Neutrophil Gelatinase-Associated Lipocalin for Assessment of Acute Kidney Injury in Cirrhosis: A Prospective Study
Abstract
Kidney biomarkers appear to be useful in differential diagnosis between acute tubular necrosis (ATN) and other types of acute kidney injury (AKI) in cirrhosis, particularly hepatorenal syndrome (HRS-AKI). Distinction is important because treatment is different. However, kidney biomarkers are still not used in clinical practice. The aim of the current study was to investigate the accuracy of several biomarkers in differential diagnosis of AKI and in predicting kidney outcome and patient survival. This was a prospective study of 320 consecutive cases of AKI in patients hospitalized for decompensated cirrhosis. Evaluation of AKI was made with a diagnostic algorithm that included identification and removal/treatment of precipitating factors and albumin administration (1 g/kg for 2 days) to patients with AKI stage 1B or greater. Urinary neutrophil gelatinase–associated lipocalin (NGAL), monomeric NGAL (mNGAL), interleukin-18, and standard biomarkers were measured at diagnosis and on days 3, 7, and 14. Of the 320 cases, 153 were hypovolemia-induced AKI (48%), 93 were HRS-AKI (29%), 39 were ATN (12%), and 35 were due to miscellaneous causes (11%). Among all biomarkers, urinary NGAL measured at day 3 had the greatest accuracy for differential diagnosis between ATN and other types of AKI (area under the receiver operating characteristic curve, 0.87; 95% confidence interval, 0.78-0.95). The cutoff with the best predictive accuracy for ATN diagnosis was 220 µg/g creatinine. Progression of AKI during hospitalization was associated with persistently high NGAL levels, and NGAL was an independent predictive factor of AKI progression. Likewise, NGAL was also an independent predictive factor of 28-day mortality together with Model for End-Stage Liver Disease score. Conclusion: These results support the use of NGAL in clinical practice within the context of a diagnostic algorithm for differential diagnosis of AKI and outcome prediction in cirrhosis.
Abbreviations
-
- AKI
-
- acute kidney injury
-
- ATN
-
- acute tubular necrosis
-
- AUROC
-
- area under the receiver operating characteristic curve
-
- BA
-
- bile acid
-
- FeNa
-
- fractional excretion of sodium
-
- HRS-AKI
-
- hepatorenal syndrome
-
- ICA
-
- International Club of Ascites
-
- IL-18
-
- interleukin-18
-
- MELD
-
- Model for End-Stage Liver Disease
-
- mNGAL
-
- monomeric NGAL
-
- NGAL
-
- neutrophil gelatinase–associated lipocalin
-
- OR
-
- odds ratio
-
- RRT
-
- renal replacement therapy
-
- sCr
-
- serum creatinine
Acute kidney injury (AKI) is a common complication of patients with advanced cirrhosis hospitalized for an acute decompensation.1-6 Importantly, the occurrence of AKI impairs prognosis. The 3-month probability of mortality after AKI is very high, ranging from 28% to 47%.3-8 Mortality risk depends on several factors, particularly severity and type of AKI, associated complications, and degree of liver failure. Therefore, AKI should be regarded as one of the most relevant complications of cirrhosis, and efforts should be made to mitigate its impact in terms of morbidity and mortality.
The introduction and widespread use of diagnostic criteria of AKI in the area of cirrhosis has contributed to an increased awareness and earlier detection of AKI.9 However, some important problems remain. One of the main issues is the differential diagnosis of AKI, particularly between acute tubular necrosis (ATN) and hepatorenal syndrome (HRS-AKI). This is important because treatment is different; renal replacement therapy (RRT) is used for the former, and vasoconstrictors and albumin are used for the latter.9, 10 Currently, there are no objective tools with high accuracy for use in the differential diagnosis. Diagnosis is made on clinical grounds and/or with some classical urine biomarkers, including urine sodium concentration or fractional excretion of sodium (FeNa), the accuracy of which has never been convincingly proven in cirrhosis.11-13 Recently, published clinical studies have reported that neutrophil gelatinase–associated lipocalin (NGAL) and interleukin-18 (IL-18) may be of value in the differential diagnosis between ATN and HRS-AKI.14-20 NGAL and IL-18 are proteins that are hardly expressed in kidney tubular cells in normal conditions but are highly overexpressed when tubular cells are damaged.21, 22 Despite the results of these studies, urine biomarkers are still not used in clinical practice. This may be due to the relatively low number of patients included and the fact that the information provided was not completely persuasive with respect to the clinical relevance of biomarker assessment. We report the results of a prospective study performed in a large series of consecutive patients with cirrhosis and AKI, in whom several biomarkers were measured at different time points during hospitalization to assess the biomarker with the greatest accuracy in the differential diagnosis between ATN and other types of AKI. The correlations between biomarkers and important clinical outcomes, including AKI progression and mortality, were assessed as secondary objectives. Moreover, the ideal time point for biomarker measurement was also assessed.
Patients and Methods
Study Population
This was a prospective study performed in consecutive patients with cirrhosis admitted at the Liver Unit of Hospital Clínic de Barcelona between December 2013 and July 2016 for an acute decompensation of the disease who either had AKI at admission or developed it during hospitalization. The diagnosis of cirrhosis was based on a combination of clinical, biochemical, ultrasonographic, endoscopic, and/or histological findings.
Exclusion criteria were as follows: age <18 or > 85 years, hepatocellular carcinoma beyond Milan criteria, extrahepatic malignancies, severe comorbidities (i.e., congestive heart failure with a New York Heart Association classification >2, chronic obstructive pulmonary disease with a Global Initiative For Chronic Obstructive Disease classification >2, patients requiring RRT), previous liver or kidney transplantation, human immunodeficiency virus infection, absence of AKI throughout the hospitalization, and lack of written informed consent. Patients admitted to the hospital for scheduled therapeutic or diagnostic procedures were not considered for participation.
Patients with a recurrent episode of AKI during the study period could be considered again as candidates for inclusion if the first episode of AKI had resolved completely. The disposition of patients in the study is shown in Supporting Fig. S1. There were a total of 1,074 admissions in 774 patients for acute decompensation. AKI was diagnosed in 356 admissions (45.2%). The remaining cases either didn't develop AKI (283 admissions) or had other exclusion criteria (435 admissions). Additionally, 36 admissions in 26 patients were excluded because a urine sample could not be collected at diagnosis of AKI. Characteristics of these patients were similar to those of the whole population (data not shown). Therefore, the study population consisted of 320 cases of AKI that occurred in 214 patients. Baseline demographic, clinical, and biochemical characteristics of the population are shown in Supporting Table S1. The protocol was approved by the institutional review board of the hospital, and all patients or relatives, if patients had hepatic encephalopathy, signed an informed consent.
The sample size was based on logistic and availability reasons, and it was expected to gather a cohort of around 300 admissions in this study. With this sample size, it was considered feasible to test a reasonable number of variables in multivariate models (around three to five) to assess the predictive clinical and laboratory data.23-26
Study Objectives and Endpoints
The main objective of the study was to investigate the accuracy of three urinary biomarkers (NGAL, monomeric NGAL [mNGAL], and IL-18) in the prediction of ATN versus other causes of AKI. Secondary objectives were correlations between urinary biomarkers and progression of AKI during hospitalization and 28-day mortality.
Study Protocol and Patient Assessment
On inclusion, data collected from the patients consisted of demographic and clinical data and an assessment of liver and kidney tests, which were then repeated every day in patients in the intensive care unit (ICU) and every 2-3 days in patients on the regular ward.
At inclusion, urinalysis with assessment of proteinuria, red blood cells, leukocytes, or casts was performed in all patients. Urine samples were collected at inclusion and at days 3, 7, and 14 (if patients were still hospitalized) to measure urinary biomarkers. Blood samples were collected to measure plasma renin activity, plasma norepinephrine concentration, and total serum bile acids (BAs) at inclusion. Six urinary biomarkers were investigated: three modern biomarkers (NGAL, mNGAL, and IL-18) and three conventional biomarkers (β2-microglobulin, FeNa, and albumin). Other modern biomarkers, such as kidney injury molecule 1 or liver-fatty acid binding protein 1, were not measured because previous studies in patients with cirrhosis have shown that they have lower accuracy compared with NGAL and IL-18 in the differential diagnosis between ATN and HRS-AKI.16-18 During hospitalization, patients were cared for by a team of nurses and attending physicians, as well as by the research team composed of two experienced hepatologists, a hepatology research fellow, and a research nurse. During hospitalization, particular attention was paid to changes in kidney function, as assessed by serum creatinine (sCr), and identification of the type of AKI. After discharge, patients were followed up until the end of the study period, liver transplantation, or death.
Diagnosis of AKI
AKI was defined according to internationally accepted criteria for patients with cirrhosis, as an increase in sCr ≥0.3 mg/dL with respect to baseline.9 The presence of AKI was assessed at hospital admission and throughout hospitalization. For the diagnosis of AKI at admission, the baseline sCr used was the most recent stable value available in the previous 3 months before admission, as reported elsewhere.9 In patients without sCr available within the previous 3 months, the last stable value available between month 4 and 1 year before admission was used as the baseline. If sCr was not available within 1 year before hospitalization, the first value at hospitalization was considered the baseline sCr. For the diagnosis of AKI during hospitalization, the baseline sCr used was that obtained at admission. Of the 320 cases included in the study, the majority (304, 95%) had baseline sCr available before AKI; in 267 cases, sCr was available within the previous 3 months (median time, 24 days); and in 37 cases, it was available between 3 months and 1 year. Only 16 cases (5%) did not have baseline sCr available, and the value used for AKI assessment was that obtained on admission to the hospital.
AKI was categorized in four stages (stage 1A, 1B, 2, or 3) according to a modified International Club of Ascites (ICA) classification, which divides patients with stage 1 in two categories according to sCr at diagnosis of AKI: 1A = <1.5 mg/dL and 1B = ≥1.5 mg/dL.3, 4, 6 This classification of AKI into four categories is recommended by the most recent European Association for the Study of the Liver clinical guidelines.27
AKI was also classified into four types: ATN, HRS-AKI, hypovolemia-induced AKI, or miscellaneous. The type of AKI was adjudicated by the investigators’ team at the time of diagnosis of AKI on the basis of preestablished definitions (see below). To ensure nonbiased adjudication of AKI classification, an experienced nephrologist (E.P.), unaware of the classifications given for each patient, repeated the process at the end of hospitalization. As for the analysis of the results, the adjudication used was that made by the investigators’ team.
Definitions
Resolution and progression of AKI are defined in the Supporting Information.
Type of AKI
AKI was classified into four types: (1) hypovolemia-induced AKI when there was history of excessive fluid losses (i.e., excessive diuresis due to diuretic therapy with loss of body weight >500 g/day or 1,000 g/day in patients without and with edema, respectively; severe diarrhea) or bleeding (i.e., gastrointestinal bleeding as defined by presence of hematemesis and/or melena) within the days before development of AKI3, 4; (2) HRS-AKI as defined according to the ICA criteria9; (3) ATN as defined according to a combination of clinical and laboratory data, with three out of four of the following criteria: FeNa > 2%, urinary osmolality <400 mOsm/L, urinary sodium > 40 mEq/L, and presence of shock or use of nephrotoxic drugs5-7; and (4) miscellaneous, when patients could not be classified in one of the three previous categories.
Management of AKI
The management of AKI was dependent on AKI stage, type of AKI, and associated conditions following a specific algorithm, which was a modification of the algorithm proposed by the ICA, which takes into consideration the categorization of patients into four stages (1A, 1B, 2, or 3), as mentioned before9 (Fig. 1).
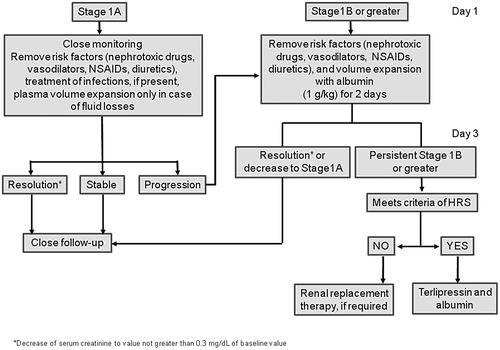
Diuretics were stopped in all patients, and causes of AKI were intensively searched for within the first 24-48 hours of diagnosis of AKI, with particular emphasis on any potential nephrotoxic drugs that patients could have received (nonsteroidal anti-inflammatory drugs, aminoglycosides, vasodilators), the presence of volume depletion (gastrointestinal fluid losses, excessive weight loss during the previous days, or gastrointestinal bleeding), hemodynamic instability, and bacterial infections. Patients with signs of dehydration, gastrointestinal bleeding, or shock were treated with standard measures that included administration of plasma volume expanders (saline in dehydrated patients, packed red blood cells in patients with bleeding, and albumin and vasopressors in those with shock). Patients with spontaneous bacterial peritonitis were treated with antibiotics together with albumin.10 In all other patients, plasma volume expansion was given according to AKI stage; patients with AKI stage 1B or greater were treated with albumin (1 g/kg 20% albumin for 2 days), whereas those with AKI stage 1A did not receive albumin unless AKI progressed to greater stages (Fig. 1). In all patients, kidney function was reassessed at 48 hours after AKI diagnosis (day 3). In patients with AKI resolution or in those in whom AKI decreased to stage 1A, no further therapy for AKI was given. Patients who still had an AKI stage ≥1B at day 3 and who met the criteria of HRS-AKI with sCr greater than 2 mg/dL were treated with terlipressin and albumin.27, 28 No specific therapy was given to patients with HRS-AKI with sCr <2 mg/dL because there is no information about the efficacy and safety of terlipressin in this subset of patients. Patients with an AKI stage ≥1B who did not meet the criteria of HRS-AKI were treated with RRT, if required. Both therapies, terlipressin and RRT, were individualized according to patient status, presence of comorbidities, and indication for transplantation.9, 10
Analytical Methods
See the Supporting Information.
Statistical Analysis
Methods used for statistical analysis are reported in the Supporting Information. Significance was set at two-tailed 0.05 for all analyses, except for in the multivariate models in which variables between 0.05 and 0.1 were not excluded. The statistical analysis was performed using SAS version 9.4 software (SAS Institute, Cary, NC) or Statistical Package for Social Sciences version 20.0 (SPSS Inc., Chicago, IL).
Results
Characteristics of the Study Population
Of the 320 cases, 153 were hypovolemia-induced AKI (48%), 93 were HRS-AKI (29%), 39 were ATN (12%), and 35 were due to miscellaneous causes (11%). There was high concordance between the first and second adjudication of types of AKI (kappa index, 0.82), indicating high reproducibility of classification used for AKI types. Baseline demographic, clinical, and biochemical characteristics of the 320 cases categorized according to the type of AKI are shown in Table 1. In addition, the evolution of renal function throughout hospitalization is shown in Supporting Fig. S2. Of note, there were no statistically significant differences in serum BAs levels among AKI types. The characteristics of patients with miscellaneous AKI are shown in Supporting Table S2. Bacterial infections were very common in the whole group of patients (62%). The types and characteristics of bacterial infections as well as their relationship with the type of AKI are shown in Supporting Table S3. Gastrointestinal bleeding occurred in 44 of the 320 cases (14%). The characteristics of these patients are shown in Supporting Table S4.
| Hypovolemia-Induced N = 153 | HRS-AKI N = 93 | ATN N = 39 | Miscellaneous N = 35 | |
|---|---|---|---|---|
| Age (years) | 62 (54-68) | 62 (54-69) | 61 (54-66) | 62 (57-70) |
| Sex (male) | 113 (73) | 71 (76) | 25 (64) | 22 (65) |
| Cause of cirrhosis | ||||
| Alcohol | 90 (59) | 49 (53) | 27 (69) | 20 (57) |
| HCV | 33 (22) | 26 (28) | 4 (10) | 4 (11) |
| Other | 30 (20) | 18 (19) | 8 (21) | 11 (32) |
| Diabetes mellitus | 65 (42) | 36 (39) | 8 (21) | 10 (29) |
| Chronic kidney disease* | 37 (24) | 17 (18) | 5 (13) | 6 (18) |
| Previous use of diuretics† | 103 (67) | 62 (67) | 20 (51) | 16 (46) |
| ICU admission | 39 (25) | 35 (38) | 35 (90) | 16 (39) |
| Ascites | 112 (73) | 80 (86) | 30 (77) | 23 (68) |
| Hepatic encephalopathy | 39 (26) | 40 (43) | 23 (59) | 17 (50) |
| Bacterial infection | 66 (43) | 77 (83) | 32 (82) | 22 (65) |
| Gastrointestinal bleeding | 32 (21) | 4 (4) | 8 (21) | 0 (0) |
| Shock | 19 (12) | 0 (0) | 23 (59) | 12 (34) |
| Mean arterial pressure (mm Hg) | 78 (71-89) | 78 (70-87) | 77 (67-92) | 77 (69-86) |
| Heart rate (bpm) | 76 (66-87) | 76 (67-89) | 80 (69-90) | 76 (68-89) |
| Serum bilirubin (mg/dL) | 1.9 (0.7-4) | 3.3 (1.2-5) | 3.4 (1-15.2) | 4.1 (1.8-25) |
| Serum BAs (mol/L)‡ | 51 (17-98) | 47 (23-96) | 33 (8-86) | 74 (23-133) |
| INR | 1.4 (1.3-1.9) | 1.6 (1.4-2.1) | 1.7 (1.3-2.3) | 1.7 (1.4-1.9) |
| sCr (mg/dL) | 1.8 (1.5-2.2) | 1.7 (1.4-2.3) | 2.4 (1.7-3.3) | 1.8 (1.2-2.2) |
| Serum sodium (mEq/L) | 134 (131-136) | 132 (129-135) | 133 (129-135) | 135 (131-139) |
| Leukocyte count (cells 103/µL) | 5.5 (4-7.4) | 7.1 (4.7-13.4) | 9 (6.1-14) | 6.3 (5.6-9.9) |
| Plasma renin activity (ng/mL per hour) | 2.8 (0.5-6.8) | 5 (1.5-9.8) | 3.9 (0.3-14.6) | 2.8 (0.8-7.1) |
| Plasma norepinephrine (pg/mL)§ | 450 (279-752) | 578 (359-900) | 1,193 (521-2,199) | 484 (293-1,363) |
| Urinalysis | ||||
| Red cells (n) | 2 (0-13) | 2 (0-14) | 50 (8-126) | 5 (2-20) |
| Leucocytes (n) | 0 (0-10) | 3 (0-15) | 3 (0-16) | 2 (0-15) |
| Granular casts (patients) | 7 (13) | 5 (13) | 7 (18) | 4 (25) |
| Proteinuria (mg/L) | 111 (59-207) | 155 (81-388) | 292 (180-819) | 143 (89-402) |
| MELD score | 20 (16-26) | 23 (17-28) | 30 (25-36) | 23 (17-27) |
| Child-Pugh|| | ||||
| Score | 9 (7-10) | 10 (8-11) | 10 (8-12) | 9 (8-11) |
| Class (A/B/C) | 24/72/55 | 9/33/50 | 3/14/22 | 4/14/16 |
| Community/nosocomial AKI (n) | 121/33 | 71/22 | 35/4 | 24/10 |
- Data are presented as median (IQR) for quantitative variables and n (%) for qualitative values.
- * As defined by GFR < 60 mL/minute/1.73 m2 for ≥ 3 months.
- † Median (IQR) dose of spironolactone was 50 mg/day (25-200) in hypovolemia-induced AKI, 100 mg/day (75-125) in AKI-HRS, 100 mg/day (25-100) in ATN, and 100 mg/day (50-200) in miscellaneous. Median (IQR) dose of furosemide was 40 mg/day (40-80) in hypovolemia-induced AKI, 40 mg/day (40-80) in AKI-HRS, 40 mg/day (10-40) in ATN and 40 mg/day (40-80) in miscellaneous AKI.
- ‡ Available in 136 patients with hypovolemia-induced AKI, 86 patients with HRS-AKI, 32 patients with ATN, and 31 patients with miscellaneous AKI.
- § Patients in each group treated with norepinephrine: 14 with hypovolemia (9%), 24 (62%) with ATN, 5 (5%) with HRS-AKI (used instead of terlipressin), and 10 (29%) in the miscellaneous group.
- || Available in 316 patients.
- Abbreviations: bpm, beats per minute; GFR, glomerular filtration rate; HCV, hepatitis C virus; INR, international normalized ratio; IQR, interquartile range.
Urinary Biomarkers in the Differentiation Between ATN and Other Types of AKI
Table 2 shows the values of the three urinary biomarkers in all patients categorized according to the type of AKI, both at diagnosis (day 1) and at day 3. The urinary levels of NGAL, mNGAL, and IL-18 at day 1 were markedly different across AKI types; patients with ATN had significantly higher values of the three biomarkers compared with patients with hypovolemia-induced AKI and HRS-AKI. Patients with miscellaneous AKI had high values of all three biomarkers, yet these were slightly lower than those of patients with ATN. NGAL and mNGAL had good accuracy in differentiating ATN from other types of AKI (with an area under the receiver operating characteristic curve [AUROC] of 0.80 for both), whereas IL-18 had lower accuracy (AUROC, 0.70).
| Hypovolemia-Induced | HRS-AKI | ATN | Miscellaneous | P | ATN vs. Other | ||||
|---|---|---|---|---|---|---|---|---|---|
| Continuous Variable | Binarized Variable | ||||||||
| AUROC (95% CI) | OR (95% CI)* | Cut-off Point† | OR (95% CI) | ||||||
| Day 1 (all patients)‡ | |||||||||
| n | 153 | 93 | 39 | 35 | |||||
| NGAL (µg/g) | 48 (22-97) | 76 (18-177) | 799 (153-2,114) | 317 (49-759) | <0.001 | 0.80 (0.72-0.88) | 1.05 (1.02-1.08)(100) | 110 | 9.3 (3.9-21.9) |
| mNGAL (µg/g) | 33 (14-67) | 44 (13-115) | 543 (108-1,838) | 236 (41-570) | <0.001 | 0.80 (0.72-0.88) | 1.07 (1.03-1.10)(100) | 73 | 8.9 (3.8-20.9) |
| IL-18 (pg/g) | 9 (3-21) | 8 (1-26) | 33 (11-107) | 14 (3-53) | <0.001 | 0.70 (0.60-0.79) | 1.01 (0.40-2.57)(10) | 23 | 4.9 (2.4-10.0) |
| Day 3§ | |||||||||
| n | 87 | 59 | 35 | 21 | |||||
| NGAL (µg/g) | 63 (37-92) | 114 (49-149) | 674 (317-2,611) | 246 (186-671) | <0.001 | 0.87 (0.78-0.95) | 1.13 (1.07-1.19)(100) | 220 | 42.9 (13.9-132.3) |
| mNGAL (µg/g) | 32 (20-57) | 66 (27-115) | 293 (94-762) | 188 (74-648) | <0.001 | 0.80 (0.71-0.89) | 1.29 (1.13-1.48)(100) | 164 | 11.5 (5.0-26.6) |
| IL-18 (pg/g) | 4 (0-7) | 5 (2-10) | 9 (4-32) | 7 (3-12) | 0.006 | 0.66 (0.54-0.76) | 1.04 (0.35-3.11)(10) | 9 | 2.9 (1.4-6.2) |
- Values of NGAL, mNGAL, and IL-18 are median (IQ range) and are expressed as micrograms or picograms per gram of creatinine, respectively.
- * OR (95% CI) risk estimates for (10) 10-units change or (100) 100-units change.
- † Cut-off point that maximizes the Youden index.
- ‡ Includes all patients in the study classified according to AKI type assigned at day 3 (n = 320). This is because AKI type cannot be assigned until the effect of albumin administration has been assessed at day 3.
- § Only patients with persistent AKI at day 3 (n = 202).
- Abbreviations: CI, confidence interval; IQ, interquartile.
At day 3, resolution of AKI had occurred in 118 of the 320 cases (37%); only 4 of the 118 patients (3.4%) had ATN. When urinary biomarkers were assessed for differentiating ATN from other types of AKI, in the 202 patients in whom AKI still persisted at day 3, NGAL had the highest diagnostic accuracy, followed by mNGAL, whereas the accuracy of IL-18 was much lower (AUROC of 0.87, 0.80, and 0.66, respectively) (Table 2). Comparison of AUROC curves between day 3 and day 1 showed that the predictive accuracy of NGAL in differentiating ATN from other types of AKI was greater at day 3. In multivariate analysis including values of urinary biomarkers at days 1 and 3, NGAL at day 3 was independently associated with the presence of ATN. The cut-off value of NGAL with the highest diagnostic accuracy for ATN at day 3 was 220 µg/g of creatinine. Most patients with ATN (30 out of 35, 86%) had NGAL above this value, whereas the majority of patients with HRS-AKI or hypovolemia-induced AKI had values below the 220-µg/g threshold (52 of the 59 [88%] and 81 of the 87 [93%], respectively). Combination of the three biomarkers did not significantly improve the predictive accuracy of NGAL at day 3.
Changes in urinary biomarker levels throughout hospitalization were also assessed. Patients with ATN showed markedly higher levels of NGAL during the whole hospitalization, and levels were statistically significant from day 1 to day 7 compared with those of patients with HRS-AKI or hypovolemia-induced AKI (Fig. 2). Patients with miscellaneous AKI had intermediate values. By contrast, IL-18 levels were significantly different between ATN and other AKI types at day 1 but not at days 3, 7, and 14. Changes in mNGAL levels were similar to those of NGAL.
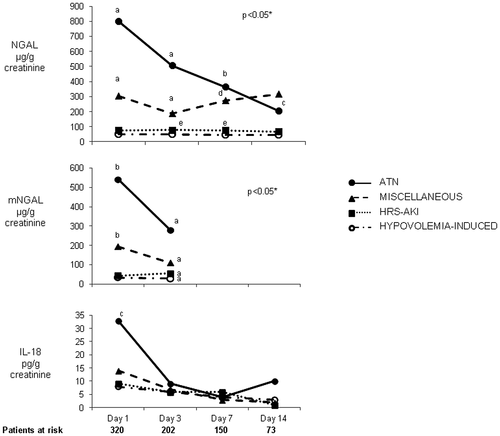
Conventional urinary biomarkers, including β2-microglobulin, FeNa, and albumin, were also assessed, showing significant differences among groups. Patients with ATN had higher values compared with the other groups (Table 3). The predictive accuracy of these biomarkers in differentiating ATN from other types of AKI was significantly lower than that of NGAL and mNGAL (AUROCs at day 3 of 0.63, 0.76, and 0.69, respectively). In multivariate analysis, conventional urinary biomarkers were not independent predictors of ATN. There was no significant correlation between serum BAs and NGAL or any other biomarkers.
| Hypovolemia-Induced | HRS-AKI | ATN | Miscellaneous | P | ATN vs. Other | ||||
|---|---|---|---|---|---|---|---|---|---|
| Continuous Variable | Binarized Variable | ||||||||
| AUROC (95% CI) | OR (95% CI)* | Cut-off Point† | OR (95% CI) | ||||||
| Day 1 (all patients)‡ | |||||||||
| n | 153 | 93 | 39 | 35 | |||||
| β2-microglobulin (µg/g) | 299 (180-1,035) | 211 (83-1,091) | 1,912 (303-34,441) | 413 (319-1,886) | <0.001 | 0.68 (0.58-0.78) | 1.00 (0.91-1.10)(1) | 1,053 | 4.2 (2.1-8.4) |
| FeNa (%) | 0.2 (0.08-0.7) | 0.09 (0.04-0.23) | 1.7 (0.6-3.5) | 0.3 (0.1-1) | <0.001 | 0.65 (0.54-0.75) | 1.58 (1.42-1.75)(1) | 1 | 3.4 (1.7-6.8) |
| Albumin (mg/g) | 16 (2-86) | 21 (6-51) | 131 (58-212) | 63 (38-220) | <0.001 | 0.79 (0.71-0.86) | 1.01 (1.00-1.01)(10) | 66 | 9.2 (3.9-21.6) |
| Day 3§ | |||||||||
| n | 87 | 59 | 35 | 21 | |||||
| β2-microglobulin (µg/g) | 383 (185-2,392) | 462 (110-2,050) | 1,027 (395-13,833) | 983 (226-4,467) | 0.001 | 0.63 (0.50-0.76) | 1.00 (0.88-1.14)(1) | 228 | 4.8 (1.3-17.5) |
| FeNa (%) | 0.3 (0.1-0.7) | 0.1 (0.04-0.4) | 1.1 (0.4-2.6) | 0.5 (0.1-1.3) | <0.001 | 0.76 (0.67-0.86) | 1.90 (1.72-2.10)(1) | 0.9 | 6.6 (3.0-14.5) |
| Albumin (mg/g) | 29 (12-120) | 27 (11-63) | 131 (30-231) | 140 (39-204) | <0.001 | 0.69 (0.60-0.78) | 1.00 (0.99-1.01)(10) | 138 | 4.1 (1.9-8.9) |
- Values are median (IQ range) and are expressed as micrograms or picograms per gram of creatinine for β2-microglobulin and albumin, respectively.
- * OR (95% CI) risk estimates for (1) 1 unit change, (10) 10 units change, or (100) 100 units change.
- † Cut-off point that maximizes the Youden index.
- ‡ Includes all patients in the study classified according to AKI type assigned at day 3 (n = 320). This is because AKI type cannot be assigned until the effect of albumin administration has been assessed at day 3.
- § Only patients with persistent AKI at day 3 (n = 202).
- Abbreviations: CI, confidence interval; IQ, interquartile.
Outcome of AKI
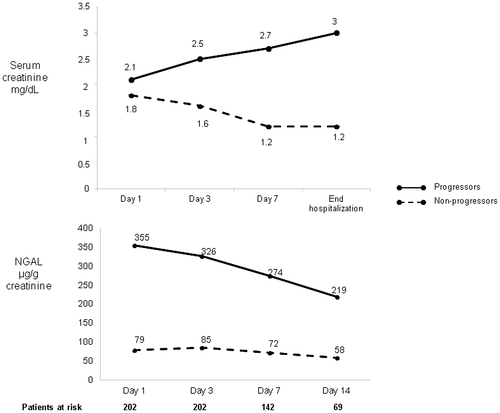
The outcome of AKI at the end of hospitalization in the 202 patients with persistent AKI at day 3 was as follows: AKI progressed in 28 (14%), remained in the same stage in 74 (37%), and resolved in 100 (49%) (in 3 of these patients, resolution occurred after a transient progression). Types of AKI in the 31 progressors were as follows: 12 had ATN, 7 had miscellaneous AKI, 6 had HRS-AKI, and 6 had hypovolemia-induced AKI. Factors related to progression are shown in Supporting Table S5. In multivariate analysis, independent predictive factors of progression were NGAL at day 3, plasma norepinephrine, and ATN. The cut-off value of NGAL that best predicted progression of AKI was 280 µg/g of creatinine (AUROC, 0.75). Figure 3 shows the evolution of sCr and time course of NGAL during hospitalization in patients in whom AKI progressed versus those in whom AKI did not progress. Twenty-six patients required RRT during hospitalization. Independent predictive factors of RRT were NGAL at day 3 and ATN. The cut-off value of NGAL that best predicted RRT was 173 µg/g of creatinine (AUROC, 0.77).
Overall, resolution of AKI at the end of hospitalization occurred in 213 (67%) of the 320 cases. There was a clear relationship between AKI type and resolution of AKI. In fact, AKI resolved more frequently in patients with hypovolemia-induced AKI and HRS-AKI (77% and 72%, respectively), compared with miscellaneous cases and ATN (43% and 33%, respectively). Fifteen of the 93 patients with HRS-AKI were treated with terlipressin, and a complete response was achieved in 9 of the 15 patients treated (60%). The remaining patients with HRS-AKI did not receive terlipressin because sCr was <2 mg/dL (n = 58), the patients had contraindications to therapy, or the patients were in terminal condition and were not candidates for transplant (n = 20). There was no relationship between NGAL levels and response to terlipressin (100 µg/g vs. 90 µg/g of creatinine in responders vs. nonresponders, respectively).
Urinary Biomarkers and Mortality
Considering only patients with the first episode of AKI, 62 of the 214 patients (29%) died during the 28-day follow-up period, 9 (4%) underwent transplant, and 6 (3%) were lost to follow-up. The remaining 137 (64%) were alive at the end of 28-day follow-up. Overall, the probability of transplant-free survival from the diagnosis of AKI was 72% at discharge from the hospital and 69% at 28 days.
There was a strong relationship between the 28-day mortality rate and AKI stage at diagnosis: 17%, 28%, 39%, and 42% for stages 1A, 1B, 2, and 3, respectively. As for the type of AKI, the highest 28-day mortality rates were observed in patients with ATN and miscellaneous type, and the lowest rates were observed in patients with HRS-AKI and hypovolemia-induced AKI (64%, 43%, 23%, and 17% respectively; P < 0.001). In patients with persistent AKI at day 3, 28-day mortality was associated with liver, kidney, and circulatory function; leukocyte count; serum BAs; Model for End-Stage Liver Disease (MELD) and Child-Pugh scores; and urinary biomarker levels (Supporting Table S6). Interestingly, mortality was also strongly correlated with AKI resolution at day 3 (27% and 50% with and without resolution of AKI at day 3; P < 0.001). In multivariate analysis, independent predictive factors of 28-day mortality for patients with persistent AKI at day 3 were MELD score and NGAL. The cut-off value of NGAL that best predicted 28-day mortality was 110 µg/g creatinine. The time course of NGAL levels in patients who survived or died during hospitalization is shown in Fig. 4.
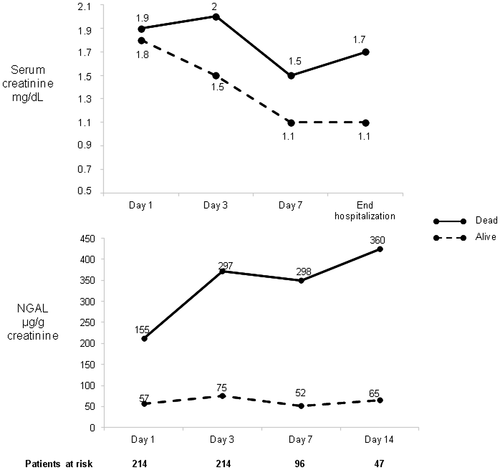
In patients with HRS-AKI, NGAL levels were associated with prognostic significance. Patients who survived had significantly lower NGAL levels at diagnosis of AKI compared with patients who died (48 vs. 127 µg/g of creatinine, respectively; P < 0.002). No prognostic significance for NGAL was observed within the group of patients with ATN or hypovolemia-induced AKI.
Internal Validation
We conducted an internal validation using bootstrap simulations. Results reported in Supporting Table S7 show that odds ratio (OR) and AUROC estimates from the constructed models and when using bootstrap simulations were both very similar and led to the same conclusions.
Discussion
The results show that urinary NGAL has high accuracy in the differential diagnosis between ATN and other types of AKI, including HRS-AKI and hypovolemia-induced AKI. By contrast, urinary IL-18 and conventional biomarkers (β2-microglobulin, FeNa, and albumin) had lower accuracy compared with NGAL. In addition, urine NGAL was also associated with important clinical outcomes, particularly with progression of AKI during hospitalization, need for RRT, and 28-day mortality.
The current investigation included a very large number of consecutive patients hospitalized for complications of cirrhosis, encompassing the whole the spectrum of AKI types. Selection of biomarkers evaluated in the present study was made on the basis of reported data in smaller series and a recent meta-analyses.15-19, 21 Biomarker evaluation was performed in the context of an algorithm for diagnosis of AKI in patients with cirrhosis.9 Aside from this, biomarkers were measured at diagnosis of AKI and also at several time points after diagnosis to determine the best point for differential diagnosis assessment and also to understand biomarker evolution in different types of AKI. Overall, all biomarkers were significantly increased in patients with ATN compared with patients with hypovolemia-induced AKI and HRS-AKI, indicating that all biomarkers were able to identify, to some extent, the existence of tubular cell damage of ATN.20-22 Nonetheless, the most important finding was that NGAL had very high accuracy to differentiate ATN from other types of AKI, an accuracy higher than that of all other biomarkers. In addition, our results demonstrate that NGAL is slightly more accurate when measured after 2 days of plasma expansion with albumin compared with measurement at diagnosis of AKI. Of note, mNGAL, which is claimed to be a specific isoform of the kidney,29, 30 did not have higher accuracy compared with NGAL. Multivariate analysis showed that NGAL measured at day 3 was independently associated with ATN. The best cut-off value of NGAL for the differential diagnosis between ATN and other AKI types was 220 µg/g of creatinine. Urine IL-18, which was shown to be highly accurate in previous studies, had lower accuracy than NGAL.20, 21, 31 Time course assessment showed that the highest difference in IL-18 levels between ATN and other types was observed at diagnosis of AKI, but this significant difference disappeared thereafter. This is probably related to the profile of activation of IL-18 gene after injury of kidney tubular cells, which occurs very early after injury and rapidly disappears thereafter.20 All classical biomarkers evaluated had lower accuracy than NGAL.
Dynamics of NGAL levels during hospitalization in patients with ATN showed that levels are very high early after diagnosis of AKI and decrease rapidly thereafter (Fig. 2). This dynamic has important practical implications because it indicates that measurement of NGAL should be done as early as possible. The observed decrease in NGAL levels was not only due to early ATN mortality because a similar trend was observed when patients who survived up to day 7 were analyzed, suggesting that it is at least in part related to the dynamics of protein elimination through urine (Supporting Fig. S3).
In addition, NGAL was also relevant in predicting AKI progression and short-term mortality. Patients in whom AKI progressed to higher stages had urine NGAL levels significantly higher than those of patients in whom AKI did not progress, and NGAL was maintained at high levels throughout hospitalization. Interestingly, the best cut-off level for predicting AKI progression was slightly higher than the best cutoff to predict ATN (280 vs. 220 µg/g of creatinine, respectively). Remarkably, aside from NGAL, plasma norepinephrine was also an independent predictive factor of AKI progression. This finding probably reflects the importance of the sympathetic nervous system as a marker of circulatory dysfunction and also as a key factor in shifting the kidney autoregulation curve in cirrhosis, which may increase the risk of AKI.32, 33 Finally, NGAL was also an independent predictive factor of 28-day survival, together with MELD score. NGAL levels of patients who died were markedly higher throughout hospitalization compared with those of patients who survived. These findings are consistent with previous studies assessing the prognostic value of NGAL.17, 18, 31 In fact, MELD score and urinary NGAL were also independent predictors of survival in patients from the CLIF Acute-on-Chronic Liver Failure in Cirrhosis (CANONIC) study.34 In keeping with results from previous studies,35 serum BAs levels were also predictive of short-term mortality; however, they did not reach independent prognostic value. Of interest, NGAL was a prognostic marker not only in the whole series of patients but also in specific AKI types, such as HRS-AKI. Whether this relationship of NGAL levels with mortality in HRS-AKI is due to greater severity of kidney damage related to tubular dysfunction or other factors is not known and would require further investigation. All in all, the findings of the current study represent a step forward in the management of AKI in cirrhosis by providing an objective tool (urine NGAL) that is simple, applicable in most settings, and very accurate in the differential diagnosis of ATN while also providing information relevant to kidney and patient outcome.
Other relevant findings that also deserve discussion are as follows. (1) The study provides confirmatory evidence of the good outcome of patients with AKI stage 1A.3, 4 In fact, AKI resolved at the end of hospitalization in 95% of these patients. Therefore, these data strongly suggest that biomarkers should not be measured in patients with AKI stage 1A. (2) Another interesting piece of information derived from the application of the diagnostic algorithm was that 16.5% of patients with an AKI stage ≥1B at diagnosis had resolution of AKI after 2 days of albumin administration; moreover, the persistence of AKI after albumin administration was associated with poor prognosis. (3) Community-acquired AKI was more frequent than nosocomial AKI; however, kidney and patient outcomes were worst in nosocomial AKI. (4) In patients with HRS-AKI, urinary NGAL levels were not predictive of response to terlipressin, but this would require validation in larger series of patients.
Our study has some limitations. First, the study was performed in a single tertiary center with experience in research on complications of cirrhosis, and it therefore could be argued that results may not be generalized to all centers caring for patients with cirrhosis. Nevertheless, the results were obtained in the context of a simple diagnostic algorithm that is easily applicable in most settings because it only requires usual clinical care and standard laboratory tests, such as that for sCr. Treatment with albumin for patients with stages ≥1B may be an issue in countries with shortages of albumin. In this context, it is not known whether plasma expansion with crystalloids in substitution of albumin could also have a beneficial effect on AKI resolution in a proportion of patients similar to that shown with albumin in the current study. Second, it could be argued that the diagnosis of ATN was not evaluated by histological findings with a kidney biopsy, and therefore some patients classified as having ATN may not have had ATN on the basis of histology; alternatively, some patients classified into non-ATN groups could have had some degree of ATN not detected with the diagnostic criteria used. Because of the high risk of severe complications in patients with cirrhosis, kidney biopsy is hardly used in the diagnosis of ATN in clinical practice. In addition, the incorporation of kidney biopsy in a study of biomarker assessment would have to be performed in all patients to avoid bias. For these two reasons, use of kidney biopsy is unrealistic in clinical studies in patients with cirrhosis. Last, the findings of this study would ideally require validation in external series of patients. However, we could not find any large prospective series in which urine was collected in the setting of the application of the diagnostic algorithm of AKI in cirrhosis proposed by the ICA. For this reason, we performed an internal validation. Using a bootstrapping approach, the predictive models led to the same results, and NGAL can thus be considered as a good predictive biomarker.
In conclusion, the results of the study indicate that urinary NGAL levels are highly accurate in the differential diagnosis between ATN and other types of AKI in cirrhosis. In patients with an AKI stage ≥1B, the accuracy of urine NGAL is greatest when assessed following albumin administration for 2 days after diagnosis of AKI. Patients with AKI stage 1A do not require NGAL assessment because of good prognosis. NGAL is more accurate than urinary mNGAL and IL-18, as well as conventional urine biomarkers. Moreover, NGAL measured at day 3 is also highly predictive of kidney outcome as well as patients’ outcome. Results were internally validated using a bootstrap method. Therefore, this study strongly supports the use of urine NGAL in clinical practice in the context of a diagnostic algorithm for differential diagnosis of AKI in cirrhosis.
Acknowledgment
We are very grateful to Nicki van Berckel and Roser Poblet for their help in the preparation of the manuscript. We also appreciate the help of all faculty and nurses of the Liver Unit who cared for the patients included in this study. Finally, we would like to thank the patients and their families for participating in this investigation.




