Transcriptome Profiling Identifies TIGIT as a Marker of T-Cell Exhaustion in Liver Cancer
Abstract
BACKGROUND AND AIMS
Programmed death 1 (PD-1) checkpoint inhibition has shown promising results in patients with hepatocellular carcinoma, inducing objective responses in approximately 20% of treated patients. The roles of other coinhibitory molecules and their individual contributions to T-cell dysfunction in liver cancer, however, remain largely elusive.
APPROACH AND RESULTS
We performed a comprehensive mRNA profiling of cluster of differentiation 8 (CD8) T cells in a murine model of autochthonous liver cancer by comparing the transcriptome of naive, functional effector, and exhausted, tumor-specific CD8 T cells. Subsequently, we functionally validated the role of identified genes in T-cell exhaustion. Our results reveal a unique transcriptome signature of exhausted T cells and demonstrate that up-regulation of the inhibitory immune receptor T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitor motif domains (TIGIT) represents a hallmark in the process of T-cell exhaustion in liver cancer. Compared to PD-1, expression of TIGIT more reliably identified exhausted CD8 T cells at different stages of their differentiation. In combination with PD-1 inhibition, targeting of TIGIT with antagonistic antibodies resulted in synergistic inhibition of liver cancer growth in immunocompetent mice. Finally, we demonstrate expression of TIGIT on tumor-infiltrating CD8 T cells in tissue samples of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma and identify two subsets of patients based on differential expression of TIGIT on tumor-specific T cells.
CONCLUSIONS
Our transcriptome analysis provides a valuable resource for the identification of key pathways involved in T-cell exhaustion in patients with liver cancer and identifies TIGIT as a potential target in checkpoint combination therapies.
Abbreviations
-
- Ahr
-
- aryl hydrocarbon receptor
-
- Blimp1
-
- B lymphocyte–induced maturation protein 1
-
- CCR7
-
- chemokine (C-C motif) receptor 7
-
- CD
-
- cluster of differentiation
-
- CTLA-4
-
- cytotoxic T lymphocyte–associated 4
-
- DE
-
- differentially expressed
-
- EF1α
-
- elongation factor 1 alpha
-
- FACS
-
- fluorescence-activated cell sorting
-
- HCC
-
- hepatocellular carcinoma
-
- HDI
-
- hydrodynamic tail vein injection
-
- iCCA
-
- intrahepatic cholangiocarcinoma
-
- IFN
-
- interferon
-
- Ig
-
- immunoglobulin
-
- IL
-
- interleukin
-
- IRES
-
- internal ribosome entry site
-
- IVIS
-
- in vivo imaging system
-
- LAG-3
-
- lymphocyte activation gene 3
-
- LM-OVA
-
- Listeria monocytogenes that expresses ovalbumin
-
- MHC
-
- major histocompatibility complex
-
- myr
-
- myristoylated
-
- OVA
-
- ovalbumin
-
- PD-1
-
- programmed death 1
-
- PLGA
-
- polylactic coglycolic acid
-
- poly(I:C)
-
- polyinosinic:polycytidylic acid
-
- pPGK-SB
-
- phosphoglycerate kinase-sleeping beauty
-
- shRNA
-
- short hairpin RNA
-
- shRp53
-
- shRNA against p53
-
- TCR
-
- T-cell receptor
-
- TIGIT
-
- T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitor motif domains
-
- TIM-3
-
- T-cell immunoglobulin and mucin domain–containing 3
-
- TNF
-
- tumor necrosis factor
-
- Tox
-
- thymocyte selection associated high mobility group box
Liver cancers belong to the most frequent malignant tumor entities, ranking sixth in incidence and fourth in mortality globally.(1) Curative treatment options including resection, transplantation, and local ablation are limited to early tumor stages, which represent only a minor fraction of the affected patients.(2) More frequently, patients present with inoperable, multilocular disease and advanced cirrhosis and are therefore limited in the choice of treatments to palliative locoregional or systemic therapies. For many years, systemic treatment options were confined to the administration of sorafenib, a pleiotropic tyrosine kinase inhibitor which prolonged survival of patients with hepatocellular carcinoma (HCC) in the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol and Asia-Pacific trials.(3, 4) This narrow spectrum of treatment options has been broadened in recent years with positive clinical trials for the programmed death 1 (PD-1) checkpoint inhibitors nivolumab(5) and pembrolizumab,(6) the anti–vascular endothelial growth factor antibody ramucirumab,(7) and the tyrosine kinase inhibitors regorafenib,(8) lenvatinib,(9) and cabozantinib.(10) The initial clinical results with PD-1 checkpoint inhibitors demonstrated high overall response rates and durable remissions, which led to early approval of PD-1 checkpoint inhibitors after positive phase 2 studies in the United States.(5, 6) While these results raised high hopes for the therapeutic potential of immunotherapy in patients with HCC, recent reports have dampened the enthusiasm due to negative outcomes of the phase 3 trials CHECKMATE-459 and KEYNOTE-240(11) in first-line and second-line HCC therapy.
The conflicting results for checkpoint inhibition in HCC indicate that HCC is susceptible to immunotherapy but that therapeutic efficacy of PD-1 monotherapy may be limited. In general, the liver represents an exceptional immunotolerant niche in which immune responses are controlled by a number of different intrahepatic cell populations, including regulatory T cells, Kupffer cells, B cells, liver sinusoidal endothelial cells, and hepatic stellate cells.(12)
Immunotherapies with antagonistic antibodies targeting both the cytotoxic T lymphocyte–associated 4 (CTLA4) and the PD-1 axis have been shown to induce clinical remission in patients with HCC.(5, 6, 13) Upon administration, these antibodies engage in a complex network of interactions between the different intrahepatic cell populations. In responding patients, these interactions result in the reinvigoration of exhausted T cells, which then recognize and kill liver cancer cells. Intriguingly, expression of the PD-1 ligand (PD-L1) has failed to predict clinical responses in patients with HCC,(5) in contrast to other tumor entities (e.g., melanoma and lung cancer(14)). These observations hint at a complex regulation of T-cell activation in liver cancer which is incompletely understood and insufficiently explained by the direct interactions of tumors with T cells. One approach to enhance the therapeutic efficacy of HCC immunotherapy which is pursued in a number of clinical trials is to combine PD-1 antagonism with antibodies targeting other coinhibitory molecules, small molecules, or locoregional therapy and ablation.(15-17) Many of these combination therapies, however, follow an empiric approach and are insufficiently supported by animal studies or analysis of human HCC samples.
We therefore sought to perform a whole-genome microarray analysis to identify key mediators of cluster of differentiation 8 (CD8) T-cell exhaustion in liver cancer. Because many costimulatory and coinhibitory molecules undergo dynamic changes during T-cell differentiation, we directly compared transcriptomes of naive, functional effector, and exhausted CD8 T cells. Our results demonstrate a dynamic regulation of both coinhibitory and costimulatory molecules in HCC-specific CD8 T cells and uncover a key role for T-cell immunoreceptor with immunoglobulin (Ig) and immunoreceptor tyrosine-based inhibitor motif domains (TIGIT) in T-cell exhaustion. Finally, our in vivo studies in mice and the analysis of human HCC and intrahepatic cholangiocarcinoma (iCCA) samples suggest therapeutically relevant synergistic effects of a combined PD-1/TIGIT blockade and the presence of two subsets of liver cancer patients with differential regulation of PD-1 and TIGIT on tumor-specific CD8 T cells.
Materials and Methods
Animals
C57BL/6 female mice (6-8 weeks old) were bred at the Animal Care facility of the Hannover Medical School, Germany. All animal experiments were performed according to German legal guidelines for animal care and experimentation (TierSchG) and approved by institutional and governmental boards (LAVES).
Cell Culture
The murine hepatoma cell line Hep-55.1C (Cell Lines Service) was cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium + GlutaMAX-I (Gibco) supplemented with 10% fetal calf serum (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin (Biochrom). The cells were tested for mycoplasma contamination on a regular basis.
Bacteria
Attenuated ovalbumin (OVA)–expressing Listeria monocytogenes of an actA–deficient strain (18) were grown and quantified as described. (19) For vaccination, mice were injected intravenously with 5 × 106 colony-forming units/mouse.
PLGA Immunization
Polylactic coglycolic acid (PLGA) microspheres (2 μm size) were purchased from Phosphorex (Hopkinton, MA) and conjugated to antigen as described.(20) SIINFEKL peptide was purchased from ProImmune (Oxford, UK). Conjugated microspheres were mixed together with 200 μg polyinosinic:polycytidylic acid (poly[I:C]) (Invivogen, San Diego, CA). For immunization, mice received intravenous injection of 1 mg PLGA microspheres conjugated to 100 μg of peptide antigen and 200 μg poly(I:C).
Tetramer Staining
Biotinylated major histocompatibility complex (MHC) class I monomers (H-2Kb) specific for OVA257–264 (SIINFEKL) were obtained from Glycotope Biotechnology (Heidelberg, Germany) and conjugated with fluorochrome-labeled streptavidin (eBioscience, San Diego, CA) according to standard protocols.
Adoptive Transfer of OT-I
For experiments with adoptive transfer of naive T cells, CD8 T cells from Thy1.1/1.2 OT-I transgenic mice were obtained from spleen and injected intravenously into naive or tumor-bearing Thy1.2 C57BL/6 mice. In some experiments, the day after the adoptive T-cell transfer, mice were either infected with L. monocytogenes that expresses OVA (LM-OVA) or received the PLGA immunization followed by LM-OVA infection 7 days later.
Quantification and Phenotypic Analysis of Antigen-Specific T Cells
The magnitude of the epitope-specific CD8 T-cell response was determined either by intracellular interferon gamma (IFN-γ) staining or MHC class I peptide tetramer staining, as described.(19) OT-I T-cell responses were determined using the Thy1.1 marker (CD90.1). Tetramer staining, extracellular antibody staining, and intracellular cytokine staining were performed according to standard protocols. Intracellular cytokine staining for IFN-γ and tumor necrosis factor alpha (TNF-α) was performed in total splenocytes, blood, lung, or liver of sacrificed mice. In order to analyze organ-specific distribution of antigen-specific CD8 T cells, mice were sacrificed and subjected to cardiac perfusion with phosphate-buffered saline (PBS; Gibco, Germany) prior to harvesting the organs. A detailed description of the tissue-processing procedure of mouse organs is included in the Supporting Information.
Treatment of Subcutaneous Tumor-Bearing Mice Using Blocking Antibodies
Blocking antibody against PD-1 (RMP1-14) was purchased from BioXCell, and blocking antibody against TIGIT (10A7) was kindly provided by Genentech. Mice were injected intraperitoneally twice a week using anti-PD-1 (RMP1-14) 150 μg or anti-TIGIT (10A7) 250 μg diluted in 0.9% NaCl solution.
Induction of Subcutaneous Tumors
Subcutaneous tumors in C57BL/6 mice were generated by subcutaneous injection with 107 cells/mouse of the previously described hepatoma cell line Hep-55.1C cells in PBS.(21) Tumors were monitored 3 times a week, and mice were sacrificed when tumor diameter exceeded 1.5 cm3 or when tumors showed signs of exulceration. Tumor volume was determined by the published formula 0.5 × length (mm) × width2 (mm).(22)
Transposon Plasmids for Generation of Autochthonous Liver Cancer
Cloning of murine NRasG12V was performed as described.(20) Myristoylated mouse Akt1 (myrAkt1) was cloned from the RCAS-myrAkt plasmid (Sandra Orsulic, Memorial Sloan Kettering Cancer Center; Addgene plasmid 11547; RCAS-myrAkt was a gift from Sandra Orsulic [Addgene plasmid # 11547; http://n2t.net/addgene:11547; RRID:Addgene_11547]) and inserted into the pT3/EF1α (elongation factor 1 alpha) transposon plasmid, resulting in the generation of pT3/EF1α-myrAkt1. The short hairpin RNA (shRNA) against p53 (shRp53), a 22-mer nucleotide sequence complementary to p53 mRNA sequence from position 844 to 866 (Genbank: NM_001127233), was obtained from the LMP-construct (Open Biosystems, Huntsville, AL). The shRNA was inserted into the pT3/EF1α transposon plasmid, leading to generation of pT3/EF1α-shRp53. For transposase-mediated integration, a plasmid encoding the sleeping beauty transposase (pPGK-SB)(23) was used.
Induction of Autochthonous Liver Cancer
To establish orthotopic liver tumors, a total amount of 20 μg DNA per mouse (5 μg pPGK-SB, 5 μg pT3/EF1α-OVA-IRES-NRasG12V, 5 μg pT3/EF1α-myrAkt1, 5 μg pT3/EF1α-shRp53) was diluted in 0.9% NaCl solution and administered by hydrodynamic tail vein injection (HDI).
In Vivo Monitoring Of Tumor Growth
The IVIS Imaging System 200 (in vivo imaging system; Xenogen Corporation, Alameda, CA) was used to observe the growth of orthotopic liver tumors. Mice received HDI with a plasmid combination (5 μg pPGK-SB, pT3/EF1α-NRasG12V, 5 μg pT3/EF1α-myrAkt1, 5 μg pT3/EF1α-shRp53, and pT3/EF1α-FlucL272A(24)) to generate luciferase-positive tumors. Prior to the measurement of firefly bioluminescence, mice were injected intravenously with D-luciferin (30 mg/kg) substrate (AppliChem, Germany) and anesthetized intraperitoneally with ketamine/xylazine (100 mg/kg and 10 mg/kg, respectively).
Isolation of OT-I Cells for RNA Isolation and Microarray Analysis
OT-I T cells from livers of OVA+ tumor-bearing mice, livers from control mice, or from the spleen of naive mice were isolated and subjected to RNA extraction. After isolation of total RNA from OT-I cells, microarray analyses of naive, effector, and exhausted CD8 T cells were performed using a refined version of the Whole Mouse Genome Oligo Microarray 4x44K v2 (Design ID 026655; Agilent Technologies). A detailed description of the isolation procedure of OT-I cells, RNA isolation, and microarray analysis is included in the Supporting Information. All microarray data are available in Gene Expression Omnibus (GEO) under the accession code GSE137610.
Analysis of CD8 T Cells From Human Liver and Tumor Tissue
A detailed description of the tissue processing procedure of human liver/tumor tissue is included in the Supplemental Materials section. Informed consent in writing was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. All experiments with human samples were approved by the ethics committee of Hannover Medical School.
Statistical Analysis
Statistical significance outside of microarray analysis was assessed using the two-tailed t test with a confidence interval of >95%. Data are presented as mean (± SD or SEM). For comparison of survival curves, the log-rank test was applied. All experiments were repeated at least once to ensure reproducibility. Levels of significance are indicated by asterisks: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
All authors had access to the study data and reviewed and approved the final manuscript.
Results
To analyze the role of different coinhibitory molecules and novel markers of T-cell exhaustion in the liver, we chose a well-established model for HCC which is based on hydrodynamic tail vein injection of transposon-flanked transgenes and coinjection of a plasmid that encodes for the transiently expressed transposase “sleeping beauty.”(20, 25) To induce malignant hepatocyte transformation, a combination of mutant NRAS, shRNA targeting the tumor suppressor p53, and myrAKT1 was chosen, while a firefly luciferase reporter was added for in vivo tumor growth monitoring (Fig. 1A). This combination of oncogenes and tumor suppressors has been shown to induce highly aggressive HCC nodules, which become detectable after 1 week and result in multilocular disease after 4-6 weeks.(20) In accordance with published results, IVIS scans revealed the presence of intrahepatic tumors after 2 weeks, which continued to grow progressively in the ensuing weeks (Fig. 1B).
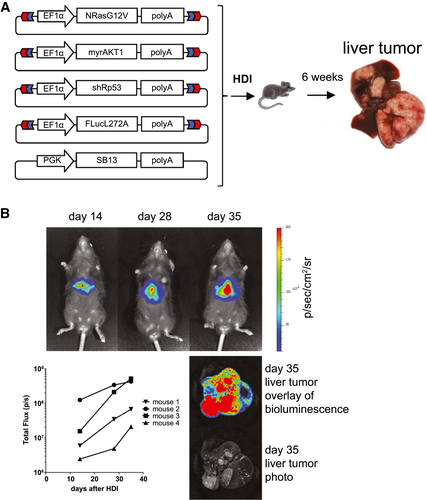
Functional Validation of The Autochthonous HCC Model
Analysis of cancer-specific CD8 T cells in vivo is hampered by the difficulty of isolating them at high purity and in sufficient numbers. To facilitate isolation of HCC-specific CD8 T cells directly from the liver, we chose OVA as a model antigen, for which both tetramers and T-cell receptor (TCR)–transgenic OT-I T cells specific for the OVA epitope SIINFEKL in the context of the murine MHC allele H-2Kb are available.(26) Autochthonous HCC was induced, and expression of the model antigen was genetically linked to NRAS-G12V expression through an internal ribosome entry site (IRES) in an OVA-IRES-NRAS-G12V transposon construct (Fig. 2A). As a second group, tumors with identical genetic composition but without OVA expression were generated. To induce sufficient expansion of SIINFEKL-specific CD8 T cells for the analysis of T-cell kinetics and phenotype, these two tumor-bearing groups and a control group without tumors were infected with an OVA-expressing, attenuated L. monocytogenes vector (ActA-deficient LM-OVA). Analysis of the CD8 T-cell response in the three groups revealed high numbers of SIINFEKL-specific CD8 T cells in peripheral blood samples of tumor-free mice and in the group with OVA-negative tumors (Fig. 2B). In contrast, the frequency of SIINFEKL-specific CD8 T cells was significantly lower in mice with tumors expressing the target antigen OVA. SIINFEKL-specific T cells from all groups were predominantly low for CD62L and chemokine (C-C motif) receptor 7 (CCR7) expression, in accordance with the described phenotypes of effector and exhausted CD8 T cells (Supporting Fig. S1A,B). Furthermore, SIINFEKL-specific CD8 T cells from the OVA+ tumor group exhibited high expression of the exhaustion markers PD-1 and T-cell Ig and mucin domain–containing 3 (TIM-3) (Fig. 2C) and other exhaustion markers including lymphocyte activation gene 3 (LAG-3), 2B4, and thymocyte selection associated high mobility group box (Tox; Supporting Fig. S1C,D), while these markers were expressed more transiently and at lower mean fluorescence intensity in the other two groups. Animal survival did not differ between the tumor-bearing groups, regardless of OVA expression (Fig. 2D). However, within the group with OVA-positive tumors we did observe a correlation between the frequency of SIINFEKL-specific T cells in peripheral blood and the survival of the mice (Fig. 2E). In summary, these results suggest a correlation between the tumor-specific CD8 T-cell response and survival in individual mice but that the magnitude of the SIINFEKL-specific CD8 T-cell response following LM-OVA infection is unable to achieve a significant survival benefit in the treated mice.

Autochthonous Liver Cancer Induces Exhaustion of Adoptively Transferred HCC-Specific CD8 T Cells
In contrast to endogenous T cells, adoptively transferred TCR-transgenic T cells can be readily identified in vivo without the need for tetramer-staining or peptide stimulation. To mimic the naive precursor frequency of endogenous immune responses, we adoptively transferred low numbers of CD90.1+ OT-I T cells (1 x 103) into CD90.2 hosts. Because the magnitude of the T-cell response after LM-OVA infection was too low to achieve sufficient numbers for microarray analyses, we employed a previously described prime-boost protocol consisting of priming with SIINFEKL-conjugated PLGA, followed by LM-OVA boosting 7 days later(20) (Fig. 3A). To ensure that the prime-boost vaccination did not clear the tumor, the vaccination was delayed to 14 days after tumor induction when the tumors had advanced to a diffuse, multilocular stage.
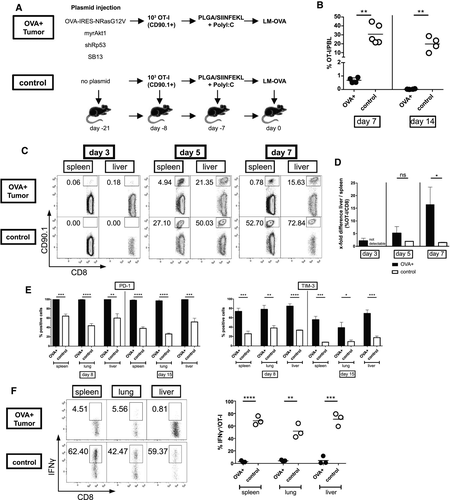
In tumor-free control mice the PLGA/LM-OVA prime-boost protocol resulted in rapid and potent expansion of the OT-I cells, reaching a frequency of 30% of all peripheral blood leukocytes by day 7 (Fig. 3B). In contrast, OT-I frequency did not exceed 1% in mice bearing OVA-positive tumors and rapidly declined to barely detectable levels by day 14. To analyze T-cell trafficking in tumor-bearing mice, we sacrificed mice at days 3, 5, and 7 after boosting and assessed OT-I frequencies in the spleen and liver. Surprisingly, OT-I T cells were readily detectable in tumor-bearing mice 3 days after boosting but not in tumor-free control mice. At days 5 and 7, however, OT-I frequencies in tumor-free mice exceeded those detected in mice with OVA+ tumors significantly (Fig. 3C). When T-cell frequencies in spleen and liver were compared to analyze organ-specific T-cell trafficking, a remarkable shift from spleen to liver was noted in the OVA+ tumor group between days 3 and 7 (Fig. 3D), indicating preferential trafficking of T cells to the liver in mice harboring autochthonous HCC. In all organs examined, expression of the two exhaustion markers PD-1 and TIM-3 was higher compared to the control group (Fig. 3E). Nevertheless, in some organs including the liver, even in the control group up to 60% of OT-I cells were PD-1+, demonstrating that PD-1 expression was not restricted to exhausted T cells. To further corroborate that OT-I T cells in the OVA+ tumor group were exhausted, we performed intracellular cytokine staining for IFN-γ (Fig. 3F) and dual staining for IFN-γ/TNF-α and IFN-γ/interleukin-2 (IL-2; Supporting Fig. S2). In the OVA+ tumor group, IFN-γ production and combined IFN-γ/TNF-α and IFN-γ/IL-2 secretion were nearly absent from the OT-I cells in all investigated organs, indicating T-cell exhaustion, while OT-I cells in the control group produced IFN-γ in up to 80% of all T cells.
Transcriptome Analysis Demonstrates TIGIT Up-regulation in Exhausted T Cells
Next, we set up an experiment to isolate HCC-specific, exhausted OT-I T cells at high purity. Because our results had demonstrated high expression of coinhibitory molecules even on non-exhausted T cells, a tumor-free control group was included, which allowed for the isolation of non-exhausted, functional T cells (Fig. 4A). As a third group we isolated naive OT-I T cells from spleen of naive mice. Following fluorescence-activated cell sorting (FACS) for CD90.1+ OT-I cells, we obtained highly pure OT-I cell populations from all three groups (Fig. 4B). mRNA was extracted from OT-I T cells of individual mice and mRNA expression profiling was performed. Clustering analysis using dendrograms and principal components separated the samples into the three major groups "naive," "effector," and "exhausted" (Supporting Fig. S3). Visualization of the expression of the 10,354 differentially expressed (DE) probe sets (corresponding to 8,710 unique DE gene IDs) that are DE in at least one of the three comparisons showed that the underlying gene expression pattern most likely causes this observed separation (Fig. 4C; Supporting Fig. S3).
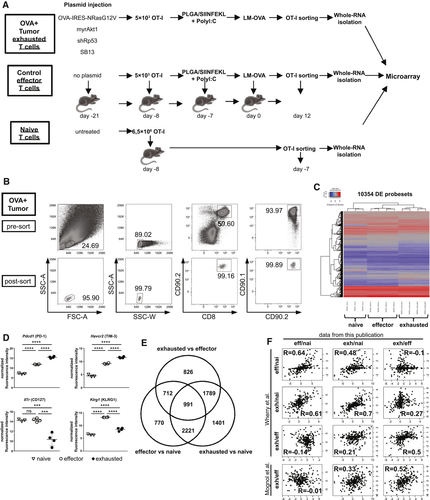
As examples of differentially expressed genes, all mice within the “effector” and “exhausted” groups displayed a gradual increase in PD-1 and TIM-3 mRNA expression. However, a selective down-regulation of the IL-7 receptor alpha chain (CD127) was observed in the exhausted CD8 T cells, and pronounced up-regulation of the killer cell leptin-like receptor (KLRG-1) in effector CD8 T cells (Fig. 4D), as expected. To better compare the transcriptome of the three groups, we calculated ratios of mRNA expression (effector/naive, exhausted/naive, exhausted/effector) for all genes. As shown in the Venn diagrams, the transcriptome of exhausted CD8 T cells differed substantially from both naive and effector CD8 T cells, revealing a larger number of genes with differential regulation within this subset when compared to either naive or effector T cells (Fig. 4E). We compared the ratios obtained from our transcriptome analysis to publicly available data sets from two studies which analyzed naive, effector, and exhausted T cells in acute and chronic lymphocytic choriomeningitis virus infection(27) and effector and exhausted T cells in a melanoma model.(28) Despite the fact that these public data sets were originated from chronic infection and an unrelated tumor entity, there was a good correlation of our HCC transcriptome ratios with the corresponding ratios derived from these two published studies (Fig. 4F).
Analysis of the transcriptome ratios revealed a number of genes with previously known up-regulation in exhausted CD8 T cells, including the coinhibitory molecules PD-1 and LAG-3 as well as the recently identified transcription factor TOX, which has been shown to drive T-cell exhaustion in cancer.(29) However, we also observed up-regulation of numerous genes with little or no known function in T-cell exhaustion, including the transcription factors aryl hydrocarbon receptor (Ahr) and Helios, the cell adhesion molecules CD301 and mammalian ependymin-related protein 1 (MERP1), the lipid signaling molecule autotaxin (ectonucleotide pyrophosphatase/phosphodiesterase 2 [Enpp2]), and the intracellular signal transducers calcitonin-2 and G protein–coupled receptor 56 (GPR56) (see Table 1 for a selected list of genes, Supporting Table S1 for the complete list of genes, and Supporting Fig. S4 for a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of pathway signaling). As a group of primary interest, we analyzed the mRNA expression of various costimulatory and coinhibitory molecules (Fig. 5A). As suggested by the FACS analysis, expression of CD137 (4-1BB), a well-recognized marker for tumor-specific T cells,(30) was markedly up-regulated in exhausted T cells compared to naive and effector T cells. Regarding the regulation of coinhibitory molecules, the vast majority of genes displayed the same gradual increase in mRNA expression (naïve < effector < exhausted T cells) as PD-1 and TIM-3, except for B and T lymphocyte attenuator 1 (BTLA) and leukocyte-associated Ig-like receptor 1 (Lair1). Of interest, the most pronounced elevation of expression between exhausted/naive and exhausted/effector was detected for TIGIT, a transmembrane protein with Ig and ITIM domains.
| Gene | Alias | Effector / Naive | Exhausted / Naive | Exhausted / Effector | Gene | Alias | Effector / Naive | Exhausted / Naive | Exhausted / Effector | Gene | Alias | Effector / Naive | Exhausted / Naive | Exhausted / Effector | Gene | Alias | Effector / Naive | Exhausted / Naive | Exhausted / Effector |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcription | Chemokine receptors | Ig-domain or Ig-like | Intracellular signal transduction | ||||||||||||||||
| Aff3 | −3.2 | −15.2 | −4.8 | Ccrl2 | Cmkbr1l2 | 6.9 | 33.9 | 4.9 | Cd80 | B7-1 | 14.3 | 66.7 | 4.7 | Adrb1 | 81.7 | 207.5 | 2.5 | ||
| Ahr | 2.3 | 44.8 | 19.7 | Ccr2 | CD192 | 25.1 | −1.0 | −25.4 | Cd86 | B7-2 | 1.9 | 9.4 | 5.1 | Arap2 | CENTD1 | 1.1 | 5.5 | 4.9 | |
| Atf4 | CREB2 | 3.2 | 2.0 | −1.6 | Ccr5 | CD195 | 15.9 | 6.9 | −2.3 | Cd200 | −2.8 | 3.4 | 9.5 | Arap3 | CENTD3 | 2.3 | 25.9 | 11.1 | |
| Camk4 | −1.5 | 1.8 | 2.6 | Ccr6 | CKR-6 | −17.0 | 1.0 | 17.4 | Cd200r1 | MOX2R | −1.2 | 108.1 | 128.8 | Areg | 5.3 | 150.4 | 28.4 | ||
| Cited4 | MRG2 | −1.1 | 24.7 | 26.4 | Ccr7 | CD197 | −27.4 | −112.7 | −4.1 | Cd200r2 | 1.0 | 582.6 | 582.6 | Arhgef2 | GEFH1 | 2.2 | −1.2 | −2.6 | |
| Creb3 | LUMAN | 1.2 | −1.8 | −2.1 | Ccr9 | CDw199 | −246.7 | −359.6 | −1.5 | Cd200r4 | 2.1 | 19.2 | 9.0 | Arhgef18 | 1.3 | −3.9 | −5.0 | ||
| Cxxc5 | CF5 | −50.2 | −3.2 | 15.5 | Cx3cr1 | GPR13 | 240.6 | 51.8 | −4.6 | Cd244 | 2B4 | 1.3 | 2.8 | 2.2 | Calcb | 1.0 | 122.9 | 122.9 | |
| Dmrta1 | DMRT4 | 4.1 | −6.2 | −25.2 | Cxcr3 | CD183 | 6.9 | 2.5 | −2.8 | Gp49a | Lilr4b | 19.8 | 59.8 | 3.0 | Cblb | −1.1 | 3.3 | 3.5 | |
| E2f2 | 4.7 | 1.2 | −3.9 | Cxcr4 | CD184 | 4.1 | 1.4 | −3.0 | Havcr2 | TIM-3 | 55.8 | 288.3 | 5.2 | Cd38 | ADPRC 1 | 3.0 | 10.8 | 3.6 | |
| E2f3 | −1.4 | 2.1 | 2.9 | Cxcr5 | Blr1 | −18.8 | −9.5 | 2.0 | Ildr1 | 9.0 | 51.2 | 5.7 | Cd81 | −40.8 | −3.2 | 12.9 | |||
| Egr2 | −6.4 | −1.5 | 4.3 | Cxcr6 | CD186 | 4.3 | 6.9 | 1.6 | Lag3 | CD223 | 95.8 | 1313.2 | 13.7 | Cd274 | PD-L1 | 2.3 | 1.5 | −1.5 | |
| Eid1 | RBP21 | 1.2 | 3.1 | 2.5 | Hrh4 | AXOR35 | 1.4 | 3.3 | 2.4 | Lair1 | CD305 | 2.5 | −5.3 | −13.1 | Chn2 | Chimerin 2 | 5.0 | 132.5 | 26.3 |
| Eomes | −1.3 | −1.8 | −1.4 | Lilrb4 | CD85K | 15.1 | 60.9 | 4.0 | Cish | SOCS | 25.8 | 25.9 | 1.0 | ||||||
| Epas1 | HIF2A | 2.4 | −4.3 | −10.3 | Pvr | CD155 | 5.1 | 1.7 | −3.0 | Ctla4 | CD152 | 73.2 | 304.6 | 4.2 | |||||
| Ets2 | −2.9 | −6.3 | −2.2 | Cytokine receptors | Sema4a | 2.4 | −2.2 | −5.3 | Cysltr2 | 6.9 | 13.7 | 2.0 | |||||||
| Fhl2 | SLIM3 | 4.0 | 3.4 | −1.2 | Fas | CD95 | −1.9 | −5.3 | −2.8 | Sema4f | 1.3 | −2.7 | −3.5 | Ddit4 | −3.0 | −1.2 | 2.5 | ||
| Fos | 42.0 | 84.9 | 2.0 | Ifngr1 | CD119 | 3.3 | 2.7 | −1.2 | Sema7a | CD108 | 5.9 | 43.7 | 7.4 | Dusp2 | PAC-1 | 5.7 | 3.4 | −1.7 | |
| Gata3 | 2.1 | 2.0 | −1.0 | Ifngr2 | −82.3 | −101.8 | −1.2 | Slamf1 | CD150 | 4.2 | 4.2 | −1.0 | Dusp6 | MKP-3 | −1.4 | 3.2 | 4.5 | ||
| Hdac10 | HD10 | 2.0 | −3.2 | −6.3 | Il2ra | CD25 | 9.8 | 3.1 | −3.2 | Slamf6 | CD352 | −2.6 | −5.6 | −2.2 | Dusp7 | 1.1 | −2.9 | −3.2 | |
| Hic1 | 22.4 | 59.6 | 2.7 | Il2rb | CD122 | 3.0 | 4.0 | 1.3 | Slamf7 | CD319 | 16.8 | 8.3 | −2.0 | Entpd1 | CD39 | 21.0 | 74.5 | 3.6 | |
| Hopx | 6.5 | 1.7 | −3.8 | Il2rg | CD132 | −1.4 | −2.1 | −1.5 | Tigit | VSTM3 | 90.8 | 1577.9 | 17.4 | Farp1 | CDEP | 12.9 | 47.9 | 3.7 | |
| Id2 | 10.7 | 10.4 | −1.0 | Il3ra | CD123 | 1.1 | 2.1 | 1.8 | Fcer1g | FceRI γ | −4.8 | −1.3 | 3.7 | ||||||
| Id3 | −20.7 | −26.7 | −1.3 | Il4ra | CD124 | −2.0 | −9.7 | −4.9 | Fcgr2b | CD32B | 35.3 | −1.8 | −63.3 | ||||||
| Ikzf1 | Ikaros | −1.6 | −2.2 | −1.3 | Il6ra | CD126 | −26.9 | −631.1 | −23.5 | Cell division, cell cycle, mitosis | Fcgr3 | CD32A | 8.7 | −2.4 | −20.6 | ||||
| Ikzf2 | Helios | −13.4 | 2.8 | 37.6 | Il6st | CD130 | −11.9 | −2.7 | 4.5 | Anxa1 | ANX1 | 29.2 | 1.5 | −19.5 | Flna | Filamin-1 | 1.7 | −2.9 | −5.0 |
| Ikzf3 | Aiolos | 3.9 | 3.8 | −1.0 | Il7r | CD127 | 1.2 | −14.2 | −17.0 | Anxa2 | ANX2 | 5.3 | 5.3 | 1.0 | Gna15 | 4.4 | 1.3 | −3.4 | |
| Ikzf4 | Eos | −4.9 | 6.4 | 31.0 | Il10ra | CD210 | 5.0 | 4.1 | −1.2 | Cdc14b | −1.8 | −5.5 | −3.0 | Gpr56 | ADGRG1 | 7.7 | 437.9 | 56.8 | |
| Irf4 | 6.1 | 8.8 | 1.4 | Il12rb1 | CD212 | 4.8 | 3.0 | −1.6 | Cdc34 | UBE2R1 | 2.2 | −1.1 | −2.5 | Gpr114 | ADGRG5 | −5.9 | −203.5 | −34.7 | |
| Irf7 | 1.4 | −3.1 | −4.4 | Il15ra | CD215 | 2.8 | 2.9 | 1.0 | Ccna2 | Cyclin A2 | −2.3 | 5.9 | 13.4 | Hspa1a | HSP70-1A | 1152.7 | 1656.1 | 1.4 | |
| Irf8 | 1.2 | 8.6 | 7.2 | Il17ra | CD217 | −1.3 | −5.4 | −4.0 | Cdk2ap2 | −1.0 | −2.7 | −2.7 | Ifit3 | 1.7 | −3.1 | −5.1 | |||
| Irf9 | −1.2 | −2.4 | −2.0 | Il18r1 | CD218a | 5.8 | 3.2 | −1.8 | Cdk19 | −1.3 | −2.7 | −2.1 | Insrr | 3.4 | 140.5 | 41.2 | |||
| Jdp2 | 5.8 | 23.2 | 4.0 | Il18rap | CD218b | 34.0 | 18.7 | −1.8 | Cdkn2a | P19ARF | 5.4 | 150.4 | 27.6 | Lat2 | NTAL | 1.8 | 14.8 | 8.1 | |
| Lass4 | CerS4 | 3.5 | 3.7 | 1.0 | Il27ra | −1.5 | −3.8 | −2.6 | Cdkn2c | P18-INK4C | 1.2 | 3.6 | 3.0 | Litaf | PIG7 | 10.1 | 37.1 | 3.7 | |
| Lef1 | TCF10 | −5.8 | −61.9 | −10.7 | Tnfrsf1b | CD120b | 3.2 | 8.1 | 2.5 | Cdkn2d | P19-INK4D | 1.2 | −3.3 | −4.0 | Nsg2 | −13.6 | −203.8 | −14.9 | |
| Kat6a | MYST3 | 1.1 | −1.9 | −2.1 | Tnfrsf4 | CD134 | −1.0 | 3.0 | 3.1 | Map3k8 | c-COT | 3.7 | 1.1 | −3.3 | Pdcd1 | PD-1 | 32.5 | 368.4 | 11.3 |
| Klf2 | LKLF | 2.1 | −2.7 | −5.5 | Tnfrsf9 | CD137 | −2.1 | 15.0 | 31.4 | Mapre2 | 5.2 | 4.5 | −1.2 | Pdcd1lg2 | CD273 | 4.8 | 25.1 | 5.2 | |
| Klf3 | BKLF | 1.0 | −14.1 | −14.7 | Tnfrsf10b | TRAILR2 | −6.0 | −12.9 | −2.1 | Mki67 | Ki-67 | 2.1 | 9.1 | 4.4 | Pdgfb | 6.0 | 16.4 | 2.7 | |
| Klf7 | UKLF | −1.6 | −3.7 | −2.4 | Tnfrsf12a | CD266 | 1.4 | −1.9 | −2.7 | Plekho1 | OC120 | −6.8 | −3.5 | 1.9 | Penk | 4.4 | 51.1 | 11.6 | |
| Mdfic | HIC | 18.0 | 47.0 | 2.6 | Tnfrsf13b | CD267 | −3.1 | −5.8 | −1.9 | Rassf2 | −2.1 | −3.4 | −1.6 | Plcd1 | 28.3 | 1.9 | −15.2 | ||
| Med21 | SRB7 | 1.3 | −1.6 | −2.0 | Tnfrsf13c | CD268 | −1.4 | −3.5 | −2.4 | Rgcc | Rgc32 | −1.8 | −17.3 | −9.7 | Prr5l | Protor-2 | 6.4 | 117.8 | 18.3 |
| Myb | Cmyb | −11.9 | −8.2 | 1.4 | Tnfrsf25 | APO3 | 2.4 | −7.3 | −17.8 | S100a4 | 53.8 | 27.4 | −2.0 | Ptpn9 | 1.3 | 2.8 | 2.1 | ||
| Myc | C-Myc | −2.3 | −1.1 | 2.2 | Tnfrsf26 | −2.6 | −20.8 | −7.8 | S100a6 | 19.4 | 13.1 | −1.5 | Ptpn11 | SHP2 | 1.7 | 4.4 | 2.6 | ||
| Nfil3 | E4BP4 | 13.6 | 37.8 | 2.8 | S100a9 | −440.6 | −7.9 | 55.9 | Ptpn13 | 17.8 | 35.5 | 2.0 | |||||||
| Nfkbia | IKBA | 5.6 | 2.0 | −2.8 | S100a10 | ANX2L | 2.5 | 1.2 | −2.2 | Rab27a | 1.1 | 2.9 | 2.8 | ||||||
| Nfkbib | TRIP9 | 2.6 | 6.6 | 2.6 | Cytolytic effector molecules | Smc4 | CAPC | −2.8 | −1.3 | 2.2 | Rab33b | 2.4 | 1.1 | −2.1 | |||||
| Notch2 | 2.1 | −1.3 | −2.8 | Gzma | CTLA3 | 84.9 | 74.4 | −1.1 | Rap2a | KREV | 6.9 | 1.6 | −4.2 | ||||||
| Nr1d2 | BD73 | 2.7 | 1.4 | −2.0 | Gzmb | CTLA1 | 195.0 | 90.5 | −2.2 | Rgs1 | 144.1 | 355.4 | 2.5 | ||||||
| Nr4a2 | TINUR | 109.6 | 599.4 | 5.5 | Gzmm | LMET1 | 4.1 | −6.0 | −24.7 | Apoptosis regulation | Rgs3 | 2.6 | 3.7 | 1.4 | |||||
| Pbx3 | 5.0 | 15.5 | 3.1 | Fasl | CD95L | 72.0 | 49.8 | −1.4 | Bcl2a1c | 21.3 | 30.2 | 1.4 | Rgs10 | −17.0 | −21.0 | −1.2 | |||
| Pou6f1 | TCFB1 | 2.5 | 1.2 | −2.1 | Prf1 | Perforin | 4.0 | 2.0 | −2.0 | Bcl2a1d | 19.3 | 30.1 | 1.6 | Rgs16 | 31.0 | 299.2 | 9.7 | ||
| Prdm1 | BLIMP1 | 18.1 | 21.4 | 1.2 | Bcl2l11 | BIM | −1.5 | 7.1 | 10.8 | Sdcbp2 | SITAC | 9.8 | 29.4 | 3.0 | |||||
| Rbpj | 1.9 | 7.5 | 4.0 | Bcl2l1 | 3.5 | 2.8 | −1.3 | Spry1 | 30.2 | 98.3 | 3.3 | ||||||||
| Rora | RZRA | 20.8 | 5.8 | −3.6 | Selectins | Birc5 | API4 | −2.2 | 6.0 | 13.4 | Tank | TRAF2 | −1.3 | 2.0 | 2.7 | ||||
| Runx1 | CBFA2 | 6.4 | 1.2 | −5.2 | Sell | CD62L | −135.7 | −594.3 | −4.4 | Bmf | −1.9 | 3.3 | 6.3 | Tnfaip8l2 | TIPE2 | −1.1 | −4.9 | −4.3 | |
| Satb1 | −3.4 | −14.2 | −4.2 | Selplg | CD162 | 1.3 | −2.1 | −2.8 | Capn2 | 2.8 | 1.5 | −1.9 | Trat1 | −24.7 | −38.1 | −1.5 | |||
| Smad3 | HSPC193 | 3.2 | −1.2 | −3.9 | Card10 | BIMP1 | 3.3 | 7.2 | 2.2 | Vipr1 | −9.7 | −204.4 | −21.1 | ||||||
| Srebf2 | SREBP2 | −1.2 | −2.5 | −2.1 | Casp1 | 22.0 | 25.7 | 1.2 | Vipr2 | 1.0 | 137.3 | 137.3 | |||||||
| Tbx21 | TBET | 27.6 | 7.4 | −3.7 | Integrins | Casp3 | 2.1 | 29.6 | 14.1 | Wls | 1.3 | 4.6 | 3.5 | ||||||
| Tcf3 | ITF1 | 2.0 | −1.1 | −2.1 | Itga1 | CD49a | 35.9 | 42.1 | 1.2 | Casp4 | 17.8 | 32.1 | 1.8 | Zyx | HED-2 | 2.6 | −1.2 | −3.2 | |
| Tcf4 | ITF2 | −1.1 | 4.4 | 4.7 | Itga4 | CD49d | 10.5 | 12.2 | 1.2 | Casp7 | 2.4 | 2.4 | −1.0 | ||||||
| Tcf7 | TCF-1 | −3.7 | −33.9 | −9.1 | Itga6 | CD49f | −2.0 | −3.1 | −1.6 | Dapk2 | 19.6 | 19.9 | 1.0 | ||||||
| Tef | 2.5 | 2.1 | −1.2 | Itgae | CD103 | −96.1 | −151.7 | −1.6 | Fgl2 | 7.8 | 16.8 | 2.2 | Protein kinases | ||||||
| Tfdp1 | DRTF1 | −1.4 | 1.6 | 2.3 | Itgal | CD11a | 2.9 | −1.2 | −3.5 | Gas2 | 2.2 | 10.9 | 4.9 | Adk | −2.9 | −3.5 | −1.2 | ||
| Tox | −1.3 | 13.7 | 18.1 | Itgam | CD11b | 7.9 | 2.5 | −3.1 | Hip1 | SHON | 72.6 | 161.4 | 2.2 | Cpne3 | Copine III | 2.0 | −1.6 | −3.4 | |
| Trip4 | SMABF1 | 2.7 | 1.0 | −2.6 | Itgav | CD51 | 4.2 | 20.5 | 4.9 | Igf1r | CD221 | −75.1 | −49.7 | 1.5 | Dclk2 | −1.6 | 4.7 | 7.8 | |
| Trps1 | 5.0 | 13.5 | 2.7 | Itgax | CD11c | 39.4 | 12.0 | −3.3 | Map3k5 | ASK1 | −1.5 | 1.4 | 2.1 | Dgka | DAGK1 | −1.8 | −4.7 | −2.6 | |
| Zbtb7b | THPOK | 25.6 | 21.6 | −1.2 | Itgb1 | CD29 | 15.3 | 6.5 | −2.4 | Naip2 | 6.0 | 4.6 | −1.3 | Dgkh | DGKη | 6.3 | 9.0 | 1.4 | |
| Zbtb32 | 86.8 | 130.4 | 1.5 | Itgb2 | CD18 | 2.4 | 1.2 | −2.1 | Nod1 | CARD4 | 2.7 | −1.4 | −3.7 | Ikbkb | IKK2 | −2.1 | −2.0 | 1.0 | |
| Zeb2 | SMADIP1 | 100.7 | 13.8 | −7.3 | Itgb3 | CD61 | −3.1 | −5.2 | −1.7 | Osgin1 | 13.9 | 93.3 | 6.7 | Ikbke | IKKE | −3.3 | −5.9 | −1.8 | |
| Itgb7 | 1.4 | −6.8 | −9.1 | Perp | 134.6 | 302.2 | 2.2 | Kit | CD117 | −6.4 | 8.6 | 55.0 | |||||||
| Rnf216 | 16.4 | 12.5 | −1.3 | Kndc1 | RASGEF2 | 1.9 | 54.9 | 28.8 | |||||||||||
| Cytokines / Chemokines | Rassf6 | 1.0 | 47.6 | 47.6 | Lrrk1 | RIPK6 | 11.1 | 16.5 | 1.5 | ||||||||||
| Ccl1 | TCA3 | 2.4 | 26.2 | 11.1 | Cell adhesion | Serpinb9 | 9.6 | 8.0 | −1.2 | Map4k2 | MEKKK 2 | −1.2 | −2.0 | −1.7 | |||||
| Ccl3 | Mip1a | 347.1 | 1094.0 | 3.2 | Actn1 | −10.1 | −3.6 | 2.8 | Sgk1 | 2.1 | −2.5 | −5.1 | Map4k4 | MEKKK4 | −2.3 | −2.5 | −1.1 | ||
| Ccl4 | Mip1b | 18.6 | 20.2 | 1.1 | Alcam | CD166 | 39.9 | 86.2 | 2.2 | Tnfaip8 | SCCS2 | −1.3 | 2.0 | 2.7 | Mapkapk3 | 2.6 | 2.7 | 1.0 | |
| Ccl5 | RANTES | 9.3 | 7.6 | −1.2 | Ambp | Trypstatin | 1.6 | 14.3 | 8.7 | Mlkl | 6.3 | 13.4 | 2.1 | ||||||
| Ccl9 | MRP-2 | 24.3 | 1.1 | −22.4 | Camk2n1 | 17.1 | 59.8 | 3.5 | Pdk1 | −7.8 | −5.5 | 1.4 | |||||||
| Ccl25 | TECK | −1.1 | −2.1 | −2.0 | Cd44 | 5.4 | 2.0 | −2.7 | Lipid metabolism | Pik3cd | −1.7 | −2.8 | −1.7 | ||||||
| Ccl27a | CTAK | 3.0 | 5.4 | 1.8 | Clec2d | LLT1 | 1.1 | −2.3 | −2.6 | Apobec3 | −1.0 | 2.9 | 3.0 | Pik3ip1 | −3.7 | −7.7 | −2.1 | ||
| Cd163l1 | −12.4 | −197.3 | −16.0 | Clec10a | CD301 | −1.8 | 79.3 | 144.5 | Afp | −11.9 | −3.6 | 3.4 | Pik3r1 | 2.7 | 3.9 | 1.4 | |||
| Cklf | UCK-1 | 1.9 | −1.3 | −2.6 | Dst | Dystonin | −2.6 | 13.5 | 34.7 | Agpat9 | GPAT-3 | 2.4 | 30.3 | 12.6 | Pik3r5 | 2.1 | −1.9 | -−4.1 | |
| Csf1 | MCSF | 1.9 | 15.4 | 8.1 | Epdr1 | MERP1 | −1.3 | 171.8 | 217.2 | Echdc2 | 8.2 | 17.6 | 2.1 | Pip4k2a | −1.7 | 1.5 | 2.5 | ||
| Csf2 | GM-CSF | 1.9 | 13.8 | 7.3 | Lamc1 | 33.0 | 31.8 | −1.0 | Enpp2 | 1.6 | 69.4 | 43.0 | Pkdcc | SGK493 | −1.5 | 150.6 | 225.9 | ||
| Cxcl10 | INP10 | 36.2 | 35.7 | −1.0 | Lgalsl | HSPC159 | 8.2 | 22.0 | 2.7 | Fabp5 | −1.1 | 4.1 | 4.7 | Plaur | CD87 | −60.4 | −65.2 | −1.1 | |
| Ifng | 749.0 | 497.5 | −1.5 | Lgals1 | 7.7 | 3.5 | −2.2 | Osbpl3 | 68.0 | 102.5 | 1.5 | Plk3 | 7.1 | 6.7 | −1.1 | ||||
| Il1b | −9.8 | −1.3 | 7.3 | Lims1 | 1.3 | 3.8 | 3.0 | Osbpl5 | 1.9 | −2.0 | −3.7 | Prkcb | −1.8 | −2.1 | −1.1 | ||||
| Il2 | TCGF | 12.3 | 1.0 | −12.2 | Nrp1 | CD304 | 30.1 | 48.2 | 1.6 | Soat2 | 22.6 | 6.8 | −3.3 | Prkch | −1.9 | 1.8 | 3.5 | ||
| Il10 | CSIF | 1.1 | 27.7 | 25.9 | Nrp2 | −2.2 | −159.7 | −73.8 | Prkcq | PKC-θ | −1.1 | −2.6 | −2.3 | ||||||
| Il15 | 9.4 | 2.2 | −4.3 | Pecam1 | CD31 | −5.3 | −3.6 | 1.5 | Prkcz | PKC-ζ | 4.5 | 5.8 | 1.3 | ||||||
| Il16 | −1.2 | −2.5 | −2.0 | Plec | 1.9 | −1.8 | −3.3 | Proteolysis | Prkd2 | −1.2 | −2.8 | −2.4 | |||||||
| Il18 | 4.5 | 2.8 | −1.6 | Tjp2 | 5.2 | 36.8 | 7.1 | Adam8 | CD156 | 9.3 | 54.4 | 5.8 | Ripk1 | RIP1 | 1.3 | −1.5 | −2.1 | ||
| Il21 | 1.0 | 16.9 | 16.9 | Tjp3 | 3.7 | 2.9 | −1.3 | Adam19 | Meltrin β | 2.6 | 5.2 | 2.0 | Ryk | JTK5A | 40.2 | 62.5 | 1.6 | ||
| Lta | TNF-β | −1.5 | −3.1 | −2.1 | Atg4d | 2.6 | −1.1 | −2.9 | Sbk1 | −1.0 | −5.9 | −5.6 | |||||||
| Il6st | −11.9 | −2.7 | 4.5 | Killer-cell lectin-like receptors | Cd55 | DAF | −3.0 | −2.8 | 1.1 | Stk16 | −1.5 | −3.1 | −2.2 | ||||||
| Spp1 | OPN | 1.0 | 153.8 | 147.2 | Gm156 | Klrh1 | 1.1 | 8451.1 | 8024.2 | Ctla2a | 7.3 | 3.7 | −2.0 | Stk38 | NDR | −1.3 | −3.6 | −2.9 | |
| Tnf | TNF-α | 5.4 | 1.8 | −3.0 | Klra7 | Ly49G | −2.3 | 29.8 | 70.0 | Ctla2b | 7.1 | 3.6 | −2.0 | Stk39 | SPAK | 2.2 | 14.0 | 6.5 | |
| Tnfsf9 | CD137L | 13.2 | 8.4 | −1.6 | Klra8 | Ly49H | 4.4 | 19.1 | 4.4 | Ctsb | Cathepsin B | 1.1 | 2.8 | 2.5 | Tec | −5.1 | −3.9 | 1.3 | |
| Tnfsf10 | TRAIL | 1.8 | 2.4 | 1.3 | Klra9 | Ly49I | 4.0 | 19.4 | 4.8 | Ctsc | Cathepsin C | −1.0 | 2.4 | 2.4 | Tk1 | 4.2 | 22.0 | 5.3 | |
| Tnfsf13b | CD257 | 8.3 | 45.7 | 5.5 | Klra22 | Ly49V | −2.7 | 4.5 | 11.9 | Dennd4a | MYCPBP | 3.1 | 4.3 | 1.4 | Trib2 | −23.5 | −3.7 | 6.4 | |
| Tnfsf14 | CD258 | 7.9 | 2.2 | −3.7 | Klra23 | Ly49W | −4.5 | 6.6 | 29.9 | Dennd4b | 1.3 | −1.6 | −2.1 | Vrk3 | 1.1 | −2.1 | −2.2 | ||
| Xcl1 | Lptn | 3.4 | 6.2 | 1.8 | Klrb1 | Ly-55 | 21.0 | 5.3 | −4.0 | Dpp4 | CD26 | −2.6 | −2.3 | 1.1 | |||||
| Klrb1c | CD161 | 21.9 | 2.3 | −9.6 | Ephx1 | −11.8 | −1.3 | 8.9 | |||||||||||
| Klrc1 | CD159a | 31.7 | 38.0 | 1.2 | Gzmk | TRYP2 | 37.6 | 63.4 | 1.7 | ||||||||||
| Klrc3 | Nkg2e | 30.7 | 37.4 | 1.2 | Napsa | Napsin-1 | −2.4 | 5.0 | 12.2 | ||||||||||
| Klre1 | NKG2I | 20.2 | 5.7 | −3.6 | Prss12 | 1.6 | −118.3 | −186.0 | |||||||||||
| Klrg1 | MAFA | 159.5 | 5.5 | −28.8 | Prss53 | POL3S | 1.8 | −1.2 | −2.0 | ||||||||||
| Klrk1 | Nkg2d | 15.2 | 7.8 | −1.9 | Rnf144a | UBCE7IP4 | −3.8 | −49.8 | −13.0 | ||||||||||
| St14 | 7.9 | 50.9 | 6.4 | ||||||||||||||||
| Tfrc | CD71 | −4.3 | −1.3 | 3.3 | |||||||||||||||
| Usp28 | −10.8 | −17.8 | −1.6 | ||||||||||||||||
- mRNA expression ratios of selected genes for the three calculated ratios effector/naive, exhausted/naive, and exhausted/effector. Genes were grouped into families according to their known function in immune cells. Table shows mean increase (positive values) or decrease (negative values) for each group. Bold ratios indicate statistical significance (p adjusted value < 0.05).
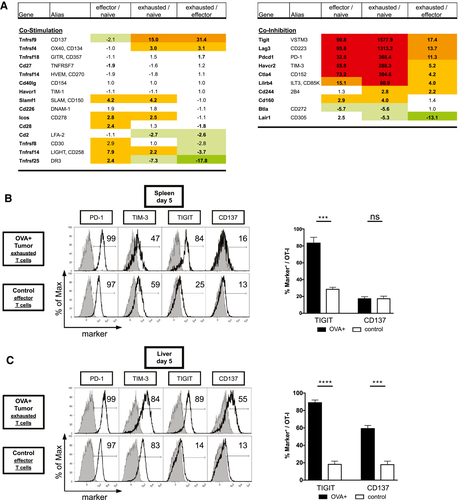
Validation of the mRNA Transcriptome Profiles
To validate protein expression of the checkpoint molecules, we performed FACS analysis of exhausted T cells in vivo. In accordance with the microarray analysis, TIGIT expression was high on exhausted T cells in the spleen but low on effector T cells (Fig. 5B) in tumor-free control mice. In contrast, PD-1 expression was detected at high levels in both exhausted and effector T cells, albeit at different fluorescence intensities. Other coregulatory molecules, including TIM-3 and CD137, failed to exhibit a differential expression in the spleen. To analyze the expression of the candidate exhaustion markers in the tumor microenvironment, we performed the same FACS analysis in the liver. For both PD-1 and TIGIT, expression levels in the liver were similar to those observed in the spleen. In contrast, TIM-3 expression in HCC increased compared to the control group, as did the percentage of CD137+ T cells (Fig. 5C). Altogether, these results suggest that TIGIT has promising properties as a marker of T-cell exhaustion and that the expression of some, but not all, potential exhaustion markers is tissue-dependent.
To rule out the possibility that the use of transgenic T cells specific for a high-affinity antigen influenced expression of coinhibitory markers, we repeated the experiment with tumors devoid of OVA expression and analyzed the endogenous CD8 T-cell response (Fig. 6A). Analysis of polyclonal T-cell populations isolated from the liver of tumor-bearing mice revealed higher expression of PD-1, TIGIT, and TIM-3 compared to tumor-free control mice (Fig. 6B). In conclusion, these observations from endogenous, polyclonal T-cell responses in autochthonous HCC confirm the results from TCR-transgenic OT-I T cells and suggest a role for TIGIT as a marker of HCC-specific CD8 T cells.
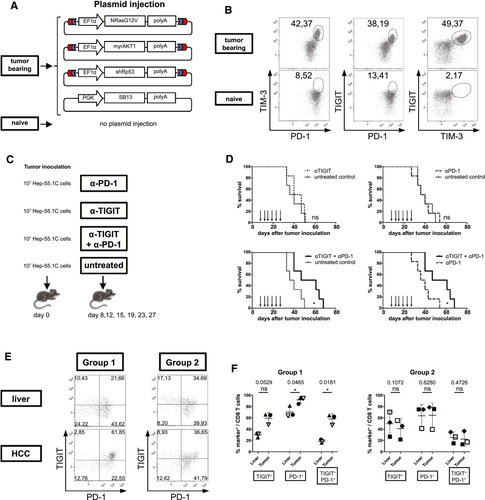
Combined PD-1 and TIGIT Blockade Improves Survival of HCC-Bearing Mice
Next we sought to determine whether inhibition of TIGIT provides synergistic effects with PD-1 inhibition in vivo. We therefore used PD-1 and TIGIT checkpoint blockade either as monotherapy or as combined therapy in a model of a subcutaneously injected, syngenic HCC cell line (Fig. 6C). While neither PD-1 nor TIGIT blockade alone was able to significantly prolong survival of the mice compared to the control group, combined PD-1/TIGIT inhibition significantly prolonged animal survival compared to both the control group and PD-1 monotherapy (Fig. 6D).
TIGIT is Selectively Up-regulated in a Subset of Patients With HCC
Lastly, we obtained fresh tumor samples from 6 patients with primary liver cancers (5 patients with HCC, 1 patient with intrahepatic CCA) undergoing hepatic resection and performed FACS analyses for TIGIT and PD-1 expression in tumor tissue and adjacent, tumor-free liver tissue (see Supporting Fig. S5A for gating strategy). All patients had underlying cirrhosis and underwent resection of 1-2 tumor nodules without prior therapy (see Supporting Table S2 for patient information). Similar to our observations in mice, we observed up-regulation of both PD-1 and TIGIT in tumor-infiltrating CD8 T cells (Fig. 6E). However, a clear dichotomy was observed in the patients, with one group (n = 3) displaying remarkable up-regulation of coinhibitory molecules in the tumor compared to surrounding liver tissue and the while the second group (n = 3) had similar expression of PD-1 and TIGIT in liver and HCC (Supporting Fig. S5B). In group 1, the higher number of PD-1+, TIGIT+, and double-positive CD8 T cells was also accompanied by an up-regulation of these two coinhibitory molecules in FACS analysis, which was not apparent in group 2 (Fig. 6E,F). In surrounding liver tissue, PD-1 expression was high in both groups, questioning the value of PD-1 as a marker of cancer-specific T cells in the liver. Overall, these data suggest that expression of coinhibitory molecules is differentially regulated in patients with primary liver cancer and that analysis of combined PD-1 and TIGIT expression identifies two subsets of patients with differential regulation of checkpoint molecules between benign and malignant liver tissue.
Discussion
Immunotherapy has shown promising results for the treatment of HCC and CCA in phase 2 trials. Recent results of phase 3 trials, however, have failed to demonstrate significant improvement of overall survival with PD-1 inhibitors, highlighting the need for a better understanding of the immunosuppressive mechanisms that control adaptive immunity in liver cancer.
A number of studies have addressed the role of coinhibitory molecules in tumor-infiltrating T cells in HCC,(31, 32) but until now a number of problems have hampered a comprehensive analysis of cell-mediated immunosuppression in liver cancer. In humans, T-cell numbers in HCC samples are typically low, and intratumoral T-cell populations are often dominated by bystander T cells which do not recognize cancer antigens, constituting up to 70% of all intratumoral T cells.(33) Here, we used an established model of autochthonous liver cancer with a model antigen to detect tumor-specific, exhausted T cells.(20) More importantly, we compared the profile of exhausted T cells to fully functional T cells specific for the same antigen, allowing us to discriminate exhaustion from effector function. Our data represent to our knowledge the first comparative analysis of genome-wide transcriptomes from naive, effector, and exhausted HCC-specific T cells.
The results of our transcriptome study demonstrate a unique signature for CD8 T cells that undergo exhaustion in liver cancer. As expected, the exhausted transcription profile showed parallels with the effector signature, corroborating the notion that exhaustion results from permanent antigen exposure. Following this argument, exhausted CD8 T cells revealed a stepwise loss of cytokine secretion (IL-2 > TNF-α > IFN-γ) and were finally deleted. In many aspects, however, exhausted T cells revealed a unique mRNA expression profile, which differed significantly from T cells in acute and chronic infections. As reported for other tumor entities, our analysis of coinhibitory and costimulatory molecules revealed up-regulation of PD-1 as well as a plethora of other coinhibitory receptors including TIGIT, LAG-3, and TIM-3. Of the costimulatory receptors, CD137 was up-regulated specifically in exhausted T cells, in accordance with data suggesting a role for CD137 as a tumor-specific marker.(30) For coinhibitory molecules, the highest relative mRNA expression was found for TIGIT and LAG-3. While LAG-3 is believed to exert its suppressive effect on CD8 T cells rather indirectly by interaction with MHC class II on CD4 T cells and antigen-presenting cells,(34) the exhaustive effect of TIGIT seems to rely predominantly on CD8 T cells and, to a lesser extent, on natural killer and regulatory T cells.(35-38) While no data from clinical phase 2/3 trials are yet available on TIGIT, a number of reports from other tumor entities support a decisive role for TIGIT signaling in cancer-mediated T-cell exhaustion. TIGIT-mediated T-cell exhaustion has been shown in melanoma,(39, 40) renal cell carcinoma,(41) Hodgkin lymphoma,(42) glioblastoma,(43) gastric cancer,(44) multiple myeloma,(45) acute myeloid leukemia,(46) and more than 80 other tumor entities.(47) In most of these cancers the expression pattern of TIGIT was similar but not identical to PD-1. The coordinated expression of PD-1 and TIGIT suggests the presence of a common transcriptional regulator. Indeed, recent studies have identified B lymphocyte–induced maturation protein 1 (Blimp-1) as a master transcriptional regulator of several checkpoint inhibitors, including PD-1 and TIGIT.(48, 49) However, in our analysis Blimp expression in exhausted T cells was only mildly up-regulated compared to effector T cells, suggesting that other transcription factors (e.g., Tox and Ahr) may be involved in the regulation of coinhibitory receptor expression.
Despite the positive correlation between PD-1 and TIGIT expression, the roles of the two coinhibitory receptors have been shown to be nonredundant, with synergistic effects on T-cell exhaustion in chronic infection and cancer.(50, 51) Due to the pleiotropic effects of TIGIT on multiple immune cell populations, therapeutic targeting of TIGIT seems ideally suited to complement the immunostimulatory features of PD-1 inhibition. Furthermore, animal studies with TIGIT inhibitors have demonstrated safe application in vivo without detrimental side effects. These results led to the proposal of a checkpoint inhibitor hierarchy with differential toxicity profiles(34, 52) (CTLA-4 > PD-1 > TIGIT/LAG-3/TIM-3), suggesting superior clinical safety of PD-1/TIGIT inhibition compared to the more toxic PD-1/CTLA-4 regimens.
In contrast to other tumor entities, biomarkers which predict responses to checkpoint blockade are lacking in patients with HCC. In this context, our observation of two patient subgroups, one with and another without up-regulation of PD-1 and TIGIT in liver cancer compared to adjacent liver tissue, suggests that these two subgroups might respond differently to checkpoint inhibition. This hypothesis is corroborated by findings from other groups which found better clinical responses to checkpoint inhibition in patients with HCC with high PD-1 expression on HCC-specific CD8 T cells.(31, 53) Further studies will be required to confirm this hypothesis in patients with HCC or iCCA receiving checkpoint inhibition in future clinical trials.
In summary, our study provides a comprehensive analysis of T-cell kinetics, function, and gene expression in liver cancer. As illustrated by the identification of TIGIT and the synergistic effect of a combined PD-1/TIGIT expression, our transcriptional profile of exhausted CD8 T cells bears the potential to identify novel molecules and pathways which control T-cell exhaustion in liver cancer. Although it remains unclear whether patients with HCC will clinically benefit from TIGIT inhibition—either as monotherapy or in combination with other checkpoint inhibitors—our results support the use of antagonistic, TIGIT-specific antibodies for the treatment of hepatocellular carcinoma.
Acknowledgment
Open access funding enabled and organized by Projekt DEAL.
Author Contributions
D.O., S.D., and J.W. were responsible for the acquisition of data. D.O. and T.C.W. designed the experimental approach. D.O., N.W., F.K., T.E., and T.C.W. wrote the manuscript. D.O. and O.D.B. performed the microarrays. D.O., O.D.B., S.R., S.N., and S.C. performed the microarray data analysis. K.T., M.K., and W.R. provided human samples. M.P.M. supervised the research and provided resources. All authors contributed to the writing of the manuscript.




