A Polarizing Case of Elevated Liver Enzymes
Case Presentation
A 25-year-old male was referred to a hepatology clinic for abnormal liver enzymes: aspartate aminotransferase = 242 IU/L, alanine aminotransferase = 507 IU/L, alkaline phosphatase = 139 IU/L, international normalized ratio = 1.1, bilirubin = 1.3 mg/dL, and albumin = 4.2 g/dL. His hemoglobin was 13.3 g/dL and platelet count was 130 × 109/L. The patient reported heavy alcohol use in his teenage years but quit 1 year before presentation. Serologic testing was negative for viral hepatitis, autoimmune markers, Wilson’s disease, schistosomiasis, and alpha-1-antitrypsin deficiency. Transferrin saturation was 3%, ferritin was 21 ng/mL, and 25-OH-vitamin D was 14.1 ng/mL. A liver biopsy showed bilirubinostasis, portal inflammation, and bridging fibrosis.
He was lost to follow-up and re-referred 4 years later for persistently abnormal liver chemistries. A second liver biopsy performed through the transjugular route showed a wedged hepatic pressure of 17 mm Hg, free of 10 mm Hg, and gradient of 7 mm Hg. Biopsy demonstrated bridging fibrosis with focal nodularity concerning for cirrhosis, cholestasis, and red-brown protoporphyrin deposits in the canaliculi, hepatocytes, and Kupffer cells of the liver. On polarization, deposits revealed Maltese cross configuration typical of porphyrin metabolism disorders (Fig. 1).
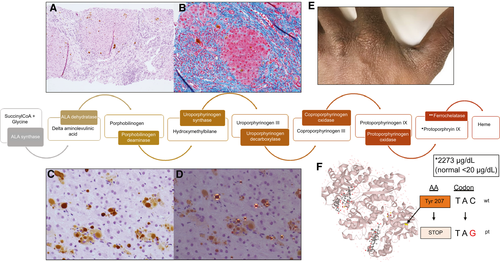
In retrospect, the patient recalled exquisite, unexplained photosensitivity as a child living in Africa, such that he became scarred on the bridge of his nose, pinnae, and dorsum of hands, forcing him to remain indoors. He also had mild splenomegaly on exam and ultrasound imaging (11.5 cm in 2015; 11.7 cm in 2019). Subsequent testing revealed elevated serum porphyrins of 10 µg/dL (normal 0-1 µg/dL), free protoporphyrins of 2,273 µg/dL (normal < 20 ug/dL), and zinc protoporphyrins of 85 µg/dL (normal < 60 μg/dL). Genetic testing (Prevention Genetics Chronic/Cutaneous Porphyria Panel; Marshfield, WI) using next generation sequencing of exome with copy number variant analysis confirmed two mutations in the FECH gene–c.621C>G p.Tyr207*, undocumented dbSNP ID number, and c.315-48T>C, intronic, dbSNP ID rs2272783, allele frequency in African population 0.018 (https://gnomad.broadinstitute.org/variant/18-55238820-A-G?dataset=gnomad_r2_1).
Due to evidence of portal hypertension and biochemical liver dysfunction, the patient has been referred for bone marrow and liver transplant evaluation.
Discussion
Protoporphyrias are rare inborn errors of heme synthesis that result in accumulation of porphyrins in bone marrow and liver. Autosomal recessive mutations in the ferrochelatase (FECH) gene leads to erythropoietic protoporphyria (EPP), resulting in porphyria uptake by hepatocytes and endothelial cells.(1)
Our patient’s first mutation, c.621C>G, is unique and pathogenic as it alters tyrosine at amino acid position 207 to a stop codon (Combined Annotation Dependent Depletion 39; scores above 30 suggest high likelihood for deleterious effect [https://cadd.gs.washington.edu/snv/GRCh38-v1.5/18:57562958_G_C]). This nonsense mutation has not been reported. The patient’s second mutation, c.315-48T>C, a known hypomorphic allele located within intron 3, has been shown to lead to clinical disease in association with other trans null FECH alleles, and is a necessary but not sufficient variant to cause overt clinical symptoms.(2)
Patients present with acute, severe, nonblistering phototoxicity within minutes of sun exposure. Half of patients have chronic anemia, and many have iron deficiency and low vitamin D levels. Only 25% of patients have elevated liver enzymes, and 2%-5% will develop chronic liver disease. There are no approved therapies for protoporphyrias. Recommendations include annual monitoring of liver enzymes, complete blood count, ferritin, vitamin D levels, screening for gallstones, and wearing sun protective clothing.(3) Bone marrow transplantation corrects the underlying genetic defect and may lead to improvement in hepatic function. In the rare instance of combined cirrhosis and liver failure, bone marrow and liver transplantation have been described. Case series suggest that pre-operative plasmapheresis, red cell exchange, hemin infusions to lower blood protoporphyrin levels, and operating room light filters with wavelengths smaller than 470 nm improve patient outcomes.(4, 5)
In summary, our patient with nonacute EPP developed cirrhosis and portal hypertension in part due to delayed diagnosis, and was referred to hematology, medical genetics, and transplant hepatology for a multidisciplinary approach in management.




