Organic Solute Transporter Alpha Deficiency: A Disorder With Cholestasis, Liver Fibrosis, and Congenital Diarrhea
Solute carrier family 51 alpha subunit (SLC51A) encodes the alpha subunit of the heteromeric organic solute transporter alpha–beta (OSTα–OSTβ), an important contributor to intestinal bile acid (BA) reabsorption in the enterohepatic circulation.1, 2 Here, we identified the first case of OSTα deficiency in a child with unexplained elevated liver transaminases, cholestasis, and congenital diarrhea.
Case Report
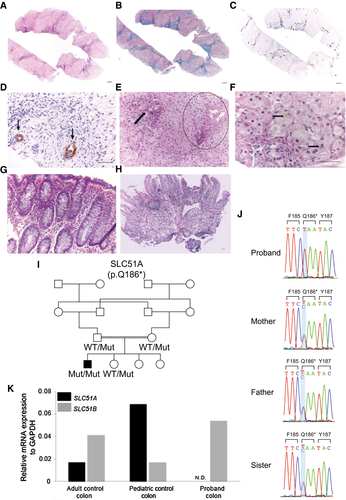
A 2.5-year-old Pakistani boy, born from a first-cousin union, was evaluated in a liver clinic. His past medical history is significant for chronic malabsorptive diarrhea of 10-15 oily daily stools, easy bruising, two episodes of prolonged bleeding that required blood transfusions, and failure to thrive. Stool studies included normal pH, and negative occult blood, reducing substance, and ova cysts. The patient was exclusively breastfed until 1 year of age, when he was started on soy-based formula milk, nutritional rehabilitation, and fat-soluble vitamin supplementation, with improvement in his weight and height. At age 2.5, he was found to have elevated transaminases and alkaline phosphatase (Table 1). Liver biopsy revealed a lobular architecture suggestive of early cirrhosis with portal and periportal fibrosis and many thin fibrous septa (Fig. 1A,B). Portal areas showed minimal lymphocytic inflammation, no interface activity, and patchy mild bile ductular proliferation (Fig. 1C,D). Bile ducts were missing in three of the portal tracts (Fig. 1E), not meeting criteria for bile duct paucity. The hepatocytes showed rare foci of subtle hepatocytic cholestasis (Fig. 1F). Duodenal and colonic biopsies showed no significant abnormality (Fig. 1G,H). Since a comprehensive workup was unrevealing (Supporting Table S1), his exome was sequenced. Consistent with consanguinity, the proband harbors 23 rare homozygous protein-altering variants, including 22 missense variants predicted to be tolerated by a meta-analytic support vector machine (MetaSVM) and therefore unlikely to be pathogenic in this child. Notably, the other homozygous mutation led to a premature termination at codon 186 in SLC51A (NM_152672,c.556C>T,p.Gln186*), which encodes OSTα. This variant allele is extremely rare, with only one instance among more than 251,400 alleles sequenced from diverse populations, including South Asians (gnomAD: https://gnomad.broadinstitute.org, last accessed September 25, 2019). Sanger sequencing confirmed the homozygous variant in the proband and showed the heterozygous carrier state of both his parents and an unaffected sister (Fig. 1I,J). Furthermore, the proband’s colon tissue showed undetectable SLC51A transcripts (Fig. 1K). Analysis of the BA concentrations in the proband’s blood spot by liquid chromatography–tandem mass spectrometry (LC-MS/MS) showed that BA concentrations were within the normal range despite underlying cholestasis. He was started on ursodiol at 5 years and 1 month and 9 months later on cholestyramine. While the coagulopathy resolved, his growth is adequate and he has two or three bulky daily bowel movements; his transaminases, direct bilirubin, and gamma-glutamyltransferase (GGT) levels remain elevated (Table 1).
| Laboratory Test | Reference | Proband’s Age in Years | |||
|---|---|---|---|---|---|
| 2.5 | 3.8 | 4 | 5 | ||
| AST (U/L) | 15-40 | 126 (H) | 112(H) | 98 (H) | 66 (H) |
| ALT (U/L) | 4-25 | 227(H) | 126 (H) | 117 (H) | 90 (H) |
| Bilirubin total/direct (mg/dL) | 0.1-1.2/<0.3 | 0.7/n.a. | 1.6 (H)/n.a. | 1.8/1.2 (H) | 2.3/1.9 (H) |
| GGT (U/L) | 5–32 | n.a. | n.a. | n.a. | 104 (H) |
| ALP (U/L) | 100–320 | 708 (H) | 1320 (H) | 661 (H) | 268 |
| INR | 0.9-1.1 | 3.1 (H) | 1.6 (H) | 1.6 (H) | 1.1 |
| Vitamin D, 25-OH (ng/mL) | 16-65 | 8.7 | n.a. | n.a. | 28* |
| Albumin (mg/dL) | 3.5-5.5 | n.a. | n.a. | 3.7 | n.a. |
| BAs measured in DBS samples† | |||||
| Cholic acid | 0.003-0.19 | n.a. | n.a. | n.a. | 0.02 |
| Dihydroxycholanoates‡ (μM) | 0.09-2.21 | n.a. | n.a. | n.a. | 0.11 |
| Glychocholic acid (μM) | 0.005-2.52 | n.a. | n.a. | n.a. | 0.11 |
| Glycodihydroxycholanoates§ (μM) | 0.22-4.16 | n.a. | n.a. | n.a. | 0.33 |
| Taurocholic acid (μM) | 0.001-0.612 | n.a. | n.a. | n.a. | 0.06 |
| Taurodihydroxycholanoates|| (μM) | 0.03-0.43 | n.a. | n.a. | n.a. | 0.07 |
- * On vitamin D supplement.
- † Prior to initiation of ursodeoxycholic acid supplement.
- ‡ Chenodeoxycholic acid + deoxycholic acid.
- § Glycochenodeoxycholic acid + glycodeoxycholic acid.
- || Taurochenodeoxycholic acid + taurodeoxycholic acid.
- Abbreviations: ALP, alkaline phosphatase; DBS, dried blood spots; H, high; INR, international normalized ratio; n.a., not available.
Discussion
Our findings provide the first demonstration of human OSTα deficiency in a child with elevated liver transaminases, cholestasis, liver fibrosis, and congenital diarrhea. The evidence implicating SLC51A genotype as the cause of the proband’s phenotype is strong. First, the proband represents the first homozygote for an extremely rare premature termination mutation in SLC51A. Second, there is an absence of SLC51A mRNA expression in the proband’s colon tissue, consistent with his recessive premature termination mutation in SLC51A leading to mRNA decay. Third, the proband’s clinical phenotype is consistent with a defect in BA metabolism. Fourth, human deficiency for OSTβ, which forms a heterodimer with OSTα in the active transporter, has been described in two siblings who also presented with congenital diarrhea and features of cholestasis at pediatric age.3 Altogether, these 3 patients with either OSTα or OSTβ deficiency have elevated transaminases (alanine aminotransferase [ALT] > aspartate aminotransferase [AST]) and GGT and low-normal circulating BAs. It is noteworthy that the liver biopsy from our proband revealed a more advanced liver disease than the liver injury reported in the two OSTβ-deficient siblings.3 The full clinical spectrum attributable to the loss of SLC51A will need to be determined by the identification and study of additional patients with OSTα deficiency.
Collectively, this case underscores how an unbiased genomic approach is highly valuable in clinical hepatology practice,4 while uncovering gene contributions in human health and disease.
Acknowledgment
We thank the proband and his family for their invaluable contribution to this work and the staff of the Yale Center for Genome Analysis for exome sequencing.
Author Contributions
P.K.M., R.P.L. and S.V. developed study concept and design; E.G., H.C., N.E., C.N.-W., D.J., C.J.S., J.L.B., Y.K., P.T.C., P.K.M., R.P.L., S.V. performed research and/or analyzed and/or interpreted data; H.C., N.W., I.M. participated in patient’s recruitment and/or patient’s ascertainment and management; E.G. and S.V. wrote the first draft of the manuscript and all authors critically revised the manuscript draft.




