Prospective Multicenter Study of Early Antiviral Therapy in Liver and Kidney Transplant Recipients of HCV-Viremic Donors
Abstract
Background and Aims
Organs from hepatitis C virus (HCV)-viremic donors have been used in HCV-uninfected recipients (D+/R-), but the optimal treatment approach has not been defined. We evaluated the kinetics of HCV infection following transplant in D+/R- kidney-transplant (KT) and liver-transplant (LT) recipients when a preemptive antiviral strategy was used.
Approach and Results
Six US transplant programs prospectively treated D+/R- primary LT and KT recipients with sofosbuvir-velpastasvir for 12 weeks starting once viremia was confirmed following transplant and the patients were judged to be clinically stable, including estimated glomerular filtration rate >30 mL/min. Primary endpoints were sustained virologic response at 12 weeks following transplant and safety (assessed by proportion of treatment-related adverse and serious adverse events). Of the 24 patients transplanted (13 liver, of whom 2 had prior-treated HCV infection; 11 kidney), 23 became viremic after transplant. The median (interquartile range) time from transplant to start of antiviral therapy was 7.0 (6.0, 12.0) versus 16.5 (9.8, 24.5) days, and the median (interquartile range) HCV-RNA level 3 days after transplant was 6.5 (3.9, 7.1) versus 3.6 (2.9, 4.0) log10 IU/mL in LT versus KT recipients, respectively. By week 4 of treatment, 10 of 13 (77%) LT, but only 2 of 10 (20%) KT, had undetectable HCV RNA (P = 0.01). At the end of treatment, all LT recipients were HCV RNA–undetectable, whereas 3 (30%) of the kidney recipients still had detectable, but not quantifiable, viremia. All achieved sustained virologic response at 12 weeks following transplant (lower 95% confidence interval bound: 85%). Serious adverse events considered possibly related to treatment were antibody-mediated rejection, biliary sclerosis, cardiomyopathy, and graft-versus-host disease, with the latter associated with multiorgan failure, premature treatment discontinuation, and death.
Conclusions
Despite differing kinetics of early HCV infection in liver versus non-liver recipients, a preemptive antiviral strategy is effective. Vigilance for adverse immunologic events is warranted.
Abbreviations
-
- AE
-
- adverse event
-
- ALT
-
- alanine aminotransferase
-
- D+/R-
-
- donor positive/recipient negative
-
- eGFR
-
- estimated glomerular filtration rate
-
- GVHD
-
- graft-versus-host disease
-
- HCV
-
- hepatitis C virus
-
- IQR
-
- interquartile range
-
- KDPI
-
- kidney donor profile index
-
- LLOQ
-
- lower limit of quantification
-
- MELD
-
- Model for End-Stage Liver Disease
-
- NAT
-
- nucleic-acid test
-
- PRO-ACT
-
- Prevention of De Novo HCV with Antiviral HCV Therapy Post-Liver and Post-Kidney Transplant Recipients
-
- SAE
-
- serious adverse event
-
- SOF-VEL
-
- sofosbuvir-velpatasvir
-
- SVR
-
- sustained virologic response
-
- TEAE
-
- treatment-emergent AE
-
- UNOS
-
- United Network of Organ Sharing
The disparity between the number of patients listed for solid organ transplants and the number of potential available organs continues to widen. Strategies to increase the organ supply include the use of organs that are suboptimal, but in which the risk/benefit ratio remains favorable when compared with the risk for the patient remaining on the transplant list without an organ. Expanded criteria donors have been used for decades, with the boundaries on what is acceptable pushed to maximize recipient access to organs and minimize donor organ discard. Donors with acute (e.g., bacterial infection) or chronic infections (hepatitis viruses) may be considered suitable donors if an effective therapy to prevent or treat the infection in the recipient is available. The use of hepatitis C virus (HCV) infected donors in HCV-negative recipients is the latest donor/recipient pairing to gain traction in North America. This reflects three converging phenomena: (1) a marked increase in number of HCV-viremic donors available that are related to the epidemic of drug use and overdose deaths, (2) the availability of safe and highly effective antivirals to prevent or treat HCV infection, and (3) the decreasing number of HCV-viremic recipients on the waiting list, largely reflective of the high cure rates with current antivirals.
In the short span of 2 years, the use of HCV-viremic donors in HCV-negative solid organ transplant recipients (HCV D+/R-) has rapidly evolved,(1) although important questions remain regarding the optimal therapeutic approach. Single-center experiences highlight high rates of posttransplant HCV eradication among recipients of HCV-viremic donors, but also report potential direct and indirect consequences of HCV viremia.(2) Different strategies may be needed for liver versus non-liver recipients. Broadly, the treatment approaches for preventing the consequences of HCV infection in recipients can be categorized as prophylactic (treatment given to prevent posttransplant viremia), preemptive (treatment started very early following transplant, before any clinical manifestations), or delayed (typically weeks to months after transplant, when clinical disease may be present). There is robust argument in the transplant community regarding the beneficial or detrimental effects of using prophylactic and preemptive approaches versus delayed. Understanding the kinetics of early viral infection in the recipient, the response to antiviral therapy and the immunologic and clinical consequences of viremia in the recipient are relevant to the debate.
PRO-ACT (Prevention of De Novo HCV with Antiviral HCV Therapy Post-Liver and Post-Kidney Transplant Recipients) is a multicenter prospective study evaluating the safety and efficacy of the pangenotypic regimen, sofosbuvir-velpatasvir (SOF-VEL), for 12 weeks in HCV-uninfected liver and kidney transplant recipients with an HCV-viremic donor (ClinicalTrials.gov No. NCT03619837). In PRO-ACT, a preemptive therapeutic approach was evaluated, with antiviral therapy started early following transplant, once viremia in the recipient was confirmed.
Methods
Study Oversight
The study protocol was reviewed and approved by the relevant institutional review boards of participating centers. The study was performed in accordance with the Declaration of Helsinki, the principles of Good Clinical Practice, and local regulatory requirements. The trial was designed and conducted by the site-study investigators, with provision of study drug and support for study procedures from Gilead Sciences (Foster City, CA). Trial data management was contracted to United Network of Organ Sharing business services with regular monitoring of trial conduct. An independent data and safety monitor reviewed the progress of the trial. All listed authors had access to the data and assumed responsibility for the integrity and completeness of the reported data from their centers and the fidelity of the trial to the protocol.
Study Design and Participants
This is a prospective multicenter, open-label, proof-of-concept study conducted at six US transplant centers (Supporting Table S1). The study sought to enroll up to 30 study patients undergoing liver and/or kidney transplantation, at least 10 of whom were undergoing liver transplant and at least 10 were undergoing kidney transplantation. Study enrollment was stopped after 24 study patients were enrolled, due to slower than expected enrollment, reflecting the broader use of HCV-viremic donors outside of study protocol. Additionally, it was determined that the study’s main findings would not be expected to change substantially by the enrollment of 6 additional patients.
Eligible patients were adults (age ≥18 years) on the waiting list for kidney or liver (alone or combined with kidney) transplant at one of the study sites. Eligible patients were without current HCV viremia at enrollment, although patients with a prior history of chronic HCV who were previously treated and HCV RNA–negative at least 12 weeks after completing treatment were eligible. As sofosbuvir was not approved in patients with estimated glomerular filtration rate (eGFR) < 30 mL/min at the time of the study start, recipients who were anticipated to have a need for prolonged dialysis following transplant, thereby delaying the start of SOF-VEL, were excluded. Patients treated with recent amiodarone before organ transplant were excluded due to risk of bradyarrhythmia when administered with sofosbuvir. In addition, individuals needing to continue other drugs with potential to interact with SOF-VEL, such as high-dose proton-pump inhibitors, were not eligible (see Supporting Table S2 for all inclusion/exclusion criteria). Finally, as the intent of the protocol was to offer transplant to patients with anticipated long waiting times, all site investigators were encouraged to particularly enroll those patients not expected to get access to a deceased donor during the study period. Recipients with a living donor were excluded. For similar reasons, kidney transplant candidates who were on dialysis more than 5 years at the time of screening were excluded, as well as those with panel-reactive antibody of more than 80%.
Deceased donors were tested for HCV-RNA positivity per protocol by the local organ procurement organizations using a sensitive nucleic-acid test (NAT) with reported qualitative results (positive vs. negative). Additional donor testing confirming the absence of human immunodeficiency virus (HIV) and hepatitis B surface antigen (HBsAg) was required. Finally, kidney donors were required to have a kidney donor profile index (KDPI) of ≤85%. Liver donors were required to have an absence of significant fibrosis (<F2 on scale of 4). These restrictions were used to reduce the use of donor organs of suboptimal quality.
Study Procedures
All participants underwent a two-part informed consent prior to any study procedures. The first part was signed at the time of screening on the wait list, and the second within 24 hours before the transplant event. Review of the potential risks and benefits of participating in this trial included a discussion regarding HCV treatment and complications of unsuccessfully treated HCV following transplant, including graft loss, fibrosing cholestatic hepatitis, cirrhosis, and death. In addition to reconfirming patient consent, study investigators also assessed and confirmed recipient and donor eligibility in the 24 hours before transplant. Transplant-related procedures at each center were performed as local standard of care, including immunosuppression protocols.
Recipient HCV RNA was checked on postoperative days 3 and 7, and every 7 days until detected. Once recipient HCV viremia was detected and study staff deemed the subject clinically stable to start antiviral therapy, SOF-VEL (400 mg/100 mg) was administered once daily. Day 1 was defined as the day that the study drug commenced. The total treatment duration was 12 weeks. If participants missed a dose (or doses) of study drug, they would continue to complete the full 84 days of study drug. For patients who did not achieve viral eradication with this 12-week course of therapy, retreatment with sofosbuvir-velpatasvir-voxilaprevir (400/100/100 mg) once daily for 12 weeks was available.
Assessments
Screening assessments of the patients included demographics, serologies for HCV, hepatitis B virus and HIV, standard laboratory testing, and clinical assessments. Liver transplant candidates had laboratory tests for Model for End-Stage Liver Disease (MELD)-Na, MELD exception score (if any), and Child-Pugh score. For kidney transplant candidates, the panel-reactive antibody result was obtained. Donor variables included age, sex, cause of death, United Network of Organ Sharing (UNOS) region, HBsAg, antibody to hepatitis B core antigen, KDPI, and fibrosis stage (for livers).
For recipients who had quantifiable HCV RNA, SOF-VEL was started when patients were deemed clinically stable to start antiviral therapy and had an eGFR > 30 mL/min. On-treatment HCV-RNA levels were measured at weeks 1 and 4, end of treatment, and weeks 4, 12, and 24 after completing therapy. For recipients who were HCV RNA–negative on day 7 after transplant, HCV RNA was retested weekly until day 28 and then monthly for 2 additional months.
The schedule for measurement of blood cell count, renal function, liver tests, as well as immunosuppression during treatment and follow-up are provided in Supporting Table S3.
Outcomes
The primary outcome was sustained virologic response, defined as HCV RNA below the lower limit of quantification 12 weeks after treatment completion (SVR12). Secondary outcomes included the proportion of patients with SVR24, defined as HCV RNA < lower limit of quantification (LLOQ) 24 weeks after the end of treatment. Viral relapse was defined as HCV RNA < LLOQ at end of treatment with subsequent quantifiable HCV RNA and on-treatment virologic breakthrough was defined as >1-log increase in viral RNA after treatment week 1.
Safety was measured as the adverse events (AEs) and serious adverse events (SAEs) attributed by the investigator to HCV infection or antiviral therapy; the proportion of recipients who prematurely discontinued antiviral therapy before the planned end of treatment; and patient and graft survival at 6 months following transplant. An exploratory endpoint was the change in wait time for kidney and/or liver transplant based on comparison with UNOS data for the participating regions.
AEs were graded using the Common Terminology Criteria for Adverse Events, Version 4.03, published June 2010. The investigator was responsible for assessing AEs and SAEs for causality and severity. Treatment-emergent AEs (TEAEs) were assessed for relationship to study drug and severity. TEAEs leading to premature discontinuation of the study drug were captured. AEs were recorded for all patients who received a NAT-positive HCV organ within 4 weeks after the last dose of study drug. SAEs were reported to the lead investigators and the independent data and safety monitor within 24 hours of recognition by study staff. SAEs were collected for the entire duration of the study. After the 10th study patient had reached treatment week 4, the lead investigators and the independent data and safety monitor conducted a safety and efficacy review of all available data. When these same patients reached follow-up week 12 (corresponding to 24 weeks after starting the study drug), the lead investigators and the independent data and safety monitor conducted another safety and efficacy review.
Statistical Analysis
No formal hypothesis testing was performed, as this is a pilot study. No inferential statistics or statistical comparisons were planned for efficacy endpoints. All participants who underwent liver and/or kidney transplant with an HCV NAT-positive organ were included in the analysis. If a quantitative PCR was missing, the value was imputed as LLOQ if the viral load before and after were both LLOQ. We assumed that none of the transplant recipients would achieve SVR12 without any antiviral therapy. The existing data for SOF/VEL have demonstrated an SVR rate above 94% in patients without cirrhosis. Along with the percentage of patients with SVR12, a two-sided 95% confidence interval was constructed using the Clopper-Pearson method. With a sample size of 24 patients, if the SVR12 rate was 95%, the lower and upper bound of the 95% CI would be 82% and 100%, respectively.
Treatment-emergent data from the first dose of study drug through the date of the last dose of study drug plus 30 days were included. TEAEs were summarized by relationship to study drug and severity. In addition, TEAEs leading to premature discontinuation of the study drug were summarized.
HCV-RNA kinetics were described as the rate of HCV-RNA log10 change per week (1) from treatment start to 1 week on-treatment and (2) from 1 week to 4 weeks on-treatment. These intervals were selected based on the biphasic decline in viral load and the largely undetectable viral load by week 4 for liver recipients, effectively resulting in a slope of zero after 4 weeks. The distribution of the rate of HCV-RNA log10 was reasonably normal. Therefore, HCV-RNA kinetics were evaluated as means (SDs) and compared by organ using linear regression. To account for differences in HCV RNA at the start of the evaluation period (treatment start or week 1), the comparison was adjusted for HCV RNA at the corresponding start time. For comparison of proportions achieving undetectable HCV RNA at weeks 1 and 4 of treatment, missing values were assumed to be HCV RNA–detectable. Analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Disposition
From July 13, 2018, to December 21, 2019, 122 patients provided the first part of the informed consent and were enrolled in this trial, and 24 (20%) of these received organs from HCV-viremic donors (Fig. 1): 13 liver transplants and 11 kidney transplants. Of the remaining eligible patients, 47 (39%) received organs from HCV-negative donors. For potential liver recipients, 13 of 74 (18%) were transplanted with organs from HCV-viremic donors, and 36 of 74 (49%) were transplanted with organs from HCV-negative donors. Two potential liver recipients died on the wait list. The median (interquartile range [IQR]) time from activation to receiving an HCV-viremic liver was 116 (56, 169) days.
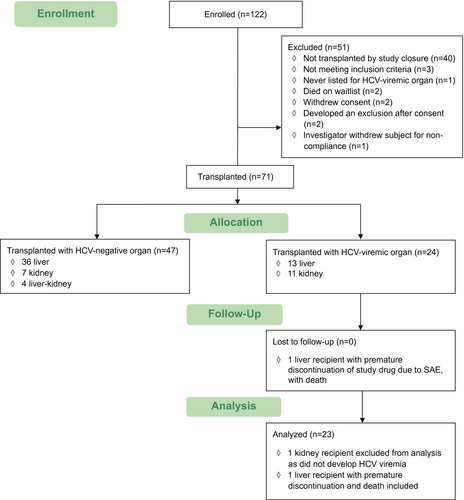
For potential kidney recipients, 11 of 40 (28%) were transplanted with organs from HCV-viremic donors, and 7 of 40 (18%) were transplanted with organs from HCV-negative donors. The median (IQR) time from activation to being able to receive an HCV-viremic kidney to transplant with an HCV-viremic kidney was 48 (34, 124) days. None of the listed liver-kidney candidates received HCV-viremic donors.
Demographics and Transplant Data
Baseline characteristics of the liver and kidney recipients of HCV-viremic organs are found in Table 1. All of the donors were located in the same UNOS regions as their recipient. The etiologies of end-stage liver disease were nonalcohol steatohepatitis (n = 5, 38%), alcohol (n = 4, 31%), HCV/alcohol (n = 2, 15%), alpha-1-anti-trypsin deficiency (n = 1, 8%), and hemochromatosis (n = 1, 8%). The median (IQR) MELD-Na score for all liver recipients was 19 (14, 22), and in 7 patients with MELD exception, the score was 22 (16.5, 24.5). The median (IQR) Child-Pugh score was 9 (7, 10). The etiologies of end-stage renal disease for the kidney recipients were hypertension (n = 4, 36%) with diabetes (n = 1, 9%), polycystic disease (n = 2, 18%), chronic nephritis (n = 2, 18%), and immunoglobulin A nephropathy (n = 2, 18%). The median (IQR) index hospitalization length of stay for the liver recipients was 10 (7, 12) days, and for kidney recipients was 4 (4, 6) days.
| Liver Recipients (n = 13) | Kidney Recipients (n = 11) | |
|---|---|---|
| Recipient Characteristics | ||
| Age, median (IQR), years | 59 (48, 62) | 54 (52, 57) |
| Sex, n (%) male | 8 (62%) | 5 (45%) |
| Recipient race, n (%) | ||
| Caucasian | 13 (100%) | 7 (64%) |
| Hispanic/Latino | 3 (23%) | 2 (18%) |
| African American | 0 | 3 (27%) |
| Asian American | 0 | 1 (9%) |
| Recipient ABO blood type | 6O, 5A, 2B | 5O, 5A, 1AB |
| Previously cured of HCV, n (%) | 2 (15%) | 0 |
| Lab MELD-Na, median (IQR) | 19 (14, 22) | — |
| Donor Characteristics | ||
| Age, median (IQR), years | 37 (32, 43) | 36 (31, 41.5) |
| Sex, n (%) male | 10 (77%) | 8 (73%) |
| Liver fibrosis stage, n (%) | 8 (62%) stage 0, 4 (31%) stage 1, 1 (8%) unavailable | — |
| Donor cardiac death, n (%) | 2 (15%) | 4 (36%) |
| KDPI, median (IQR) | — | 52 (40.5, 61.5) |
| Donor risk index, median (IQR) | 1.19 (1.13, 1.48) | — |
| Antibody to hepatitis B core–positive (%) | 1 (8%) | 1 (9%) |
| Transplant-Related Factors | ||
| Warm ischemia time, median (IQR), minutes | 29 (26, 33) | 29 (22, 36) |
| Cold ischemia time, median (IQR), minutes | 323 (261, 421) | 1,070 (963, 1,439.5) |
| Induction immunosuppression | 0 | 10 (100%)* |
| Length of index hospital stay, median (IQR), days | 10 (7, 12) | 4 (4, 6) |
- * Thymoglobulin (n = 9) and basiliximab (n = 1).
Efficacy
Recipient serum HCV RNA was quantifiable on day 3 after transplant in all 13 liver recipients and in 9 of 10 (82%) kidney recipients, with 1 kidney recipient with HCV RNA not tested until day 7 after transplant with quantifiable results at that time point. In another kidney recipient, HCV RNA remained negative at all time points following transplant; this recipient did not require antiviral treatment.
The median (IQR) time from transplant to start of antiviral therapy was 7.0 (6.0, 12.0) days for liver transplant recipients and 16.5 (9.8, 24.5) days for kidney transplant recipients (Fig. 2). More specifically, 7 of 13 (54%) liver recipients and only 1 of 10 (10%) kidney recipients started antiviral treatment in the first week. Conversely, only 1 of the 13 (8%) liver recipients and 3 of the 10 (30%) kidney recipients started antiviral treatment in the fourth week after transplant. Reasons for different starting times of antiviral therapy beyond the first week are included in Table 2. In 1 of 13 liver recipients and in 5 of 10 kidney recipients, the start of antiviral therapy was delayed beyond the first week by eGFR < 30 mL/min.
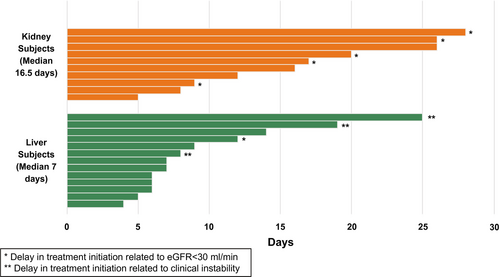
| Posttransplant Outcomes | Liver Recipients (n = 6) | Kidney Recipients (n = 9) |
|---|---|---|
| Reason study drug delayed | ||
| eGFR < 30 | 1 (17%) | 5 (56%) |
| Unstable patient | 3 (50%) | — |
| amiodarone use intraoperatively (n = 1); prolonged intubation (n = 1); hepatic artery revision (n = 1) | ||
| Site logistics* | 2 (33%) | 4 (44%) |
| ALT > 2 times normal when study drug started | 1 (17%) | 2 (22%) |
| HCV RNA week 4 of treatment | 5 (83%) undetectable | 1 (11%) undetectable |
| 1 (17%) not performed | 3 (33%) detectable, not quantifiable | |
| 5 (56%) quantifiable | ||
| SVR12 | 6 (100%) | 9 (100%) |
| Possibly related SAE | 1 (17%) GVHD | — |
- * Includes delays in return of HCV-RNA results, timing of clinic visits, and clinic closures due to holidays.
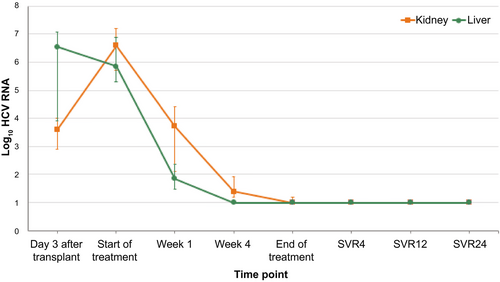
The median (IQR) HCV-RNA level 3 days after transplant was 6.5 (3.9, 7.1) log10 IU/mL in liver recipients versus 3.6 (2.9, 4.0) log10 IU/mL in kidney recipients (Fig. 3). The median (IQR) HCV RNA at the start of antiviral therapy was 6.6 (5.7, 7.2) in kidney recipients and 5.8 (5.3, 6.9) log10 IU/mL in liver recipients (Table 3). The median (IQR) HCV RNA was higher in the kidney recipients at day 7 after starting treatment, 3.7 (2.1, 4.) versus 1.9 (1.5, 2.4) log10 IU/mL in liver recipients. By week 4 of treatment, 10 of 13 (77%) liver recipients, but only 2 of 10 (20%) kidney recipients, had undetectable virus (P = 0.01). All of the liver recipients had undetectable virus at the end of treatment, while 3 (30%) of the kidney recipients still had detectable, but not quantifiable, virus at the end of the treatment period. Still, all 23 treated recipients achieved SVR12. All 22 subjects who completed 24 weeks of follow-up after treatment had SVR24. One subject died of complications of graft-versus-host disease (GVHD) prior to SVR24. Adjusted for baseline HCV-RNA levels, the mean change in HCV RNA from day 1 to week 1 was greater in liver than kidney recipients (−4.03 vs. −3.07, P = 0.008), but not statistically different from week 1 to week 4 (−0.85 vs. −1.74, P = 0.32) (Table 3). Individual viral kinetics for liver and kidney transplant recipients are shown in Supporting Fig. S1.
| Timepoint | Liver | Kidney | P Value | Adjusted P Value* | ||
|---|---|---|---|---|---|---|
| (n) | HCV-RNA log10 IU/mL | (n) | HCV-RNA log10 IU/mL | |||
| Start of treatment day 1 | 12 | 5.85 (5.29, 6.88) | 9 | 6.60 (5.73, 7.20) | 0.41 | — |
| Week 1 median (IQR) | 13 | 1.85 (1.46, 2.35) | 10 | 3.72 (2.11, 4.41) | 0.04 | — |
| Median (IQR) change from day 1 → day 7 | 12 | −4.34 (−4.82, −3.01) | 9 | −3.48 (−3.62, −2.47) | 0.06 | — |
| Mean (SD) change from day 1 → day 7 | 12 | −4.03 (1.05) | 9 | −3.07 (0.92) | 0.04 | 0.008 |
| Week 4 median (IQR) | 12 | 1.00 (1.00, 1.00) | 10 | 1.39 (1.18, 1.92) | 0.003 | — |
| Median (IQR) change from week 1 → week 4 | 12 | −0.82 (−1.25, −0.35) | 10 | −2.23 (−2.59, −0.93) | 0.05 | — |
| Mean (SD) change from week 1 → week 4 | 12 | −0.85 (0.54) | 10 | −1.74 (−1.05) | 0.02 | 0.32 |
- * Differences by organ in HCV RNA changes from day 1 to 7 and week 1 to 4 were evaluated using linear regression adjusting for HCV RNA at day 1 and week 1, respectively.
Safety
AEs and SAEs possibly or probably related to study participation are given in Table 4. One liver recipient required intraoperative amiodarone. In this case, due to the known drug interaction between amiodarone and sofosbuvir, the study drug was not started until 25 days after transplant and with telemetry monitoring for 72 hours. No bradyarrhythmias or other cardiac events occurred. SAEs were reported in 9 of 13 (69%) liver recipients and in 4 of 11 (36%) kidney recipients (Table 4).
| Relatedness | Serious Adverse Events | Adverse Events | ||
|---|---|---|---|---|
| Liver Recipients (n = 13) | Kidney Recipients (n = 11) | Liver Recipients (n = 13) | Kidney Recipients (n = 11) | |
| Possible, probable, or definite |
n = 4
|
n = 0 | n = 0 |
n = 2
|
| Unrelated |
n = 17
|
n = 5
|
n = 4
|
n = 4
|
- * Note: More than one SAE may be reported per subject.
- † This SAE resulted in premature, permanent discontinuation of study drug.
SAEs deemed potentially related to receipt of an HCV-viremic donor or HCV therapy occurred in 4 (31%) of the liver recipients. One subject who received an A2→O graft and began the study drug 5 days after transplant developed early antibody-mediated rejection that was treated with intravenous immunoglobulin G with normalization of liver biochemistries by day 36 after transplant. Another subject who began the study drug 6 days after surgery developed a progressive, intrahepatic biliary sclerosis starting by day 31 after an uneventful transplant from a 36-year-old donor after brain death. An ischemic etiology was excluded. Other than abnormal-appearing bile ducts on cholangiography and an alkaline phosphatase 3 times the upper limit of normal, there have been no other clinical sequelae 16 months after transplant. A third subject was diagnosed with GVHD beginning day 19 following transplant. The recipient was a 62-year-old male with blood type O, and the donor was male. Study drug was started 8 days after transplant but was permanently discontinued prematurely after only 7 weeks of treatment per investigator discretion due to multisystem organ complications. Still, the subject had a SVR. However, despite treatment for GVHD with corticosteroids and ruxolitinib, the subject succumbed to multisystem organ failure 152 days after transplant. The last subject who started study treatment 6 days after surgery developed an idiopathic pericardial effusion with decreased left-ventricular ejection fraction about 1 month after transplant that resolved with pericardiocentesis.
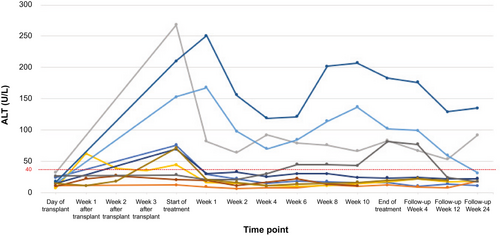
Two (20%) of the kidney recipients had delayed graft function, requiring transient hemodialysis. Renal function before, during, and after SOF-VEL therapy are shown in Supporting Fig. S2. None of the kidney recipients had acute cellular rejection or clinical evidence of glomerulopathy following transplant. Three kidney recipients with normal serum liver tests at the time of transplant developed serum transaminase elevations 4-7 times the upper limit of normal before HCV treatment was initiated that persisted throughout the SOF/VEL treatment and into the posttreatment period (Fig. 4). One patient, without known risk factors, had biopsy-proven nonalcoholic steatohepatitis on liver biopsy performed after the SVR12 visit. In another patient, the abnormal liver tests were attributed to a statin. One kidney recipient had normal alanine aminotransferase (ALT) at the time of transplant and during the first 6 weeks of antiviral treatment, but then developed mild elevation in ALT, which peaked at two times normal at the end of treatment, with normalization by the SVR12 visit. None of these 4 kidney recipients with abnormal ALT during treatment had undetectable HCV at week 4 of treatment, although all had SVR12.
Discussion
In the PRO-ACT study, preemptive SOF-VEL for 12 weeks achieved high rates of viral eradication in HCV-infected liver and kidney-transplant recipients who received HCV-viremic donors. This extends prior reports and is important in clinical situations in which genotype data are unavailable or in which waiting for genotype data would impede treatment initiation. In addition, the recent Food and Drug Administration label change for SOF-VEL to include patients with eGFR < 30 mL/min removes one of the main concerns of using this drug in the transplant setting, where renal function in the very early transplant period may be impaired. In our study, 1 of 13 liver recipients and 5 of 10 kidney recipients would have been able to start their SOF-VEL in the first week after transplant under the newer label guidance. However, the delayed approach to antiviral therapy was uniformly effective when a conventional treatment duration (i.e., 12 weeks) was used.
Expanding the donor pool for recipients of solid organ transplants remains a vitally important endeavor, with waiting lists growing for all organs annually. The American Society of Transplantation recommends that HCV D+/R- transplantation only be conducted under internal review board–approved study protocols with fully informed consent of the knowledge gaps with such transplants.(3) At the opposite side of the spectrum are experts who argue that use of “infected” donors is not new to transplantation, with organs from donors exposed to other infections (e.g., cytomegalovirus) routinely used with appropriate provisions for managing infections, and that HCV should not be viewed differently.(4) Indeed, the number of recent single-center reports attests to how rapidly the practice of using HCV-viremic donors in uninfected recipients has been embraced by transplant physicians in the United States.(5-7) Yet, there remain important questions regarding how best to approach antiviral therapy in this setting. To this end, our study adds important information.
We demonstrate that the HCV viral kinetics of recipient infection differ in liver versus non-liver (in our study, kidney) recipients. Because the initial viral burden for liver-transplant recipients is much greater than kidney (and other non-liver) transplant recipients, the ramp-up of viremia in the recipient would be anticipated to increase more rapidly in liver recipients. Indeed, while HCV-RNA levels are easily quantifiable in most recipients by post-op day 3, the post-op day 3 HCV-RNA levels are significantly higher in our liver-transplant recipients compared with kidney-transplant recipients. Beyond day 3, the HCV viral levels were higher in kidney-transplant recipients compared with liver-transplant recipients before antiviral treatment started, and several factors may be contributing: (1) the longer delay between transplant and initiating antiviral therapy in the kidney recipients; (2) use of more intensive immunosuppression in kidney recipients; and (3) differences in the local immunologic control, with liver recipients potentially having transplanted immune cells that limit local spread of HCV between hepatocytes. Because livers from HCV-viremic donors were required to have only minimal or no fibrosis in our study, these livers may have been selected for an immunologic milieu that limits viral replication and/or immune-mediated liver injury; prior studies in liver-transplant recipients with recurrent HCV infection highlight the importance of natural killer cells in liver disease severity following transplant.(8) Thus, the differences in viral kinetics identified suggest that treatment duration will likely need to be longer for liver versus non-liver recipients of HCV-viremic donors.
In our study, the duration of antiviral therapy for our liver and kidney recipients was the same and based on recommended treatment duration for patients with chronic HCV infection.(9) All of the liver-transplant recipients achieved SVR12. Prior reports of HCV viremic D+/R- liver-transplant recipients also used standard or extended-duration HCV therapy.(5, 6, 10-12) The median time to start of treatment for liver recipients in our study was 7 days, shorter than when antiviral therapy is acquired through the patient’s insurance (median of 42-62 days in such studies).(5, 6, 11) Earlier treatment may eliminate the risk of severe HCV recurrence, specifically fibrosing cholestatic hepatitis, and is the reason why recent guidelines have favored early rather than delayed HCV treatment.(9)
For our kidney recipients, posttransplant viremia occurred in 91%. In the single case without viral transmission, the donor was anti-HCV-negative but NAT-positive. Other studies in non-liver D+/R- transplants have reported transmission rates of 69%-100% from NAT-positive donors,(13-15) highlighting the concept that transmission rates are not 100%. This contrasts with reports in NAT-positive D+/R- liver transplants, in whom 100% transmission is reported. This likely reflects the lower HCV-RNA viral inoculum with nonliver organs as well as specific organ preservation methods that may lower HCV-RNA levels, such as duration of the organ on pump(16) and use of viral inactivating steps (e.g., ultraviolet C perfusate irradiation).(7) Additionally, false-positive NAT test results may occur, albeit rarely.(17). Thus, an antiviral strategy that awaits documentation of posttransplant viremia may be reasonable in D+/R- non-liver transplants, as those acquiring HCV infection usually have detectable viremia by day 3 following transplant. In settings where access to antiviral therapy during the index transplant hospitalization is limited, waiting to document viremia before starting treatment, as was done in our protocol, eliminates unnecessary treatment of those uninfected while still allowing early preemptive treatment. The alternative strategy of using prophylactic antiviral therapy to treat all D+/R- recipients without awaiting confirmation of transmission has been studied and shown to be a reasonable approach, given the safety of antiviral therapy. A prophylactic strategy in non-liver recipients allows for a shorter duration of therapy,(18) although the optimal duration of prophylactic therapy is not yet known, with ultrashort prophylactic therapy associated with unacceptable failure rates.(19) Of note, 3 of our 10 kidney recipients had detectable but not quantifiable HCV RNA at the end of 12 weeks of antiviral therapy, yet all achieved SVR12. Prior studies in direct-acting antiviral (DAA)–treated nontransplant patients report HCV-RNA positivity (either detectable but not quantifiable or quantifiable) in about 7% of patients at end of treatment,(20) but with no effect on SVR12 rates. Although these results are reassuring, it argues against shorter duration therapy in D+/R- kidney recipients starting treatment after onset of posttransplant viremia.
Several series have reported a higher incidence of acute and chronic rejection in HCV D+/R- transplants, raising the possibility of indirect immunologic effects related to presence or rapid clearance of HCV viremia. In our study, only 1 liver-transplant patient experienced antibody-mediated rejection (7%), so it is difficult to assert that this rate is higher than expected. Interestingly, other D+/R- liver-transplant studies have reported higher rates. In a Stanford series of 10 D+/R- liver transplants, 3 (30%) of 10 patients had acute and/or antibody-mediated rejection during or after HCV treatment.(6) In preliminary data from the Mayo Clinic of 14 liver-transplant recipients, 4 (29%) had acute or chronic rejection.(12) In both of these studies, antiviral therapy was delayed, on average, to about 4 weeks following transplant versus a median of 1 week for liver recipients in our study. Whether the delay in time to treatment influences the risk of rejection remains to be seen. Regardless, continued vigilance for virally triggered immune-related complications is important.
In addition to possible increased rates of rejection, HCV D+/R- transplant could also potentially be associated with other unique consequences, if HCV therapy is delayed. In our study, 4 liver recipients sustained otherwise unexplained SAEs with a possible immunologic origin: a fatal GVHD, an antibody-mediated rejection, an idiopathic intrahepatic biliary sclerosis, and an explained cardiomyopathy. In the Mayo case series, 1 of their liver recipients also had an event termed “ischemic cholangiopathy,” despite a donor with deceased brain and the absence of an identifiable vascular insult.(12) The rigor of SAE reporting in a registered multicenter study, such as ours, may differ from those of single-center observational experiences.(5, 6, 11) This may have contributed to differences seen and highlights the importance of complete reporting to help the transplant community better understand risks and benefits of D+/R- transplantation.
In our study, we also observed 3 kidney recipients with serum transaminase elevation that started before antiviral therapy, lasted throughout the treatment course, and persisted into the posttreatment period. The explanation for these abnormal liver tests in at least 2 of these subjects is lacking. A fourth kidney recipient developed mildly abnormal ALT during antiviral therapy that persisted following therapy. A case series of D+/R- kidney recipients from Memphis also showed 15% of HCV D+/R- kidney recipients had unexplained ALT elevations to at least three times the upper limit of normal during posttransplant follow-up, although the median time to start of antiviral therapy was 76 days.(21) While these adverse events cannot be attributed clearly to HCV D+/R- transplant due to the lack of D-/R- controls, the exceptional situation of introducing and rapidly eradicating a viral infection simultaneous with a transplanted organ and immunosuppression clearly requires close monitoring.
Our study has several limitations. First is the modest sample size, although it is similar to other published data and is strengthened by the inclusion of liver and nonliver recipients. The final sample size of 30 patients was not reached, with the slow recruitment related to the rapid uptake of this practice by many centers. However, the primary outcome of the study is unlikely to be substantially undermined by the failure to enroll the remaining 6 patients. Second was the variability in the start time of antiviral therapy, a factor reflecting the limitations in use of sofosbuvir in patients with eGFR < 30 mL/min during the study. Additionally, clinicians had latitude in determining when patients were clinically stable to start treatment. Thus, while the study intent was to use antiviral therapy preemptively, only 35% started antiviral therapy within the first week, and a few patients began treatment only after clinical manifestations (elevated ALT) were present. However, we feel this reflects the reality of transplant practice and the complexity of decision making in D+/R- transplants. Whether prophylactic therapy offers advantages over early preemptive therapy (starting with the first week) is unclear from the available data, although experts are converging on the importance of early treatment.(9) Third, the study population was selective, because of the desire to minimize risk of early posttransplant renal dysfunction that would limit treatment with SOF-VEL. With the new label for sofosbuvir-based DAAs, this concern is alleviated and should lead to use of HCV-viremic donors in an expanded pool of potential kidney and liver recipients. Finally, we did not perform liver biopsy on all kidney recipients with elevated liver enzymes, so we could not rule out causes other than HCV as contributing, and we did not include measures of liver disease (e.g., elastography) as a study endpoint; thus, we were unable to comment on whether there are unrecognized residual liver effects. However, the study has many strengths, including being a prospective, multicenter study that is inclusive of liver and kidney recipients, using detailed viral kinetics and independent safety monitoring.
In summary, in this multicenter study of HCV-viremic D+/R- liver and kidney transplantation, a pangenotypic regimen of SOF-VEL using a preemptive strategy and a standard 12-week duration of therapy was uniformly effective. The kinetics of viral infection differ in kidney versus liver recipients, with a delayed but higher HCV-RNA peak in kidney recipients and a lower proportion achieving unquantifiable HCV RNA within 4 weeks of treatment. This finding is relevant to strategies that are considering a shorter treatment duration for non-liver recipients. While our study supports the continued application of HCV-viremic D+/R- transplantation, the potential risk of immunologic consequences provide strong justification for standardized reporting of outcomes, with the goal of providing more quantitative information on direct and indirect risks to recipients.
Acknowledgments
The authors acknowledge the support of UNOS Data Engineering, Research and Solutions departments, as well as contributions from the following individuals to the success of the study: Piedmont Transplant Institute (Bethany Lane, RN, MSN, CCRC, CCRA, Natasha Morris, MS, CCRP and Roshan Shertha, MD), University of Colorado (James Pomposelli, MD, Alex Wiseman, MD, and Rachael Dran), University of California San Francisco (Chris Freise, MD, Shiang-Cheng Kung, MD, and Wesley Chan), Houston Methodist Hospital (Constance Mobley, MD, William Medrano, and Darrel Cleere), and Columbia University (Lloyd Ratner, MD, and Jean Emond, MD).
Author Contributions
N.T.: study concept and design, funding acquisition, study administration and oversight, data acquisition, data analysis and interpretation, original draft of manuscript and final editing and review of manuscript. J.B., M.G., E.V., J.B., C.K., D.V., S.M., J.T.: data acquisition and final editing and review of manuscript. J.D.: data analysis and interpretation; final editing and review of manuscript. C.N.: study concept and design, project administration and oversight, data interpretation, final editing and review of manuscript. R.R.: study concept and design, study oversight, funding acquisition, data acquisition and management, data analysis and interpretation, original draft of manuscript and final editing and review of manuscript.




