Genetic Variation in HSD17B13 Reduces the Risk of Developing Cirrhosis and Hepatocellular Carcinoma in Alcohol Misusers
Abstract
Background and Aims
Carriage of rs738409:G in patatin-like phospholipase domain containing 3 (PNPLA3) is associated with an increased risk for developing alcohol-related cirrhosis and hepatocellular carcinoma (HCC). Recently, rs72613567:TA in hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) was shown to be associated with a reduced risk for developing alcohol-related liver disease and to attenuate the risk associated with carriage of PNPLA3 rs738409:G. This study explores the risk associations between these two genetic variants and the development of alcohol-related cirrhosis and HCC.
Approach and Results
Variants in HSD17B13 and PNPLA3 were genotyped in 6,171 participants, including 1,031 with alcohol-related cirrhosis and HCC, 1,653 with alcohol-related cirrhosis without HCC, 2,588 alcohol misusers with no liver disease, and 899 healthy controls. Genetic associations with the risks for developing alcohol-related cirrhosis and HCC were determined using logistic regression analysis. Carriage of HSD17B13 rs72613567:TA was associated with a lower risk for developing both cirrhosis (odds ratio [OR], 0.79; 95% confidence interval [CI], 0.72-0.88; P = 8.13 × 10−6) and HCC (OR, 0.77; 95% CI, 0.68-0.89; P = 2.27 × 10−4), whereas carriage of PNPLA3 rs738409:G was associated with an increased risk for developing cirrhosis (OR, 1.70; 95% CI, 1.54-1.88; P = 1.52 × 10−26) and HCC (OR, 1.77; 95% CI, 1.58-1.98; P = 2.31 × 10−23). These associations remained significant after adjusting for age, sex, body mass index, type 2 diabetes, and country. Carriage of HSD17B13 rs72613567:TA attenuated the risk for developing cirrhosis associated with PNPLA3 rs738409:G in both men and women, but the protective effect against the subsequent development of HCC was only observed in men (ORallelic, 0.75; 95% CI, 0.64-0.87; P = 1.72 × 10−4).
Conclusions
Carriage of variants in PNPLA3 and HSD17B13 differentially affect the risk for developing advanced alcohol-related liver disease. A genotypic/phenotypic risk score might facilitate earlier diagnosis of HCC in this population.
Abbreviations
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- HBV
-
- hepatitis B virus
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HSD17B13
-
- hydroxysteroid 17-beta dehydrogenase 13
-
- MAF
-
- minor allele frequency
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- OR
-
- odds ratio
-
- PAF
-
- population-attributable fraction
-
- PNPLA3
-
- patatin-like phospholipase domain containing 3
-
- SNP
-
- single-nucleotide polymorphism
-
- T2DM
-
- type 2 diabetes
-
- TM6SF2
-
- transmembrane 6 superfamily member 2
Alcohol-related liver disease has a global prevalence of 12% and accounts for half of all cirrhosis-associated deaths in Europe and the United States.1-3 Alcohol-related cirrhosis is now the second-most-common indication for liver transplantation, accounting for approximately 40% of all primary liver transplants in Europe and approximately 25% in the United States.4
Chronic alcohol misuse is associated with the development of a broad spectrum of liver injury. Hepatic steatosis develops in most heavy alcohol users, but more substantial liver injury only develops with persistent alcohol misuse over time; inflammation and progressive fibrosis will develop in 10%-35% of individuals, whereas cirrhosis is observed in only 10%-15%.5-7 Between 5% and 15% of people with alcohol-related cirrhosis are at risk for developing hepatocellular carcinoma (HCC); the annual incidence is 2.5%-3.0%, with a 5-year cumulative risk of around 8%.8-11 The global incidence of HCC is increasing; it is now the fifth-most-frequent cancer and third-most-frequent cause of cancer-related mortality worldwide; one third of cases develop on a background of alcohol-related cirrhosis.12
The susceptibility to develop significant alcohol-related liver disease is determined by the interplay of a number of risk factors, including sex; ethnicity; the amount/pattern of alcohol drinking; coffee consumption; cigarette smoking; comorbidities such as obesity, type 2 diabetes, and hepatitis C virus (HCV) infection; and a number of host genetic factors.13 Carriage of the common missense variant rs738409:G in patatin-like phospholipase domain containing 3 (PNPLA3) is the most robustly validated risk locus for the development of alcohol-related cirrhosis and HCC,14-19 accounting for 26.6%14 and 43.5%18 of the variance, respectively. Two further gene variants, rs58542926 in transmembrane 6 superfamily member 2 (TM6SF2) and rs641738 in membrane-bound O-acetyltransferase domain containing 7 (MBOAT7), are additional risk factors, albeit with much lower effect sizes.19
Recently, Abul-Husn et al.,20 identified a splice variant rs72613567 in hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) that appears to protect against the development of chronic liver injury in people for European ancestry. HSD17B13 encodes an hepatic lipid droplet protein; the insertion of an adenine adjacent to the donor splice site on exon 6 (rs72613567:TA allele) results in an unstable and truncated protein with reduced enzymatic activity, suggesting a loss-of-function variant.20 Abul-Husn et al.20 found that carriage of HSD17B13 rs72613567:TA was associated with reduced serum aminotransferase activities and a reduced risk for developing alcohol-related and nonalcoholic fatty liver disease (NAFLD) and, more specifically, for developing both alcohol-related and NAFLD-related cirrhosis.20 The association with alcohol-related cirrhosis was the most compelling, but the total number of cases was very small. This group also showed that carriage of this variant attenuated the risk for developing progressive liver injury associated with carriage of rs738409:G in PNPLA3.20
Yang et al.21 recently confirmed the association between carriage of rs72613567:TA in HSD17B13 and a reduction in the risk for developing alcohol-, NAFLD-, and HCV-related liver disease and, more specifically, the risk for developing cirrhosis. They also found that carriage of this variant protected against the development of HCC, but only in patients with a background of alcohol-related liver disease. The sample size was small, and those with HCC were more frequently male and older than their counterparts without HCC. Thus, in order to validate these findings, much larger cohorts are needed to allow for adjustment for these potential confounders.21 Interactions between variants in HSD17B13 and PNPLA3 were not addressed in this study.
The aims of the present study were to determine whether: (i) carriage of rs72613567:TA in HSD17B13 protects against the development of alcohol-related cirrhosis and alcohol-related HCC; and, (ii) to explore possible risk interactions between rs72613567:TA in HSD17B13 and rs738409:G in PNPLA3.
Patients and Methods
Study Cohorts
- Alcohol-related cirrhosis and HCC (HCC; n = 1,031)
- Alcohol-related cirrhosis without HCC (CIRR; n = 1,653)
- Alcohol misusers with no evident liver disease (ALC; n = 2,588)
- Healthy controls with no history of alcohol misuse or liver disease (n = 899)
Participants in cohorts 1-3 were recruited from hepatology units and addiction centers across Europe and were of self-reported Swiss/German/Austrian/Italian/British ancestry. Ninety of the UK samples with cirrhosis and HCC were obtained from the Nottingham Digestive Diseases Centre Biomedical Research Unit Research Tissue Bank (Rec Ref: 14/WA/1234). Participants in cohort 4 were recruited from the United Kingdom.
The diagnosis of alcohol-related cirrhosis was established as described in detail previously;19 briefly, the diagnosis was based on a history of prolonged, sustained alcohol intake of a minimum of 40 g/day in women and 60 g/day in men together with histological examination of liver tissue or compatible historical, clinical, laboratory, radiological, and endoscopic features of advanced chronic liver disease. Patients were excluded if they had any other potential cause of liver injury, specifically, if they were positive for hepatitis B surface antigen, anti-HCV immunoglobulin G, antinuclear antibodies (titer > 1:80), or antimitochondrial antibodies (titer > 1:40). Patients with elevated serum ferritin concentrations and a transferrin saturation of >50%, a serum caeruloplasmin of <20 mg/dL (0.2 g/dL), or a serum alpha-1 antitrypsin of <70 mg/dL (13 µmol/L) were further investigated and excluded, as appropriate.
The diagnosis of HCC was based on histological examination of tumor tissue or evidence on imaging, preferably using two modalities, of lesions that were hypervascular in the arterial phase with washout in the portal venous or delayed phases.22 The severity of the underlying cirrhosis was assessed using Pugh’s modification of Child’s grading system.23
The patients with alcohol misuse but no evidence of significant liver injury were recruited as described in detail previously;19 in brief, they had a background of alcohol consumption of at least 60 g/day for women and 80 g/day for men for ≥10 years with or without features of alcohol dependence.24 None had historical, clinical, or laboratory evidence of liver disease, and its absence was confirmed either by a liver stiffness measurement (FibroScan, Echosens, Paris) of below 6 kPa (interquartile range <20%) or by the absence of histological liver damage.
Healthy controls were recruited from London branches of the National Health Service blood transfusion service, from general practitioners’ surgeries, from among university students, and from the general public. None currently drank alcohol above a weekly maximum of 112 g for women and 168 g for men, nor had they done so at any time in the past. None had a history or clinical evidence of liver disease.
DNA Preparation and Genotyping
Genomic DNA was extracted from venous blood samples and quantified using standard procedures.19 Genotyping of the single-nucleotide polymorphisms (SNP) of interest viz. PNPLA3 rs738409 (Assay ID: C_7241_10) and HSD17B13 rs72613567 (primer and probe sets manufactured through custom TaqMan Assay design) was performed using TaqMan SNP genotyping assays and chemistries (Applied Biosystems, Waltham, MA) on an automated platform with Tecan Freedom EVO and 384-well TeMO liquid-handling robots (Tecan, Männedorf, Switzerland) as described previously.18, 19
All process data were logged and administered with a database-driven LIMS. Reactions were completed and read in a 7900 HT TaqMan sequence detector system (Applied Biosystems, Waltham, MA). The amplification reaction was carried out with the TaqMan universal master mix at cycling conditions of 1 cycle for 10 minutes at 95°C, followed by 45 cycles for 15 seconds at 95°C and 1 minute at 60°C.
Statistical Analysis
Logistic regression and SNP*SNP interaction analyses were performed using SPSS v.25.0 (IBM Corporation, Armonk, NY). A three-way case control design was adopted: HCC versus CIRR; CIRR versus ALC; HCC versus ALC. Genotypic and allelic tests of association were assessed using two logistic regression models:
Model A: univariate logistic regression, and
Model B: adjustments for age, sex, body mass index (BMI), type 2 diabetes, and country.
Results, expressed as odds ratios (ORs) with their 95% confidence intervals (CI), were derived from beta coefficients and their standard deviations. Nominal two-sided asymptotic P values are reported for all tests.
The interactions between HSD17B13 and PNPLA3 were examined by logistic regression for the univariate additive and genotypic regression models, including both main SNP effects and the SNP*SNP interaction term.
Fixed-effect model meta-analysis using the inverse variance-weighted method to summarize effect sizes and forest plots were performed using the R package “metaphor” v.2.0-0.
Sex-specific post hoc analyses for the risk associations with HSD17B13 and PNPLA3 were performed. The Mantel–Haenszel test for trend was applied for testing a linear trend in observed genotype proportions from contingency tables for ALC < CIRR < HCC, by sex.
The population-attributable fraction (PAF) provides an epidemiological estimate of the proportion of a disorder that is attributable to a given risk factor. Thus, in this instance, it provides an estimate of how much lower the frequency of HCC would be in patients with alcohol-related cirrhosis if the risk genotype(s) were eliminated from the population.
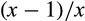
where x = (1 − p)2 + 2p(1 − p)OR1 + p2OR2(19); p is the allele frequency in the CIRR or ALC cohorts, and OR1 and OR2 are the ORs associated with hetero- and homozygosity.
Combined PAF estimates were calculated as PAF = 1−(1 − PAF1)(1 − PAF2)(1 − PAFn) based on the individual PAFs for each associated SNP, assuming no multiplicative interaction between them.
Ethics
The study protocol was approved by the ethics committees of the participating institutions; all included subjects provided written informed consent before inclusion into the study.
Results
The patient cohorts were predominantly male and middle aged. The patients with cirrhosis were generally older than those misusing alcohol and more likely to be overweight and to have type 2 diabetes. The patients with cirrhosis and HCC were generally older than the patients with cirrhosis without malignant transformation and were proportionately more likely again to be male, overweight, and to have type 2 diabetes (Table 1). Laboratory variables showed the expected gradients (Supporting Table S1).
| Cohorts | Number | Age [SD] | Men (%) | BMI [SD] | T2DM (%) | Nicotine (% Users) |
|---|---|---|---|---|---|---|
| Alcohol-related cirrhosis with HCC (HCC) | ||||||
| Total | 1,031 | 62 [10] * | 91 * | 27.8 [4.8] * | 45% * | 47% * |
| Germany | 778 | 61 [10] | 91 | 27.8 [4.8] | 45% | 50% |
| Switzerland | 115 | 61 [11] | 85 | 27.9 [5.0] | 53% | 38% |
| United Kingdom‡ | 65 | 65 [9] | 93 | 25.6 [3.1] | 0% | N/A |
| Italy | 73 | 72 [8] | 92 | N/A | 47% | 29% |
| Alcohol-related cirrhosis without HCC (CIRR) | ||||||
| Total | 1,653 | 55 [10] † | 72 † | 25.9 [4.9] † | 16% † | 61% † |
| Germany | 1,050 | 56 [10] | 72 | 26.2 [5.2] | 22% | 61% |
| Switzerland | 192 | 56 [10] | 73 | 26.2 [5.8] | 29% | 48% |
| United Kingdom* | 376 | 53 [11] | 68 | 24.6 [2.6] | N/A | N/A |
| Italy | 35 | 54 [9] | 86 | N/A | 24% | 66% |
| Alcohol misusers (ALC) | ||||||
| Total | 2,588 | 48 [10] | 84 | 24.7 [4.1] | 4% | 79% |
| Germany | 1,827 | 48 [9] | 88 | 24.8 [4.3] | 4% | 81% |
| Switzerland | 417 | 45 [12] | 74 | 24.4 [4.0] | 14% | 59% |
| United Kingdom | 344 | 49 [10] | 76 | 24.7 [2.3] | 2% | N/A |
| Italy | N/A | N/A | N/A | N/A | N/A | N/A |
- Bold numbers (n) indicate total n for each cohort (HCC, CIRR, ALC).
- * Significance of the difference between patients with alcohol-related cirrhosis with and without HCC: P < 0.001.
- † Significance of the difference between patients with alcohol-related cirrhosis and alcohol misusers: P < 0.001.
- ‡ People with a BMI > 30 and/or with type 2 diabetes were excluded a priori.
- Abbreviations: BMI, body mass index; T2DM, type 2 diabetes; N/A, not available.
Genotyping was completed for both SNPs with call rates greater than 95% (Supporting Table S2). All markers followed Hardy–Weinberg equilibrium (cutoff HWE P > 0.05).26 In the healthy controls, the minor allele frequencies (MAFs) for both HSD17B13 rs72613567:TA (0.276) and PNPLA3 rs738409 G (0.224) were comparable with those in public databases27 and previous publications18-20 and did not differ significantly from the MAFs in the ALC group (Supporting Table S2).
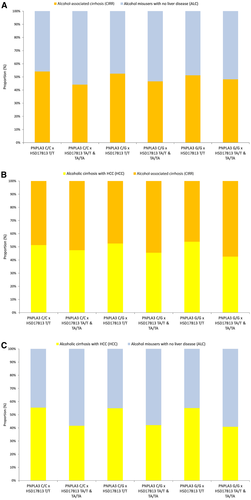
A progressive reduction in MAFs for HSD17B13 rs72613567:TA was observed between the 3 groups: ALC (26.4%); CIRR (22.0%), and HCC (17.7%; Ptrend = 1.09 × 10−15; Supporting Table S2). This contrasted with the expected stepwise increase in the MAFs for PNPLA3 rs738409:G in the same 3 groups, viz., ALC (23.9%), CIRR (35.4%), and HCC (49.8%; Supporting Table S2).
In the univariate model, allelic and genotypic associations for HSD17B13 rs72613567:TA were highly significant for the comparisons HCC versus CIRR (Pallelic = 2.27 × 10−4, Pgenotypic = 1.05 × 10−3), CIRR versus ALC (Pallelic = 8.13 × 10−6, Pgenotypic = 1.54 × 10−6), and HCC versus ALC (Pallelic = 1.69 × 10−14, Pgenotypic = 3.22 × 10−14; Table 2). The protective effect associated with carriage of HSD17B13 rs72613567:TA remained significant in the CIRR and HCC cohorts after correction for sex, age, BMI, type 2 diabetes, and country (Table 2). The protective effect for HCC was greater for homozygous than heterozygous carriage of the HSD17B13 rs72613567:TA allele (Fig. 1A,B).
| Cohorts | HSD17B13 (rs72613567) | Comparative Groups | Genotypic OR (95% CI) Regression Model: A | Genotypic OR (95% CI) Regression Model: B | Regression Model | Allelic OR (95% CI) | Significance (P) | Cases/Controls (n) | |
|---|---|---|---|---|---|---|---|---|---|
| HCC | CIRR | ||||||||
| Alcoholic-related cirrhosis and HCC (HCC) | T|T | 705 | 1,027 | ||||||
| T|TA | 287 | 524 | 0.80 (0.67-0.95) | 0.92 (0.69-1.22) | A | 0.77 (0.68-0.89) | 2.27 × 10−4 | 1,031/1,653 | |
| TA|TA | 39 | 102 | 0.56 (0.38-0.82) | 0.43 (0.23-0.80) | B | 0.79 (0.63-0.99) | 0.037 | 604/679 | |
| MAF | 0.177 | 0.220 | P = 1.05 × 10−3 | P = 0.028 | |||||
| CIRR | ALC | ||||||||
| Alcohol-related cirrhosis without HCC (CIRR) | T|T | 1,027 | 1,401 | ||||||
| T|TA | 524 | 1,009 | 0.71 (0.62-0.81) | 0.79 (0.63-0.98) | A | 0.79 (0.72-0.88) | 8.13 × 10−6 | 1,653/2,588 | |
| TA|TA | 102 | 178 | 0.78 (0.61-1.01) | 0.82 (0.54-1.23) | B | 0.85 (0.72-0.99) | 0.048 | 679/1,483 | |
| MAF | 0.220 | 0.264 | P = 1.54 × 10−6 | P = 0.085 | |||||
| HCC | ALC | ||||||||
| Alcohol misusers (ALC) | T|T | 705 | 1,401 | ||||||
| T|TA | 287 | 1,009 | 0.57 (0.48-0.66) | 0.68 (0.52-0.89) | A | 0.60 (0.53-0.69) | 1.69 × 10−14 | 10,31/2,588 | |
| TA|TA | 39 | 178 | 0.44 (0.30-0.62) | 0.39 (0.21-0.72) | B | 0.66 (0.53-0.81) | 1.14 × 10−4 | 604/1,483 | |
| MAF | 0.177 | 0.264 | P = 3.32 × 10−14 | P = 6.00 × 10−4 | |||||
Abbreviations: CI, confidence intervals; MAF, minor allele frequency; OR, odds ratio.
- Genotypic and allelic ORs were assessed by logistic regression models, model A: univariate logistic regression and model B: multivariate logistic regression, adjusted for age, sex, BMI, type 2 diabetes, and country.
Allelic and genotypic associations for PNPLA3 rs738409:G were also significantly associated in the CIRR (ORallelic 1.70 [1.54-1.88], Pallelic = 1.52 × 10−26) and HCC (ORallelic 1.77 [1.58-1.98], Pallelic = 2.31 × 10−23) cohorts (Table 3). These associations were robust to corrections for sex, age, BMI, type 2 diabetes, and country.
| Cohorts | PNPLA3 (rs738409) | Comparative Groups | Genotypic OR (95% CI) Regression Model: A | Genotypic OR (95% CI) Regression Model: B | Regression Model | Allelic OR (95% CI) | Significance (P) | Cases/Controls (n) | |
|---|---|---|---|---|---|---|---|---|---|
| HCC | CIRR | ||||||||
| Alcohol-related cirrhosis with HCC (HCC) | C|C | 269 | 697 | ||||||
| C|G | 497 | 714 | 1.80 (1.50-2.16) | 1.75 (1.30-2.36) | A | 1.77 (1.58-1.98) | 2.31 × 10−23 | 1,030/1,631 | |
| G|G | 264 | 220 | 3.11 (2.48-3.90) | 2.75 (1.88-4.01) | B | 1.67 (1.39-2.01) | 7.19 × 10−8 | 603/660 | |
| MAF | 0.498 | 0.354 | P = 2.98 × 10−22 | P = 4.37 × 10−7 | |||||
| CIRR | ALC | ||||||||
| Alcohol-related cirrhosis without HCC (CIRR) | C|C | 697 | 1,364 | ||||||
| C|G | 714 | 802 | 1.74 (1.52-2.00) | 1.64 (1.31-2.06) | A | 1.70 (1.54-1.88) | 1.52 × 10−26 | 1,631/2,319 | |
| G|G | 220 | 153 | 2.81 (2.25-3.53) | 2.65 (1.85-3.78) | B | 1.63 (1.39-1.91) | 1.67 × 10−9 | 660/1,469 | |
| MAF | 0.354 | 0.239 | P = 1.61 × 10−25 | P = 1.31 × 10−8 | |||||
| HCC | ALC | ||||||||
| Alcohol misuse (ALC) | C|C | 269 | 1,364 | ||||||
| C|G | 497 | 802 | 3.14 (2.65-3.73) | 2.75 (2.06-3.66) | A | 3.00 (2.67-3.36) | 2.17 × 10−79 | 1,030/2,319 | |
| G|G | 264 | 153 | 8.75 (6.90-11.10) | 6.76 (4.60-9.95) | B | 2.63 (2.18-3.17) | 3.89 × 10−24 | 603/1469 | |
| MAF | 0.498 | 0.239 | P = 4.14 × 10−78 | P = 5.50 × 10−23 | |||||
Abbreviations: CI, confidence intervals; MAF, minor allele frequency; OR, odds ratio
- Genotypic and allelic ORs were assessed by logistic regression models, model A: univariate logistic regression and model B: multivariate logistic regression, adjusted for age, sex, BMI, type 2 diabetes, and country.
Because patients were recruited from across Europe, a meta-analysis of the HSD17B13 and PNPLA3 loci for association with cirrhosis and HCC was performed by country (Supporting Tables S3 and S4). The associations were not necessarily significant in every European cohort but fixed-effect meta-analyses confirmed both the protective and risk-enhancing effects of the HSD17B13 and PNPLA3 loci, respectively, in the total cohort. There was no evidence of heterogeneity (Supporting Figs. S1A-C and S2A-C).
The PAF for PNPLA3 rs738409 for CIRR was 27.2% (20.7%-33.6%) and for HSD17B13 rs72613567 -14.7% (-21.2% to -7.9%). The combined PAF for CIRR was 16.5%. The PAF% for PNPLA3 rs738409 in HCC was 38.7% (29.3%-47.2%) and for HSD17B13 rs72613567 -10.0% (-16.7% to -2.7%). The combined PAF for HCC was 32.6%.
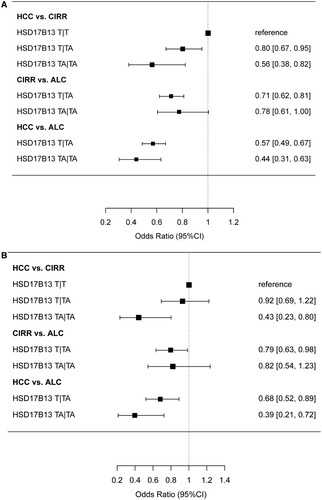
The potential modifying effect of HSD17B13 rs72613567 on PNPLA3 rs738409 was explored by calculating the proportion of the HCC, CIRR, and ALC cohorts with different “gene signatures,” combining either PNPLA3 rs739409:G (high risk variant) with wild-type HSD17B13 rs72613567:T (lack of protection) or the PNPLA3 rs739409 C (low-risk variant) with HSD17B13 rs72613567:TA (protection). A preponderance of patients in the CIRR and HCC cohorts were homozygous for PNPLA3 rs739409:G and for HSD17B13 rs72613567:T, which is in line with the estimated risk contributions of each variant (Fig. 2A,B).
Possible SNP*SNP interactions between HSD17B13 rs72613567 and PNPLA3 rs739409 were explored by testing interaction terms in the logistic regression models for the risk for developing CIRR and the further risk for developing HCC. The genotypic interactions for the risk association with CIRR was not significant in the total study population (P = 0.598; Supporting Table S5) or in the men-only cohort (P = 0.689; Supporting Table S6). None of the HSD17B13 × PNPLA3 genotype interaction pair combinations was significant. The interactions in the additive CIRR and HCC risk models were also not significant. Thus, there does not appear to be an HSD17B13 rs72613567:TA × PNPLA3 rs739409:G interaction.
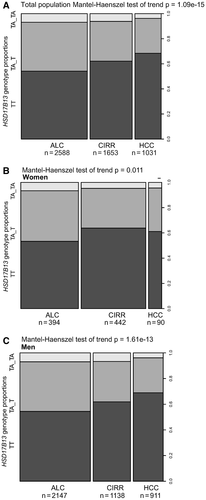
In a sex-specific post hoc analysis of the HSD17B13 locus, an association with HCC disease risk was observed in men (ORallelic, 0.75; 95% CI, 0.64-0.87; P = 1.72 × 10−4) but not in women (ORallelic, 1.07; 95% CI, 0.73-1.58; P = 0.772), whereas the protective effect for cirrhosis risk associated with carriage of HSD17B13 rs72613567:TA was observed in both sexes (Supporting Table S7; Fig. 3A-C). The association between carriage of PNPLA3 rs739409:G and cirrhosis and HCC disease risk showed no sex differential (Supporting Table S8).
Discussion
The genetic contribution to the development of significant alcohol-related liver injury is gradually being unraveled. Three genetic loci have been identified that are associated with an increased risk for developing cirrhosis at genome-wide significance, namely PNPLA3, TM6SF2, MBOAT7,19 and at least two variants, PNPLA3 rs738409 and TM6SF2 rs58542926, are associated with an increased risk for developing HCC on this background.15, 16, 18
There is little published evidence for genetic variants conferring protection against the development of significant alcohol-related liver injury. Indirect protection is afforded in individuals, primarily those of East Asian ancestry, who carry rs1229984 in alcohol dehydrogenase 1B28 and/or rs671 in aldehyde dehydrogenase 229 and, as a consequence, develop a reaction to alcohol and so tend to avoid it.
Recently, however, Abul-Husn et al.,20 identified a splice variant, rs72613567 in HSD17B13, which appeared to protect against the development of chronic liver injury in people of European ancestry. Specifically, this SNP appeared to confer protection against the development of alcohol-related and NAFLD-related cirrhosis.20 The association with alcohol-related cirrhosis was more compelling even though the total number of cases was very small, amounting to only 124 in their discovery cohort and 215 in their validation cohort.
In a later French/Belgain collaboration, Yang et al.,21 confirmed that rs72613567 in HSD17B13 confers protection against the development of alcohol-, NAFLD-, and HCV-related liver disease. They further showed that this SNP confers protection against the development of HCC, but only when arising on a background of alcohol-related liver disease. The data were adjusted for age, sex, and the degree of hepatic fibrosis but not for other important confounders, such as BMI, type 2 diabetes, and PNPLA3 genotype. Only half the patients with alcohol-related HCC in this study had cirrhosis. Thus, the numbers of patients with alcohol-related cirrhosis (n = 1,243) and alcohol-related HCC (n = 217) were relatively small, and those with HCC were more frequently male and older than those with cirrhosis, per se. Adjustments were not made for these possible confounders, leading the authors to conclude that larger cohorts were needed to validate their findings.
The cohorts included in the present study were large, consisting of 1,031 patients of European ancestry with alcohol-related cirrhosis and HCC and 1,653 with alcohol-related cirrhosis without HCC. In addition, 2,588 alcohol misusers with no evidence of liver disease were included to exclude the possibility that any observed genetic effect might relate to the susceptibility to develop problematic drinking.
The results of the present study robustly establish that carriage of HSD17B13:rs72613567:TA protects against the development of alcohol-related cirrhosis and its subsequent evolution to HCC. The results also reconfirm that carriage of PNPLA3 rs738409 is associated with a significantly increased risk of developing alcohol-related cirrhosis and HCC. However, the magnitude of these two effects is of a different order. Thus, although carriage of the HSD17B13:rs72613567:TA allele reduces the PAF for cirrhosis by 14.7% and for HCC by 10%, carriage of the PNPLA3 rs738409:G allele increases the PAFs by 27.2% and 38.7%, respectively. Consequently, the combined PAF for cirrhosis was 16.5% and for HCC 32.5%.
Thus, the risk of carriage of PNPLA3 rs738409:G appears to be attenuated, to a degree, by co-carriage of HSD17B13:rs72613567:TA. Abul-Husn et al.,20 explored the possibility of interactions between these two SNPs and observed nominally significant interactions in association analyses with serum aminotransferase activities, primarily in the obese, but there were no significant interactions in relation to chronic liver disease. They also showed, using RNA sequencing-based expression analysis, that HSD17B13 rs72613567:TA was associated with decreased PNPLA3 messenger RNA expression in an allele dose-dependent manner. However, exploration of SNP*SNP interaction in the present study showed no evidence of a significant interaction. Yang et al.,21 did not report data on possible SNP*SNP interaction, so no further comparisons can be made.
Male sex is a significant risk factor for the development of HCC in people with alcohol-related cirrhosis.12, 13 The reasons for this are unknown. Men are more likely to drink at harmful levels and hence more likely to develop alcohol-related cirrhosis, but they are still proportionately overrepresented among those developing HCC. Malignant transformation is more likely to occur in individuals with alcohol-related cirrhosis who stop drinking,30 hence the suggestion that the difference in sex-specific HCC rates may reflect lower abstinence rates among women. The possibility of sex-genetic variant interactions should also be considered. The comparative survival advantage in women with alcohol-related cirrhosis is well documented,31-33 and it has recently been shown that this may relate, at least in part, to a sex-variant interaction with rs738409:G in PNPLA3.34, 35
The possibility of sex-variant interactions was also explored in the present study. The progressive increase in the risk for developing alcohol-related cirrhosis and HCC associated with carriage of PNPLA3 rs738409:G was observed in both men and women. Likewise, the protective effect for cirrhosis risk associated with carriage of HSD17B13 rs72613567:TA was observed in both sexes. However, the protective association of HSD17B13 rs72613567:TA and HCC was only found in men. There was no significant sex difference in the MAFs for HSD17B13 rs72613567:TA in the alcohol misusers (26.3% vs. 26.6%) or in the patients with alcohol-related cirrhosis (22.4% vs. 20.5%). However, whereas the frequency was further reduced in men with HCC (17.8%), the trend partially reversed in women (21.7%). These findings are counterintuitive and are without explanation. The number of women with HCC was relatively small, so this finding needs further exploration in a larger cohort.
Neither of the previous studies explored sex differences in the effects of HSD17B13 rs72613567:TA.20, 21 However, Ferenci et al.,36 found sex-related phenotypic variation associated with carriage of HSD17B13 rs72613567:TA in patients with Wilson’s disease. Thus, they showed that none of the men who developed fulminant Wilson’s disease carried the protective HSD17B13 variant in contrast to 13.6% of their female counterparts. Clearly, sex-variant interactions should be considered in any further genetic studies in the field of liver disease.
HSD17B13 belongs to the family of pluripotent 17-hydroxysteroid dehydrogenase enzymes whose members convert 17-keto- and 17-hydroxysteroids; regulate the biological activity of sex hormones; participate in fatty acid and cholesterol metabolism; and contribute to bile acid synthesis.37 The function of HSD17B13 is incompletely understood, but it is located on the surface of lipid droplets and is mainly expressed in the liver. Su et al.,38 have demonstrated that transcriptional regulation of HSD17B13 expression is likely to be liver X receptor mediated through a sterol regulatory element binding protein 1c (SREBP-1c)-dependent mechanism; they also found evidence for a SREBP-1c response element in the promoter region of the HSD17B13 gene located on chromosome 4q22.1. HSD17B13 expression is increased in patients with NAFLD39, 40 and in murine models of fatty liver disease,41 and its overexpression in Huh7 and HepG2 hepatoma cell lines results in accumulation of HSD17B13 on the surface of lipid droplets.40, 42
Ma et al.,43 have recently shown that HSD17B13 is an hepatic retinol dehydrogenase. Retinol, retinoic acid, and retinol-binding protein have been implicated in the pathogenesis of steatosis, fibrosis, adipogenesis, and insulin resistance.44 Thus, HSD17B13 may be involved in the complex nuclear receptor interaction in NAFLD through activation of the retinoic acid receptor.45 Of greater importance, in the context of the present study, is the possibility that HSD17B13, functioning as a retinol dehydrogenase, may contribute to the depletion of hepatic retinoic acid observed in individuals chronically misusing alcohol. Retinoic acid depletion results in a functional down-regulation of liver retinoic acid receptors and a marked increase in the expression of the activator protein-1 (c-Jun and c-Fos) transcriptional complex, which is associated with hepatic cell hyperproliferation, a decrease in apoptosis, and stimulated hepatic carcinogenesis.46, 47 The retinol dehydrogenase activity of the rs72613567:TA variant is likely reduced or absent, and this may explain, at least in part, its protective effect against HCC in the context of alcohol-related cirrhosis. Accordingly, repletion of hepatic retinoic acid concentrations experimentally or in patients with HCC may have a therapeutic role.48
There are some contrary findings in relation to the protective effect of a reduction in HSD17B13 activity. Chen et al.,49 for example, reported that mRNA expression of HSD17B13 was down-regulated in Asians with hepatitis B virus (HBV)-related HCC, although not in HBV-related cirrhosis, and showed that low HSD17B13 expression in peritumor tissue was independently associated with a reduction in recurrence-free survival. They also showed that overexpression of HSD17B13 in Huh7 cell and SK-HEP-1 cell lines results in delays in cell cycle progression. Their overall conclusion, based on these findings, was that increased HSD17B13 expression might inhibit the development and progression of HBV-related HCC. This contrasts with the findings in the present study and those of others20, 21 that in Europeans, decreased expression of HSD17B13 protects against the development of both alcohol-related cirrhosis and its evolution to HCC. This apparent contradiction may attest to differences in the mechanisms of HCC development in viral- and alcohol-related liver disease. Clearly, further research on the functional role of HSD17B13 in HCC development and progression is needed.
There is considerable interest in the possibility of pharmaceutical inhibition of HSD17B13 to counteract the steatogenic effect of overexpressed HSD17B13 and mimic the loss-of-function derived from the HSD17B13 rs72613567:TA variant. For example, fenofibrate, which is an agonist of the peroxisome proliferator-activated receptor-α (PPARα) and approved for use in humans, suppresses hepatic HSD17B13 expression in mouse liver,50 a finding supported by the high expression of HSD17B13 observed in pparα knock-out mice.42
This study has a number of strengths. The study cohorts were large and well-characterized and specifically selected to explore the genetic risks associated with the development of alcohol-related cirrhosis and HCC in Caucasians of European origin. Controls were exercised for a number of known risk factors, such as age, sex, BMI, and type 2 diabetes. Similar effect sizes were observed across all individual geographical cohorts, excluding the possibility of heterogeneity and thus broadening the applicability of the findings. The specificity of the genetic associations was explored by including a large control cohort of alcohol misusers who had no evidence of liver disease.
This study also has its limitations; first, it was cross sectional and undertaken retrospectively, meaning that phenotypic data sets were not always complete. Second, it is likely that some of the patients with alcohol-related cirrhosis will develop HCC over time and equally likely that some of the individuals misusing alcohol will develop cirrhosis in the future, particularly if they continue to drink. However, the group differences were significant and remained robust to adjustment for confounders, including age. Finally, the findings cannot be used to define the genetic risks associated with the development of cirrhosis and HCC in people with liver disease of different etiologies or from outside of Europe, as evidenced by the findings of Chen and colleagues.49
In conclusion: HSD17B13 rs72613567:TA protects against the development of alcohol-related cirrhosis and, at least in men, the subsequent development of HCC. Its carriage attenuates the increased risk associated with carriage of PNPLA3 rs738409:G. Combining phenotypic and genetic signatures to score risk could facilitate management of patients with alcohol-related liver disease. Further evaluation of the function of HSD17B13 and the rs72613567 variant may identify suitable drug targets.
Acknowledgment
The authors thank the Clinical Research Support Service of the CHUV-UNIL, Lausanne, Switzerland, for providing the infrastructure for patient recruitment and collecting phenotypic data.
Author Contributions
F.S., J.H., and M.Y.M. conceived the study; F.S. raised the pivotal funding and wrote the manuscript; M.Y.M. cowrote the manuscript and reanalyzed the data; A.M. contributed biological material from healthy controls and reviewed the biostatistics analysis; F.S., P.L., and H.D.N. contributed biological material from cases and controls; I.S., V.R., J.F., K.H.W., D.G., J.R., A.M., M.E., M.K., M.C., F.L., U.S., F.E., A.V., S.M., J.v.F., H.W., R.S., S.A., A.F., S.N., V.M., C.S., L.S., C.L., R.E.S., A.C., A.L., L.V., J.I.G., G.P.A., J.U.M., W.F., S.Z., J.-F.D., J.T., C.D., P.D., S.M., T.B., and M.Y.M. contributed biological material from phenotyped cases and controls; J.H. provided the genotyping infrastructure; T.B. genotyped the healthy control samples collected in the United Kingdom; S.B. performed the biostatistics and analyzed the primary data; F.S., P.L., and H.D.N. analyzed the data; P.L., S.B., H.D.N., I.S., V.R., J.F., K.H.W., D.G., J.R., A.M., M.E., M.K., M.C., F.L., T.B., A.M., U.S., F.E., A.V., S.M., J.v.F., H.W., R.S., S.A., A.F., S.N., V.M., C.S., L.S., C.L., R.E.S., A.C., A.L., L.V., J.I.G., G.P.A., J.U.M., W.F., S.Z., J.-F.D., J.T., C.D., P.D., S.M., T.B., J.H., and M.Y.M. critically revised the manuscript; F.S., P.L., S.B., H.D.N., I.S., V.R., J.F., K.H.W., D.G., J.R., A.M., M.E., M.K., M.C., F.L., T.B., A.M., U.S., F.E., A.V., S.M., J.v.F., H.W., R.S., S.A., A.F., S.N., V.M., C.S., L.S., C.L., R.E.S., A.C., A.L., L.V., J.I.G., G.P.A., J.U.M., W.F., S.Z., J.-F.D., J.T., C.D., P.D., S.M., T.B., J.H., and M.Y.M. approved the final draft submitted.




