA levels for inflammatory markers, tumor necrosis factor α, plasminogen activator inhibitor 1, monocyte chemoattractant protein 1, and intercellular adhesion molecule 1 (Fig. 1I-L); inhibited hepatic superoxide generation (Supporting Fig. S4I,J); and decreased hepatic malondialdehyde (MDA) content (Fig. 1M). There was a slight increase in plasma triglyceride concentration (Supporting Fig. S4E). Values that were not significantly affected by FGF1△HBS were hepatic cholesterol content (Supporting Fig. S4F), plasma total cholesterol (Supporting Fig. S4G), and plasma low-density lipoprotein concentration (Supporting Fig. S4H). As expected, FGF1△HBS did not increase hepatic cell proliferative markers proliferating cell nuclear antigen, and Ki-67 declined (Supporting Fig. S5).
FGF1△HBS Treatment Preserves Antioxidant Activity and Normalizes Lipid Metabolism in db/db Liver
The transcription factor Nrf2 plays a critical role in the antioxidant response by up-regulating multiple antioxidant enzymes.(16) Thus, we tested whether FGF1△HBS treatment of T2D mice activated hepatic Nrf2 and Nrf2-regulated enzymes. FGF1△HBS treatment significantly increased translocation of Nrf2 to the nucleus (Fig. 2A,B) and induced known targets of Nrf2 transcriptional up-regulation, including nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) dehydrogenase (quinone 1) (Fig. 2A,C) and heme oxygenase 1 (Fig. 2A,D). These results suggest an important role of Nrf2-dependent up-regulation of antioxidant activity as part of FGF1△HBS-mediated protection from NAFLD.

To identify the mechanism responsible for improved hepatic lipid metabolism by FGF1△HBS, several important components of hepatic fatty acid synthesis and oxidation were tested. Treatment with FGF1△HBS markedly reduced the mRNA level (Fig. 2E) and transcriptional activity of transcription factor sterol regulatory element binding protein 1 (SREBP-1; as indicated by the ratio of mature SREBP-1 to precursor SREBP-1(13, 17); Fig. 2F,G). Changes in SREBP-1 were accompanied by significant reductions in its downstream targets, stearoyl-coenzyme A (CoA) desaturase 1 (SCD-1; Fig. 2F,H) and fatty acid synthase (FAS; Fig. 2F,I) at both mRNA and protein levels. In addition, FGF1△HBS treatment significantly up-regulated three proteins in fatty acid β-oxidation: peroxisome proliferator–activated receptor α (Supporting Fig. S6A), peroxisome proliferator–activated receptor gamma coactivator 1α (Supporting Fig. S6B), and carnitine palmitoyltransferase 1α (Supporting Fig. S6C) but was without effect on fatty acid transporter CD36 (Supporting Fig. S6D) expression. These results indicate that both inhibition of lipogenesis and promotion of fatty acid oxidation contributed to FGF1△HBS suppression of hepatic lipid accumulation in T2D.
AMPK is a key energy sensor that plays a critical role in hepatic lipid metabolism in diabetic mice.(18) Thus, we tested for a role of AMPK signaling in FGF1△HBS-mediated protection against hepatic steatosis. FGF1△HBS treatment significantly increased phosphorylation of hepatic AMPK (Fig. 2J,K) and up-regulated phosphorylation of the AMPK target, acetyl-CoA carboxylase (ACC; Fig. 2J,L). These data indicate that activated AMPK signaling is likely important to FGF1△HBS regulation of hepatic lipid metabolism.
In Hepatocytes, Nrf2 Is Required for FGF1 Protection From Palmitate-Induced Oxidative Stress but Not for FGF1 Protection From Palmitate-Induced Lipid Metabolic Dysregulation
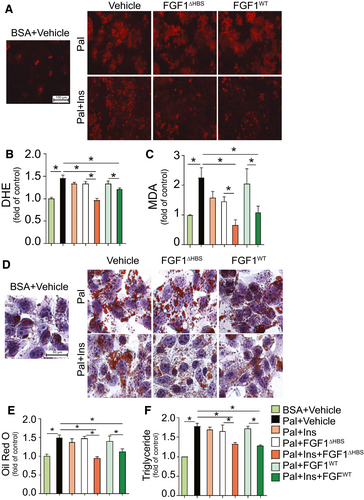
To explore the hepatocellular specific basis of FGF1 action, HepG2 cells were exposed to palmitate in vitro to mimic the diabetic milieu of oxidative damage and lipid dysregulation. Paralleling the hepatic response to diabetes in vivo (Figs. 1 and 2), palmitate exposure of HepG2 cells induced oxidative stress (Fig. 3A-C), inhibited Nrf2 nuclear translocation (Supporting Fig. S7A,B), and reduced expression of downstream antioxidant enzymes NADPH dehydrogenase (quinone 1) (Supporting Fig. S7A,C) and heme oxygenase 1 (Supporting Fig. S7A,D). Palmitate exposure of HepG2 cells augmented lipid accumulation, which was seen as increased lipid droplet formation (Fig. 3D,E) and increased triglyceride contents (Fig. 3F). Lipid accumulation coincided with activation of the lipogenic gene, SREBP-1 (Supporting Fig. S7E,F), and increased expression of the SREBP-1 target genes, FAS (Supporting Fig. S7E,G) and SCD-1 (Supporting Fig. S7E,H). Lipid accumulation also coincided with reduced phosphorylation of both AMPK (Supporting Fig. S7E,I) and ACC (Supporting Fig. S7E,J). Importantly, and as seen in diabetic livers, all of these changes that are induced by palmitate exposure of HepG2 cells were prevented by FGF1△HBS or FGF1WT treatment in an insulin-dependent manner (Fig. 3; Supporting Fig. S7).
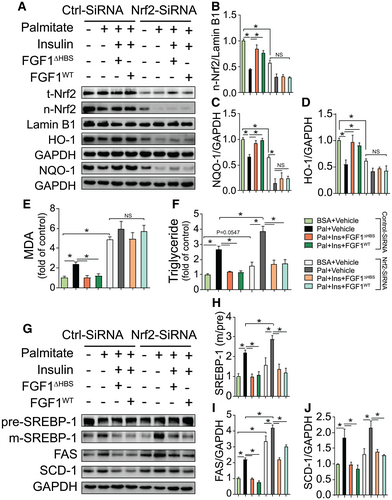
Chronic oxidative stress plays an important role in the progression of fatty liver disease, possibly because of linkage between oxidative stress and dysregulated lipid metabolism.(19) Nrf2, in addition to responding to oxidative stress, also has a critical role in lipid metabolism.(16) To test if the antioxidant and/or antisteatosis actions of Nrf2 were required for the response to FGF1 treatment, loss-of-function studies were performed using small interfering RNA (siRNA) knockdown of Nrf2 in HepG2 cells. As intended, Nrf2 siRNA greatly reduced total Nrf2 (Fig. 4A) and nuclear Nrf2 (Fig. 4A,B). Knockdown of Nrf2 was accompanied by down-regulation of Nrf2 target antioxidant genes (Fig. 4A,C,D) that resulted in a more severe oxidative stress response to palmitate exposure, as indicated by increased MDA production (Fig. 4E). Importantly, Nrf2 knockdown completely abolished the protective effects of treatment with FGF1△HBS or FGF1WT against palmitate-induced oxidative stress in HepG2 cells (Fig. 4E). However, knockdown of Nrf2 did not reduce the protective actions of either FGF1△HBS or FGF1WT treatment against lipid accumulation, as reflected by triglyceride contents (Fig. 4F) and lipogenic gene up-regulation (Fig. 4G-J). These findings indicate that FGF1 protection from hepatic oxidative stress requires Nrf2, but in contrast, Nrf2 is unnecessary for FGF1-mediated protection from dysregulated lipid metabolism.
In Hepatocytes, AMPK Is Required for FGF1 Protection From Both Palmitate-Induced Lipid Metabolic Dysregulation and Palmitate-Induced Oxidative Stress
Studies in T2D indicated that hepatic metabolic dysregulation could be prevented by AMPK activation.(20, 21) Based on our findings that FGF1 activates AMPK in vivo (Fig. 2) and in vitro (Fig. 4), we wondered whether AMPK was required for the protective effects of FGF1 against liver damage in T2D. To test this hypothesis, HepG2 cells were pretreated with Compound C, a selective AMPK inhibitor. Compared with dimethyl sulfoxide vehicle control, Compound C treatment significantly reduced AMPK expression (Supporting Fig. S8A,B) and phosphorylation (Supporting Fig. S8A,C). This was accompanied by reduced phosphorylation of the AMPK target, ACC (Supporting Fig. S8A,D) and greater maturation of SREBP-1 (Supporting Fig. S8A,E). Furthermore, inhibiting AMPK with Compound C significantly aggravated palmitate-induced lipid accumulation, as shown by increased triglyceride contents (Supporting Fig. S8F) and abolished the ability of either FGF1△HBS or FGF1WT treatment to inhibit lipid accumulation (Supporting Fig. S8F) and block up-regulation of lipogenic genes (Supporting Fig. S8A,G,H). Surprisingly, Compound C inhibition of AMPK also exacerbated palmitate-induced oxidative stress in the absence or presence of FGF1 (Supporting Fig. S8I). This was evident by more MDA production (Supporting Fig. S8I), nearly complete attenuation of Nrf2 nuclear translocation (Supporting Fig. S8J,L), and reduced expression of Nrf2 downstream antioxidant genes (Supporting Fig. S8J,M,N). These changes occurred without significant effects on total Nrf2 expression (Supporting Fig. S8J,K). These results demonstrated that pharmacological inhibition of hepatic AMPK blocked FGF1 protection against both palmitate-induced lipid metabolic dysregulation and palmitate-induced oxidative stress.
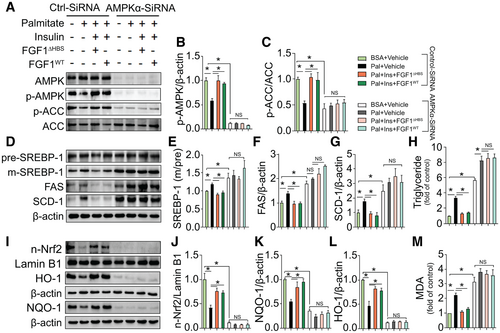
To exclude potential nonspecific actions of Compound C and to eliminate differences between HepG2 cells and primary hepatocytes, we next performed a loss-of-function study using AMPKα-siRNA in primary mouse hepatocytes. Compared with control siRNA, AMPKα-siRNA knockdown of AMPKα almost completely eliminated AMPK expression (Fig. 5A) and phosphorylation (Fig. 5A,B), which was accompanied by reduced phosphorylation of the AMPK downstream target gene, ACC (Fig. 5A,C). Furthermore, knockdown of AMPKα markedly elevated palmitate-induced lipid accumulation, which was reflected by increased triglyceride contents (Fig. 5H), and completely abolished the protective effects of both FGF1△HBS and FGF1WT against lipid accumulation (Fig. 5H) and lipogenic genes up-regulation (Fig. 5D-G). Importantly, knockdown of AMPKα also aggravated palmitate-induced oxidative stress and completely abolished the protective effects of both FGF1△HBS and FGF1WT against oxidative stress induced by palmitate in primary hepatocytes, reflected by greater MDA production (Fig. 5M). These results coincided with a near complete attenuation of Nrf2 nuclear translocation (Fig. 5I,J) and less expression of Nrf2 downstream antioxidant genes (Fig. 5I,K,L) under basal and FGF1-stimulated conditions. These results support the notion that AMPK plays a central role in FGF1-mediated protection from palmitate-induced dysregulation of hepatic lipid metabolism and hepatic oxidative stress.
In Hepatocytes, FGF1 Activation of AMPK and Protection From Palmitate-Induced Lipid Accumulation Is Mediated by FGFR4
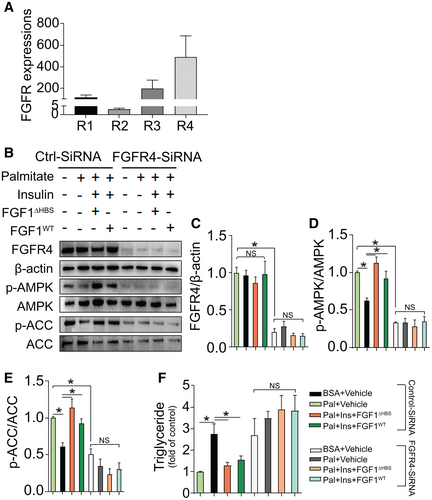
Among four major subtypes of FGFRs, FGFR4 was most abundant in isolated primary hepatocytes, followed by FGFR3 and FGFR1 (Fig. 6A). We next explored the role of FGFR4 in the hepatic actions of FGF1 on AMPK activation and lipid accumulation by knocking down FGFR4 expression using siRNA. Compared with control siRNA, FGFR4-siRNA markedly reduced FGFR4 expression (Fig. 6B,C), which was accompanied by dramatically reduced phosphorylation of AMPK (Fig. 6B,D) and its downstream target gene, ACC (Fig. 6B,E), in the absence or presence of FGF1. Notably, knockdown of FGFR4 elevated palmitate-induced lipid accumulation, which was reflected by increased triglyceride contents (Fig. 6F), and completely abolished the protective effects of both FGF1△HBS and FGF1WT against triglyceride accumulation (Fig. 6F). Taken together, these findings suggest that the effects of FGF1 on hepatic AMPK activation and lipid accumulation are predominantly mediated by FGFR4.
AMPK-LKO Abolishes FGF1△HBS Reversal of NAFLD in HFHS-Fed Mice
To ensure that the in vitro findings mentioned also apply in NAFLD in vivo, studies with AMPK-LKO mice were performed. AMPK-LKO and WT mice were fed HFHS diet for 5 months, followed by FGF1△HBS (0.5 mg/kg body weight) or PBS treatment every other day for 1 month. The complete knockout of AMPK in the liver was verified by immunoblots (Fig. 7A). As expected, FGF1△HBS treatment significantly up-regulated the phosphorylation of hepatic AMPK (Fig. 7A,B) and its downstream target ACC (Fig. 7A,C) in WT mice. But in AMPK-LKO mice, the expression and phosphorylation of AMPK (Fig. 7A,B) and its downstream target ACC (Fig. 7A,C) were almost completely abolished in the absence or presence of FGF1△HBS. Similar to FGF1△HBS protection observed in db/db mice (Fig. 1), FGF1△HBS treatment markedly reversed chronic HFHS diet feeding-induced liver steatosis in WT mice as evident in hematoxylin and eosin–stained and Oil Red O–stained liver sections (Fig. 7D,E), liver triglyceride contents (Fig. 7F), reduced hepatic oxidative stress (Fig. 7G), and plasma markers of liver injury (Fig. 7H,I). Although hepatic AMPKα deficiency did not notably aggravate the HFHS diet–induced steatosis phenotype compared with HFHS-fed WT mice, AMPKα deficiency significantly reduced the protective effects of FGF1△HBS against hepatic lipid accumulation (Fig. 7E,F), MDA production (Fig. 7G), and plasma markers of liver injury (Fig. 7H,I). The ALT effect of FGF1△HBS (Fig. 7H) was almost normal, perhaps because ALT can also come from nonhepatic tissues. Taken together, these findings strongly support an essential role of AMPK in mediating the protective effects of FGF1 that reduce both hepatic steatosis and liver injury.

FGF1△HBS Attenuates the Development of NASH in HFHC Diet–Fed ApoE-KO Mice
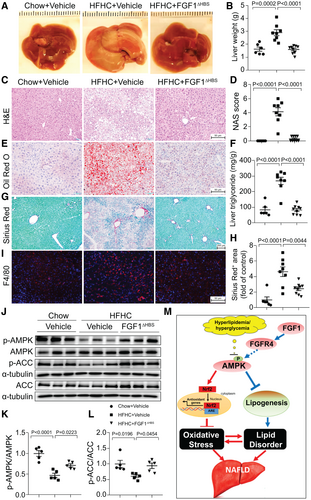
To investigate the effects of FGF1△HBS on NASH, an HFHC diet–induced apoE-KO mouse model was used.(14, 22) Compared with normal chow-fed apoE-KO mice, HFHC-fed mice had obvious morphological and pathological features of NASH, including increased liver size and weight (Fig. 8A,B), increased NAS (Fig. 8C,D), elevated hepatic lipids accumulation (Fig. 8E,F, Supporting Fig. S9A,B), aggravated hepatic fibrosis indicated by collagen deposition (Fig. 8G,H) and fibrotic marker expression (Supporting Fig. S10A), and increased hepatic inflammation shown by macrophage infiltration (Fig. 8I) and hepatic inflammatory marker expression (Supporting Figs. S10B). These changes were accompanied by dramatic elevation of the plasma markers of liver injury: ALT and AST (Supporting Fig. S9F,G). Strikingly, FGF1△HBS treatment almost completely prevented the progression of HFHC-induced NASH in apoE-KO mice (Fig. 8A-H, Supporting Figs. S9 and S10) that coincided with preservation of hepatic AMPK signaling (Fig. 8J-L). FGF1△HBS treatment also significantly decreased plasma concentrations of total cholesterol (Supporting Fig. S9C) and low-density lipoprotein fraction (Supporting Fig. S9D) but without significant effect on plasma triglycerides (Supporting Fig. S9E). In line with the hepatic proliferation results in db/db mice, FGF1△HBS significantly reduced expression of cell proliferative markers proliferating cell nuclear antigen and Ki-67 (Supporting Fig. S11) in livers of apoE-KO mice. These results clearly demonstrate that FGF1△HBS can protect against NASH without stimulating hepatic proliferative potential in mice.
Discussion
T2D and NAFLD share common characteristics and risk factors, resulting in a high prevalence of NAFLD among individuals with T2D.(23) Because the severity of NAFLD is exacerbated by concurrent T2D, the lack of effective NAFLD treatments is most serious for patients with both NAFLD and T2D. The discovery in mouse models that FGF1 has positive effects on adipose remodeling,(6) insulin resistance,(7) hyperglycemia,(7) and steatosis(11) suggests unique potential for FGF1 as a treatment for patients with NAFLD, T2D, or both conditions. However, FGF1 has mitogenic properties that elevate cancer risk and present a major obstacle to FGF1’s therapeutic use in metabolic diseases.(4) We have reported that FGF1△HBS, a modified version of native FGF1, has markedly lower mitogenic activity but retains full metabolic activity against diabetes with respect to lowering blood glucose, restoring insulin sensitivity, and preventing hepatic lipid accumulation.(10) Here, we demonstrate that nonmitogenic FGF1△HBS is similarly effective as native FGF1 in vitro and in vivo for preventing hepatic oxidative stress, inhibiting lipogenesis, reducing lipid accumulation, and decreasing inflammation. Furthermore, our mechanistic studies in hepatic cells and AMPK-LKO mice reveal that FGF1△HBS induction of antioxidant and metabolic responses requires hepatic AMPK activation through FGFR4. Moreover, nonmitogenic FGF1△HBS is able to ameliorate HFHC diet–induced NASH with hepatic fibrosis.
The most clinically relevant findings of this study are that FGF1△HBS treatment reverses NAFLD in the late stage for db/db T2D mice (Fig. 1) and prevents HFHC diet–induced NASH in apoE-KO mice. Protection from liver steatosis by FGF1△HBS had been shown only in 3-month-old db/db mice.(10) This conclusion that FGF1△HBS can reverse NAFLD in the late stage for db/db T2D mice is based on the efficacy of FGF1△HBS to reduce multiple parameters of NAFLD in 9-12-month-old db/db mice. The improved parameters include liver weight, size, lipid accumulation, inflammation, and oxidative stress as well as plasma ALT and AST. Because NAFLD is usually diagnosed in patients long after NAFLD begins, the ability to reverse pre-existing disease is a likely prerequisite to clinical value of any new treatment in this large population of patients.
A study demonstrated that native FGF1 could prevent hepatic steatosis and inflammation in ob/ob NAFLD mice. However, in choline-deficient, L-amino acid-defined, diet-induced NASH mice, FGF1 could only reduce hepatic inflammation, but it could not prevent hepatic steatosis and fibrosis.(11) The inability of FGF1 to prevent hepatic steatosis in the choline-deficient, L-amino acid-defined, diet-induced model may have been due to the defect in hepatic lipid β-oxidation produced by choline deficiency.(11) Herein, we tested the anti-NASH effects of FGF1△HBS in an HFHC diet–induced apoE-KO model. The results clearly indicate that FGF1△HBS can protect from NASH in this model, as indicated by prevention of hepatocellular ballooning, steatosis, inflammation, and fibrosis (Fig. 8). The abilities of FGF1△HBS to prevent NASH and reverse established NAFLD in mouse models provide a strong impetus to continue the evaluation of its clinical therapeutic potential.
Because FGF1 effectively lowers blood glucose in obese T2D mice,(6, 7, 10) an adequate mechanism to explain FGF1 protection against NAFLD may simply be that it normalizes elevated levels of blood glucose. However, recent studies demonstrate benefits of FGF1 on diabetic complications that do not depend on reduced hyperglycemia. Our group reported that FGF1 ameliorated diabetic nephropathy in type 1 diabetic mice despite no measurable effect on blood glucose,(8) and studies have shown that FGF1 exhibited protective effects against diabetic cardiomyopathy in type 1 diabetic mice without lowering blood glucose.(24) To assess whether FGF1 could protect liver cells by mechanisms independent of ambient glucose, we used cultures of hepatic cells exposed to palmitate to mimic diabetic-induced or obesity-induced hyperlipidemia. The results demonstrate that treatment of cultured hepatocytes with FGF1WT or FGF1△HBS protects human HepG2 cells (Fig. 3; Supporting Fig. S7) and mouse primary hepatocytes (Figs. 5 and 6) from palmitate-induced oxidative stress and lipid disorders, independent of changes in exposure to glucose. Based on these direct actions on hepatocytes, it is evident that FGF1△HBS has actions independent of lowering blood glucose, which are integral to its ability to protect from NAFLD.
Oxidative stress is elevated in diabetic liver, and the progression of NAFLD pathology is accelerated by oxidative stress.(19) Therefore, reducing hepatic oxidative stress is an obvious therapeutic strategy to attempt to slow NAFLD onset in diabetes. In the present study, we observed that FGF1△HBS administration to db/db mice inhibited hepatic superoxide generation and MDA production (Fig. 1; Supporting Fig. S4), indicating a hepatic antioxidant response. Nrf2-mediated signaling plays a critical role in activating the antioxidant response by up-regulating multiple antioxidant enzymes.(16) In livers of db/db mice, FGF1△HBS treatment activated Nrf2 signaling, as indicated by increased nuclear translocation of Nrf2 and up-regulation of the Nrf2 regulated antioxidant genes, heme oxygenase 1, and NADPH dehydrogenase (quinone 1) (Fig. 2). These findings implicate hepatocyte Nrf2 as a key mediator of FGF1△HBS protection against NAFLD associated oxidative stress in T2D.
The same liver samples that revealed FGF1△HBS-induced activation of Nrf2 signaling also demonstrated improved lipid metabolism: compared with untreated db/db liver samples, FGF1△HBS treated samples had much less lipid accumulation (Fig. 1), inhibition of lipogenic transcription factor SREBP-1, and lower expression of SREBP-1 target genes, FAS and SCD-1 (Fig. 2). Transcription factor Nrf2 not only up-regulates expression of antioxidant genes but can also regulate expression of lipid metabolism genes, leading to reduced lipogenesis and lipid accumulation.(16, 25) Because known transcriptional actions of Nrf2 can mediate major antioxidant and lipid metabolic actions in db/db liver, we hypothesized that all protective actions of FGF1△HBS against NAFLD in db/db mice may be mediated by Nrf2. To test this hypothesis, Nrf2-siRNA knockdown studies were performed in HepG2 cells. These results showed that knockdown of Nrf2 completely abolished the ability of FGF1WT and FGF1△HBS to ameliorate palmitate-induced oxidative stress and prevent palmitate-induced inhibition of antioxidant gene expression (Fig. 4). However, knockdown of Nrf2 had no significant effect on the ability of FGF1WT or FGF1△HBS to ameliorate palmitate-induced lipid accumulation or prevent palmitate-induced modulation of lipogenic gene expression (Fig. 4). These findings indicate that both FGF1WT and FGF1△HBS prevent hepatic oxidative stress by activating Nrf2 signaling but that their antisteatosis effects are independent of Nrf2 activation.
The fact that Nrf2 signaling does not mediate antisteatosis effects of FGF1 leads to our second innovative finding that AMPK is a key downstream target for FGF1 protection against NAFLD. AMPK is a central metabolic sensor and regulator with a major role in many metabolic diseases. Activation of AMPK stimulates hepatic fatty acid oxidation and ketogenesis and inhibits cholesterol synthesis, lipogenesis, and triglyceride synthesis.(26, 27) In addition, AMPK also acts as a redox sensor and regulator of redox balance through activation of multiple target proteins.(27, 28) Several recent studies demonstrate crosstalk between Nrf2 and AMPK under different pathophysiological conditions.(29, 30) In the present study, FGF1△HBS administration up-regulated the phosphorylation of AMPK and ACC and inhibited the transcription activity of SREBP-1 and the expression of its downstream target genes, FAS and SCD-1, in the liver of late-stage db/db mice (Fig. 2) and HFHS-fed mice (Fig. 7). Based on these findings, we hypothesized that FGF1 might reverse NAFLD in T2D by up-regulating AMPK-mediated lipid metabolic and antioxidative pathways. Consistent with this hypothesis, pharmacological inhibition or gene-specific knockdown of AMPK in human HepG2 cells or mouse primary hepatocytes eliminated the ability of FGF1WT or FGF1△HBS to suppress palmitate-induced lipid deposition and oxidative stress (Fig. 5; Supporting Fig. S8). AMPK inhibition or knockdown also eliminated the ability of FGF1WT and FGF1△HBS to inhibit SREBP-1–mediated lipogenic signaling and protect Nrf2-mediated antioxidative signaling (Fig. 5; Supporting Fig. S8). Most importantly, liver-specific knockout of AMPK completely abolished FGF1△HBS reversal of NAFLD and hepatic lipogenic signal derangement (Fig. 7). All these findings support an essential role of AMPK in the protective antioxidant and lipid metabolic responses of FGF1 treatment in NAFLD. Interestingly, a recent study showed that activation of AMPK by an aminopropyl carbazole compound induces FGF1 in an HFD-induced NAFLD mouse model,(31) suggesting a possible feedback mechanism by which FGF1 is able to activate AMPK and then AMPK also up-regulates FGF1 under metabolic disorder conditions. This potential feedback mechanism and its relevance to the pathogenesis and clinical treatment of NAFLD is worthy of further investigation.
There are at least seven FGFR proteins generated from FGFR1, FGFR2, FGFR3, and FGFR4 genes by alternative splicing.(32) FGF1 is considered a universal ligand because it can bind and activate all of the receptors.(4, 7, 33) In the present study, we found FGFR1, FGFR2, FGFR3, and FGFR4 isoforms in primary hepatocytes with highest abundance for FGFR4 (Fig. 6). However, the exact roles of FGFR4 in whole animal and liver metabolism remain controversial.(34, 35) A study found that global FGFR4-deficient mice exhibited systemic features of metabolic syndrome even on a normal diet.(34) Surprisingly, the same global FGFR4 deficiency alleviated HFD-induced fatty liver, whereas restoration of FGFR4 in hepatocytes restored sensitivity to HFD-induced fatty liver. In the current study, siRNA knockdown of FGFR4 in mouse primary hepatocytes (Fig. 6) markedly abolished FGF1 activation of AMPK and its downstream signaling and blunted FGF1 protection against palmitate-induced lipid accumulation. These findings demonstrate, at least in the hepatocyte, that FGFR4 mediates the direct protective actions of FGF1 against lipotoxicity. However, based on the earlier findings(34, 35) in global FGFR4-deficient mice, FGFR4 in different organs may exert complex (indirect) effects on the liver.
There are several limitations in our study. First, our observations are solely based on rodent models. In light of the fact that there is a difference in hepatic lipid metabolism between rodents and humans, the pathophysiological relevance of our findings remains to be confirmed in large animals and in clinical studies. Second, our data demonstrate that the lipid-lowering action of FGF1 requires hepatocyte FGFR4 to activate AMPK, but the complete signaling pathway and the defects that lead to impaired lipid metabolism in NAFLD need further investigation. Third, although our data demonstrate FGF1 reversal of palmitate-induced triglyceride accumulation in an insulin-dependent manner in hepatic cells in vitro, how insulin sensitizes this effect of FGF1 in vivo remains to be investigated in future studies.
In summary, our findings indicate that the nonmitogenic variant FGF1△HBS exhibits therapeutic effects against NAFLD in the T2D and HFHS model and NASH in the HFHC model through effectively improving hepatic lipid metabolism while inhibiting hepatic oxidative stress, inflammation, and fibrosis. Thus, FGF1△HBS has great potential for treatment of NAFLD and NASH with low risk of cancer. Importantly, our studies provide insight into the mechanism(s) by which FGF1△HBS prevents hepatic oxidative stress and steatosis in T2D. AMPK activates Nrf2-mediated antioxidative pathways and inhibits lipogenic pathways and thus is essential for FGF1 protection against NAFLD in T2D and obesity (Fig. 8M). These results advance the application of FGF1△HBS toward clinical use and reveal the potential of AMPK activation for protection from NAFLD and NASH.
Acknowledgment
We acknowledge the technical support of Drs. Jianxiang Xu and Jing Chen and the insightful suggestions from Drs. Wenke Feng and Leah J. Siskind.
Author Contributions
Q.L., Y.L., Y.T. conceived and designed the study. Q.L., X.F., X.Y., and H.S. performed in vitro cell culture, cell biology and molecular biology experiments. Q.L., Z.H., and H.S. performed in vivo therapeutic studies in mice. Q.L., G.C., Z.L., and Z.Z. performed in vivo loss of function mouse studies. Q.L., G.C., Z.L., and Z.Z. performed histology analysis. Q.L., G.C., M.K., L.C., Y.L., and Y.T., analyzed the data. Z.H., Jingya Li, Jia Li, M.-H.Z., and M.M. provided critical materials. Q.L., Y.L., and Y.T. wrote the manuscript. D.J.C. P.N.E., K.A.W., M.M., and X.L. edited the manuscript with important intellectual input. Y.L. and Y.T. supervised this study.




