Indeterminate pediatric acute liver failure is uniquely characterized by a CD103+CD8+ T-cell infiltrate
Potential conflict of interest: Nothing to report.
Supported by the Fred and Suzanne Biesecker Pediatric Liver Center at the Children's Hospital of Philadelphia and the Siragusa Transplantation Center at the Ann & Robert H. Lurie Children's Hospital of Chicago.
Abstract
The cause of pediatric acute liver failure (PALF) is unknown in up to 40% of cases. Evidence suggests that aberrant immune system activation may play a role. We hypothesized that indeterminate PALF cases would exhibit a unique pattern of hepatic inflammation. This was a retrospective and prospective study of PALF cases due to indeterminate (iPALF), autoimmune hepatitis, or known diagnosis (dPALF) etiology. Liver tissue sections were stained with immunohistochemical markers for cytotoxic T-cells (cluster of differentiation 8 [CD8]), perforin, and tissue resident memory T-cells (CD103) and scored as minimal, moderate, or dense. Lymphocytes were isolated from liver tissue for T-cell receptor beta sequencing and flow-cytometric studies. Thirty-three iPALF, 9 autoimmune hepatitis, and 14 dPALF cases were included. Dense hepatic infiltrates of CD8+ T-cells were found in 27 (82%) iPALF cases compared to 1 (7%) dPALF case (P < 0.0001). Perforin staining was dense or moderate in 19 (73%) of 26 iPALF cases compared to minimal in all 7 dPALF cases (P = 0.004); 16 (62%) of 26 iPALF cases had dense CD103 staining compared to none of the 6 dPALF cases (P = 0.001). T-cell receptor beta sequencing of iPALF cases demonstrated increased clonality compared to dPALF and control cases. Flow cytometry and immunohistochemistry revealed that iPALF intrahepatic leukocytes were predominantly tissue resident memory CD8+ T-cells. Conclusion: Indeterminate PALF is characterized by a dense CD8+ T-cell hepatic infiltrate consistent with expansion of a tissue resident memory T-cell phenotype; CD8+ T-cells are a biomarker of immune dysregulation in iPALF and may be used to better identify and define this group. (Hepatology 2018).
Abbreviations
-
- AA
-
- aplastic anemia
-
- AIH
-
- autoimmune hepatitis
-
- ALF
-
- acute liver failure
-
- CCR7
-
- chemokine (C-C motif) receptor 7
-
- CD
-
- cluster of differentiation
-
- CHOP
-
- Children's Hospital of Philadelphia
-
- dPALF
-
- known diagnosis PALF
-
- ICC
-
- intraclass correlation coefficient
-
- IgM
-
- immunoglobulin M
-
- IHC
-
- immunohistochemical
-
- iPALF
-
- indeterminate PALF
-
- LT
-
- liver transplantation
-
- PALF
-
- pediatric ALF
-
- Trm
-
- tissue resident memory
In up to 40% of cases of pediatric acute liver failure (PALF) no known etiology can be found despite a thorough age-appropriate diagnostic workup.1-4 Patients in this group, called indeterminate PALF (iPALF), have worse outcomes and are more likely to undergo liver transplantation (LT) than those with known diagnosis (dPALF).2, 5 To date, little is known about the pathophysiology of iPALF, but growing evidence supports the hypothesis that the liver injury is immune-mediated, resulting from an imbalance between proinflammatory and anti-inflammatory factors.6-11 iPALF patients frequently exhibit signs of immune activation and dysregulation including cytopenias, elevated soluble interleukin-2 receptor levels, natural killer cell dysfunction, and development of aplastic anemia (AA) either shortly before or after their liver injury. It is now accepted that immune dysregulation plays a key role in propagating liver injury in the majority of iPALF cases. Thus, we hypothesized that these immune-active patients would have a distinctive inflammatory hepatic infiltrate. Our primary objective was to characterize the iPALF group by types of inflammatory cells observed on liver histology and determine whether specific patterns or cell types could differentiate iPALF from autoimmune hepatitis (AIH) and/or dPALF cases.
Patients and Methods
This was a primarily retrospective study, with a small prospective component, of patients with PALF characterized as iPALF, AIH, or dPALF.
RETROSPECTIVE ARM
Medical records of patients who presented to the Ann & Robert H. Lurie Children's Hospital of Chicago from 1999 to 2015 or Children's Hospital of Philadelphia (CHOP) from 2004 to 2015 were retrospectively reviewed for PALF cases. Potential cases were identified by an International Classification of Diseases, Ninth Revision, database code search for acute liver failure (ALF) and hepatic encephalopathy.
PROSPECTIVE ARM
During 2015-2017, patients who presented to CHOP with ALF were evaluated for enrollment prospectively if they underwent LT during their admission. Patients consented to storage of their explanted liver tissue in the CHOP biorepository for use in research studies. Other than use of fresh liver tissue, all study aspects were the same for the prospective group as described for the retrospective group.
METHODS
Cases were included by meeting PALF criteria as defined by the PALF Study Group,1 were age 1-17 years, and had available formalin-fixed, paraffin-embedded liver tissue specimens. Patients with PALF due to ischemia, rheumatologic disease, or malignancy were excluded. Cases were divided into three groups: iPALF, AIH, and dPALF. As AIH is also an immune-mediated disease, often with significant inflammation on liver biopsy, we considered this group separately from the dPALF group. Archived liver tissue specimens from 4 pediatric deceased donors and 4 children who had liver biopsies prior to vascular shunt procedures and had normal liver histology on pathologist review of hematoxylin and eosin–stained slides served as a nondisease control group. This study was approved by the Lurie Children's Hospital and CHOP institutional review boards. Informed consent was obtained for patients enrolled prospectively at CHOP. For the retrospective component, the requirement for informed consent was waived.
CLINICAL AND LABORATORY DATA
Chart review was conducted for iPALF, AIH, and dPALF cases to collect demographic, laboratory, and outcome data and evaluate the diagnostic workup. Information collected included age at presentation, gender, type of liver tissue sample (explant/wedge or needle biopsy), 21-day outcome from admission (spontaneous recovery with native liver, death, or LT), and development of AA within 12 months prior to or up to 12 months following PALF presentation (diagnosed by bone marrow biopsy). Beginning in 2011 at our centers, iPALF patients with features of immune activation were considered for treatment with intravenous steroids. Duration and dose were recorded for patients who received steroid therapy prior to biopsy or LT. Additional data regarding serum biomarkers of immune activation status were collected as available for the iPALF group including soluble interleukin-2 receptor level, natural killer cell function (normal or decreased/absent), and flow-cytometric ratio of cluster of differentiation 4–positive (CD4+) to CD8+ T-cells (normal or decreased ≤1.0). iPALF cases were defined as no identifiable etiology for their liver disease despite an age-appropriate diagnostic evaluation, as determined by medical record review at each individual site. A thorough workup included a comprehensive review of possible exposures to medications and supplements, testing for common infections including hepatitis B virus with hepatitis B surface antigen or antibody to hepatitis B core antigen immunoglobulin M (IgM) or hepatitis B virus DNA PCR, hepatitis A virus with anti–hepatitis A virus IgM, and Epstein-Barr virus with Epstein-Barr virus viral capsid antigen IgM or Epstein-Barr virus PCR and screening for AIH with anti–nuclear antibody, anti–smooth muscle antibody, and anti–liver kidney microsomal antibody using institution-specific cutoff values for positive results.2, 12 Additional workup for patients 3 years of age and older included screening for Wilson disease with serum ceruloplasmin level and for patients less than 3 years of age screening for fatty acid oxidation defects with urine organic acids and for mitochondrial disorders with serum lactate and pyruvate levels. AIH cases were defined as those with positive serum autoantibodies (anti–nuclear antibody, anti–smooth muscle antibody, or anti–liver kidney microsomal antibody) per institution-specific cutoffs and liver biopsy histology consistent with AIH, in agreement with standard diagnostic criteria.13, 14 Duration and type of immunosuppressive therapy given to AIH patients prior to collection of liver tissue samples were recorded. All other non-indeterminate and non-AIH etiologies for PALF that met inclusion criteria comprised the dPALF group.
IMMUNOHISTOCHEMISTRY AND LIVER HISTOPATHOLOGY OF FORMALIN-FIXED, PARAFFIN-EMBEDDED LIVER TISSUE
Immunohistochemical (IHC) staining was performed by clinical pathology laboratories at Lurie Children's Hospital and CHOP. Formalin-fixed, paraffin-embedded liver tissue specimens were sectioned to 5-μm thickness and mounted on positively charged slides. Cases were stained with hematoxylin and eosin and antibody for cytotoxic T-cells (CD8) and macrophages (CD163). The majority of cases (determined by tissue availability) were stained with antibody for helper T-cells (CD4), B-cells (CD20), natural killer cells (CD56), perforin (a marker of lymphocyte cytolytic activity), and tissue resident memory (Trm) T-cells (CD103). A subset of cases were double-stained with CD103 (brown chromogen) and CD8 (red chromogen). IHC staining was performed at Lurie Children's Hospital using the Ventana Benchmark Ultra Immunohistochemistry Stainer (Ventana Medical Systems, Inc., Tucson, AZ), following standard protocols, and using the UltraView Universal DAB Detection Kit. IHC staining was performed at CHOP using the Leica Bond Immunohistochemistry Stainer (Leica Biosystems Inc., Buffalo Grove, IL), following standard protocols, and using the Bond Polymer Refine Brown Detection and Refine Red Detection Kits. Results were reviewed by study investigators, including independent review by two pediatric pathologists (one per institution). On preliminary review, the CD8 staining pattern appeared to differ between groups. Cases were subjectively scored by study investigators as dense, moderate, or minimal CD8 staining. Computer-assisted quantification of CD8+ cells and percent stain density were performed using ImageJ software.15 For each case, five random nonoverlapping images of hepatic tissue (three parenchymal and two portal areas) were obtained with a ×40 objective, and an automated calculation of average number of CD8+ cells per high-power field was generated. In addition, the number of pixels corresponding to CD8+ staining was measured, computed as a percentage of total pixels for each image, and expressed as area percent density per high-power field. Cases were scored as minimal if mean stain percent density was <5%, moderate if ≥5% to <10%, and dense if ≥10%. The stain percent density score was used for CD8 statistical analyses. Perforin and CD103 staining was performed on cases with available tissue and subjectively scored by one blinded pathologist as dense, moderate, or minimal. This subjective score was used for perforin and CD103 statistical analyses. CD103/CD8 double-labeling was performed on select iPALF cases and categorized as double-labeled cells present versus absent. CD4, CD20, CD56, and CD163 staining patterns were similarly reviewed by investigators and subjectively scored as dense, moderate, or minimal.
INTRAHEPATIC LYMPHOCYTE ISOLATION AND FLOW-CYTOMETRIC ANALYSIS OF FRESH LIVER TISSUE
Four patients (3 iPALF and 1 dPALF) presented to CHOP during the study and were prospectively enrolled when they underwent LT. During transplantation, a piece of fresh tissue from the explanted livers was obtained for flow-cytometric studies. Intrahepatic lymphocytes were isolated based on a published protocol.16 Briefly, 10 g of fresh liver slices were perfused 3 times with 10 mL Hank's balanced salt solution (37oC) before being cut into small (∼2 mm3) pieces and homogenized through a metal strainer in warm Hank's balanced salt solution. Tissue homogenate was then shaken in 30 mL Hank's balanced salt solution with collagenase A (1 mg/mL; Roche, Basel, Switzerland) and DNase I (50 μg/mL; Roche) for 25 minutes at 37oC. Homogenate was strained through a 70-μm cell strainer, and erythrocytes were lysed in ammonium-chloride-potassium lysis buffer (Lonza, Basel, Switzerland) for 4 minutes before resuspension in Hank's balanced salt solution and proceeding to staining for flow cytometry. Isolated lymphocytes were stained with LIVE/DEAD fixable viability dye from Life Technologies (now ThermoScientific, Waltham, MA), Fc block (BD Biosciences, San Diego, CA), and CD3, CD19, CD4, CD8, CD103, CD45RA, and chemokine (C-C motif) receptor 7 (CCR7) antibodies (BD Biosciences). Following surface antigen staining, cells were stained for perforin (BD Biosciences) using the Cytofix/Cytoperm kit (BD Biosciences).
T-CELL RECEPTOR BETA SEQUENCING OF FROZEN LIVER TISSUE
Archived frozen liver tissue samples were available for a subset of cases (5 iPALF, 3 AIH, 2 dPALF, and 1 normal liver case) for T-cell receptor beta sequencing. Genomic DNA was isolated from frozen liver sections banked in a –80°C freezer. Sequences were generated from genomic DNA using primers situated near FR2 and Jb (BIOMED2 protocol). Samples were run in duplicate, and libraries from each sample were pooled together (individual library data remain available). Libraries were created using the Illumina Nextera XT (2 × 300 bp) paired end kit and run on an Illumina MiSeq instrument in the Human Immunology Core facility at the University of Pennsylvania. Data were analyzed using an in-house pipeline for assessing degree of clonality and diversity of repertoire.
STATISTICAL ANALYSES
Data are reported as percentages if categorical, means ± standard error if normally distributed, or median with range if not normally distributed. Kruskal-Wallis, chi-squared, and Fisher's exact tests were used to compare patient characteristics. Chi-squared and Fisher's exact tests were used to compare staining pattern results between groups. The intraclass correlation coefficient (ICC) was used to determine agreement between CD8 computer-generated and CD8 subjective stain scores and between CD8 computer-generated and CD163 subjective scores. The Kruskal-Wallis test was used to compare CD8 stain mean percent density between groups. These analyses were conducted using SAS Statistical Software 9.4 (SAS Institute Inc., Cary, NC). P < 0.05 was considered statistically significant.
Results
Thirty-three iPALF, 9 AIH, and 14 dPALF cases met inclusion criteria. All iPALF cases had complete medication and supplement use history taken, and 88% had a negative serum acetaminophen level, 100% had negative hepatitis B virus and hepatitis A virus testing, 97% had negative Epstein-Barr virus testing, and 100% had negative autoantibody screening for AIH. All patients ≥3 years of age had negative screening for Wilson disease. For patients <3 years of age, 100% had negative screening for fatty acid oxidation defects and 80% had screening for mitochondrial disease. The 2 patients who did not have a pyruvate and lactate level reported were included in the iPALF group as they had no clinical features suggesting a diagnosis of mitochondrial disease. Six iPALF patients received a median of 2 days (range 1-4) of 2 mg/kg/day intravenous methylprednisolone therapy prior to liver tissue specimen collection. Two additional iPALF patients were treated with intravenous methylprednisolone after the liver biopsy was performed. The AIH group (n = 9) included 5 patients with type I and 4 patients with type II AIH. Five of the AIH patients were treated with 1 week or less of 1-2 mg/kg/day intravenous methylprednisolone prior to LT. A sixth patient diagnosed with AIH when they presented with ALF was treated with 5 months of prednisolone and azathioprine but ultimately failed therapy and underwent LT. The explant was examined for this study. The remaining 3 AIH patients did not receive any immunosuppressive therapy prior to collection of the liver tissue specimen. The dPALF group (n = 14) was comprised of 6 (43%) cases of drug toxicity (3 acetaminophen, 1 valproic acid, 1 trimethoprim-sulfamethoxazole, and 1 enflurane inhaled anesthetic), 5 (36%) cases of Wilson disease, and 3 (21%) cases of mitochondrial disease. Patient characteristics by group are reported in Table 1. iPALF patients were younger than dPALF patients (P = 0.002), and explant/wedge biopsy liver tissue was used for 28 (85%) iPALF compared to 7 (50%) dPALF cases (P = 0.03). Outcome at 21 days differed significantly between the iPALF and dPALF groups (P = 0.03). Eight (24%) iPALF compared to no AIH or dPALF patients developed AA.
|
iPALF (n = 33) |
AIH (n = 9) |
dPALF (n = 14) |
P all 3 groups | P iPALF versus AIH | P iPALF versus dPALF | |
|---|---|---|---|---|---|---|
| Median age, years (range) | 4 (1-17) | 9 (1-14) | 15 (2-17) | 0.004a | 0.11b | 0.002b |
| Male gender, n (%) | 22 (67) | 5 (56) | 5 (36) | 0.15c | 0.70 | 0.05c |
| Sample type used for study, n (%) | ||||||
| Explant/wedge biopsy | 28 (85) | 7 (78) | 7 (50) | |||
| Needle biopsy | 5 (15) | 2 (22) | 7 (50) | 0.04 | 0.63 | 0.03 |
| 21-day outcome, n (%) | ||||||
| Spontaneous recovery | 7 (21) | 2 (22) | 3 (21.5) | |||
| LT | 26 (79) | 7 (78) | 8 (57) | |||
| Death | 0 | 0 | 3 (21.5) | 0.09 | 1.0 | 0.03 |
| Developed AA, n (%) | 8 (24) | 0 | 0 | 0.05 | 0.17 | 0.08 |
- a P value from Kruskal-Wallis test.
- b P value from Wilcoxon rank sum test.
- c P value from chi-squared test. The remaining P values are from Fisher's exact test.
IHC REVEALS CD8+ T-CELL–PREDOMINANT HEPATIC INFILTRATION IN iPALF
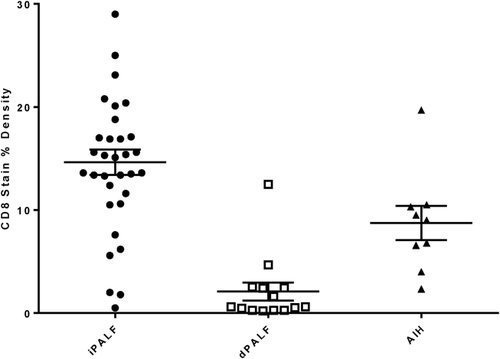
There was a significant difference in the CD8 staining pattern between all three groups with dense parenchymal and portal infiltrates of CD8+ T-lymphocytes observed in 27 (82%) iPALF compared to 3 (33%) AIH and 1 (7%) dPALF case (P < 0.0001; Table 2 and Fig. 1). Comparing CD8 stain percent density values between groups similarly showed that mean percent density was significantly higher in iPALF cases (14.6%) compared to AIH (8.7%) and dPALF (2.1%) cases (P < 0.0001; Fig. 2). The majority (n = 13, 93%) of dPALF cases, compared to only 2 (22%) AIH and 3 (9%) iPALF cases, exhibited minimal CD8 staining. All control liver specimens had minimal CD8 staining. There was no apparent difference in the CD8 staining pattern between iPALF cases who received steroids and those who did not. The one dPALF case with dense CD8 staining was a hypersensitivity reaction to trimethoprim–sulfamethoxazole with features of drug reaction with eosinophilia and systemic symptoms. The AIH cases exhibited a range of CD8 staining scores, with 2 cases having minimal, 4 cases moderate, and 3 cases dense CD8 staining. There was no apparent difference in the CD8 staining pattern between AIH patients who received pretransplant steroids (1 minimal, 3 moderate, and 2 dense) and those who did not (1 minimal, 1 moderate, and 1 dense). All of the 8 iPALF cases who developed AA had dense (n = 7) or moderate (n = 1) CD8 staining. A small subset of iPALF cases had additional immune studies data available. Eight iPALF patients had soluble interleukin-2 receptor levels measured, which were elevated in 6 (75%), with 4 having dense, 1 moderate, and 1 minimal CD8 staining. Three of the 8 iPALF patients who developed AA had peripheral blood flow cytometry performed, which was notable for a low CD4:CD8 ratio of ≤1.0 in all cases. There was excellent agreement between subjective and computer-automated CD8 density scores (ICC = 0.92, P < 0.0001), supporting use of the subjective scores for the remaining IHC stains.
| iPALF | AIH | dPALF | P all 3 groups | P iPALF versus AIH | P iPALF versus dPALF | |
|---|---|---|---|---|---|---|
| CD8 staining pattern, n (%) | (n = 33) | (n = 9) | (n = 14) | |||
| Dense | 27 (82) | 3 (33) | 1 (7) | |||
| Moderate | 3 (9) | 4 (45) | 0 | |||
| Minimal | 3 (9) | 2 (22) | 13 (93) | <0.0001 | 0.01 | <0.0001 |
| Perforin staining pattern, n (%) | (n = 16) | (n = 8) | (n = 7) | |||
| Dense | 13 (50) | 2 (25) | 0 | |||
| Moderate | 6 (23) | 0 | 0 | |||
| Minimal | 7 (27) | 6 (75) | 7 (100) | 0.004 | 0.06 | 0.002 |
| CD103 staining pattern, n (%) | (n = 16) | (n = 8) | (n = 6) | |||
| Dense | 16 (62) | 1 (12.5) | 0 | |||
| Moderate | 4 (15) | 3 (37.5) | 0 | |||
| Minimal | 6 (23) | 4 (50) | 6 (100) | 0.001 | 0.03 | 0.002 |
- P values are from Fisher's exact test.
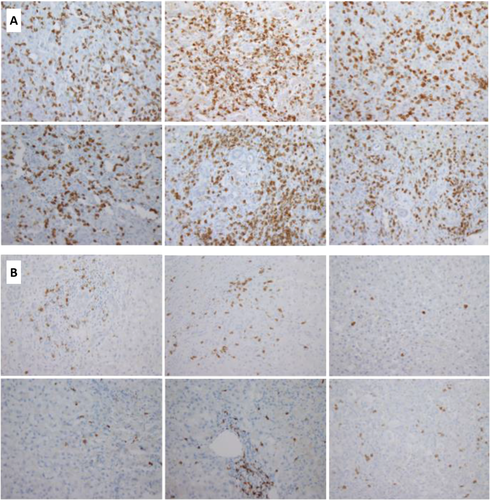
Macrophage staining (CD163) also differed significantly between groups, with all iPALF cases having dense (n = 27, 84%) or moderate (n = 5, 16%) staining compared to moderate or minimal staining in the majority of AIH and dPALF cases (Table 3). There was a significant but only moderate agreement between CD163 and CD8 staining scores (ICC = 0.5, P < 0.0001). Helper T-cells (CD4+) and B-cells (CD20+) were minimal or moderate in most cases, and the staining pattern did not differ significantly between groups. Natural killer cells (CD56+) were minimal in all cases. IHC staining for perforin was performed in 26 (79%) iPALF, 8 (89%) AIH, and 7 (50%) dPALF cases. Perforin staining was dense (n = 13) or moderate (n = 6) in 73% of iPALF cases compared to minimal in 6 (75%) AIH and 7 (100%) dPALF cases (P = 0.004; Table 2). CD8 and CD4 T-cells and natural killer cells can all express perforin; however, the majority of perforin-positive cells were thought to be CD8 cells based on their predominance compared to the rare staining of CD4 and natural killer cells.
| iPALF | AIH | dPALF | P all 3 groups | P iPALF versus AIH | P iPALF versus dPALF | |
|---|---|---|---|---|---|---|
| CD163 staining pattern, n (%) | (n = 32) (97) | (n = 9) (100) | (n = 14) (100) | |||
| Dense | 27 (84) | 3 (33) | 5 (36) | |||
| Moderate | 5 (16) | 5 (56) | 7 (50) | |||
| Minimal | 0 | 1 (11) | 2 (14) | 0.004 | 0.003 | 0.0007 |
| CD4 staining pattern, n (%) | (n = 29) (88) | (n = 8) (89) | (n = 11) (79) | |||
| Dense | 1 (3) | 0 | 0 | |||
| Moderate | 6 (21) | 2 (25) | 1 (9) | |||
| Minimal | 22 (76) | 6 (75) | 10 (91) | 0.05 | 0.27 | 0.20 |
| CD20 staining pattern, n (%) | (n = 29) (88) | (n = 8) (89) | (n = 11) (79) | |||
| Dense | 1 (3) | 0 | 0 | |||
| Moderate | 4 (14) | 1 (12) | 1 (9) | |||
| Minimal | 24 (83) | 7 (88) | 10 (91) | 0.10 | 0.34 | 0.28 |
| CD56 staining pattern, n (%) | (n = 29) (88) | (n = 8) (89) | (n = 11) (79) | |||
| Dense | 0 | 0 | 0 | |||
| Moderate | 0 | 0 | 0 | |||
| Minimal | 29 (100) | 8 (100) | 11 (100) |
- P values are from Fisher's exact test.
FLOW-CYTOMETRIC STAINING OF FRESH INTRAHEPATIC LEUKOCYTES CONFIRMS PERFORIN+CD8+ T-CELL INFILTRATION
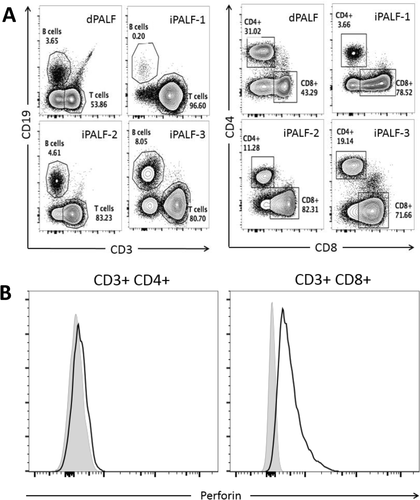
During the study, we had access to fresh liver explants from 3 iPALF cases and 1 dPALF (mitochondrial disease) case to assess intrahepatic leukocytes by flow cytometry. Consistent with the IHC results, intrahepatic leukocytes from all 3 iPALF livers showed a CD8+ T-cell predominance, while the dPALF liver showed a more evenly mixed infiltrate (Fig. 3A). Staining for intracellular perforin showed that the majority of CD8+ T-cells in iPALF livers were perforin-positive (fold change in median fluorescence intensity compared to “fluorescence minus one” control staining 2.2-10), consistent with IHC perforin staining results, while, as expected, the CD4+ T-cells were negative for perforin (fold change in median fluorescence intensity compared to “fluorescence minus one” control staining 1-1.4) (Fig. 3B).
iPALF INTRAHEPATIC CD8+ T-CELLS BEAR THE SURFACE MARKERS OF THE Trm SUBSET
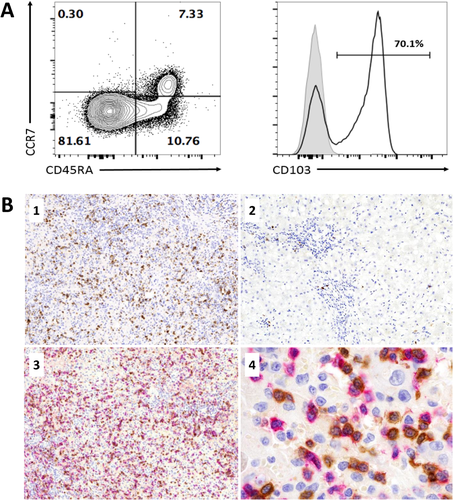
Two fresh iPALF livers were assessed by flow cytometry for CD8+ T-cell memory markers CD45RA and CCR7. The majority of CD8+ T-cells were CD45RA–CCR7–, consistent with an effector memory phenotype (Fig. 4A). We further considered that these cells may represent Trm CD8+ T-cells and therefore stained a sample for the Trm marker CD103. Sixty percent of all CD8+ T-cells and 71% of the CD45RA–CCR7– CD8+ T-cells were CD103+ (Fig. 4A). In order to confirm that CD8+ Trm infiltration was a feature of iPALF, IHC staining for CD103 was performed on liver sections from 26 (79%) iPALF, 8 (89%) AIH, and 6 (43%) dPALF cases and subjectively scored as dense, moderate, or minimal. Dense staining for CD103 was observed in 16 (62%) iPALF compared to 1 (12%) AIH case and no dPALF cases (P < 0.001; Table 2 and Fig. 4B). All 6 dPALF cases had minimal CD103 staining, while the AIH group had 3 (38%) cases with moderate and 4 (50%) cases with minimal CD103 staining. To confirm that these CD103+ cells were CD8+ T-cells, we double-labeled sections from 6 iPALF cases for both CD8 and CD103, demonstrating staining on the same cell (Fig. 4B).
iPALF INTRAHEPATIC T-CELLS SHOW AN INCREASE IN CLONALITY
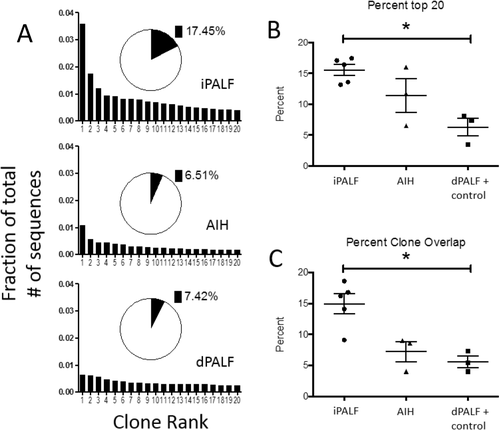
We considered whether CD8+ T-cells were responding to antigen or if they were nonspecifically being activated and recruited to the liver. We performed next-generation sequencing of PCR products using common Vβ primers from frozen liver specimens from 5 iPALF cases, 3 AIH cases, 2 dPALF (mitochondrial disease and Wilson disease) cases, and 1 normal control to assess the clonality of T-cell receptor beta usage of the intrahepatic lymphocytes. Samples were run in duplicate and showed no differences in quality between runs. We assessed clonality by measuring percentage of reads that composed the top 20 clones (Fig. 5A,B) and by measuring percent overlapping clones between the duplicates of each sample, which is expected to be higher if specific clones dominate the specimen (Fig. 5C).17, 18 By both measures, iPALF cases showed significantly increased clonality compared to dPALF and control cases (P < 0.01). AIH cases exhibited increased clonality compared to dPALF and control cases but decreased clonality compared to iPALF cases (the difference between groups was not statistically significant).
Discussion
iPALF cases are characterized by dense CD8+ T-cell hepatic infiltrate, and we propose that CD8+ inflammation is a biomarker of the iPALF immune dysregulation phenotype. This study demonstrates that a histopathologic feature, specifically CD8 staining, differentiates iPALF from dPALF cases. Our findings agree with those of McKenzie et al., who published a case series of patients with iPALF (n = 7) and acute hepatitis (n = 2) thought to have immune dysregulation and found to have numerous CD8+ T-cells on IHC staining of liver biopsies.8 Similarly, Patel et al. reported a CD8 predominant T-cell infiltrate on IHC staining of liver tissue from 5 patients with iPALF.19 CD8 staining is rapidly performed in most clinical pathology laboratories, with results available in 1-2 days, and could be easily integrated into clinical practice as a biomarker of immune-active iPALF. Macrophage density was also significantly increased in iPALF compared to AIH and dPALF cases and appeared to correlate with degree of hepatocyte necrosis. However, the pattern of CD163 staining overall did not clearly differentiate PALF groups and only moderately correlated with CD8 density, which was a strong marker of iPALF. Further studies are needed to determine whether iPALF macrophages are primarily proinflammatory and involved in propagating the liver injury or if they are anti-inflammatory and assist in tissue repair.
We further characterized the iPALF CD8 T-cell phenotype and found that these cells are perforin-positive, suggesting that they are armed for rapid effector function. This apparent activation of CD8+ T-cells in iPALF was not seen in dPALF, where the majority of cases showed minimal perforin staining. Consistent with T-cell activation in iPALF, 6 of 8 (75%) cases had an elevated soluble interleukin-2 receptor level, a serum marker for lymphocyte activation. Furthermore, CD8+ T-cells of iPALF are largely positive for the Trm marker CD103, which was not seen among the dPALF cases. Trm cells are a recently identified bona fide population of CD8 T-cells characterized to be a memory population that does not reside in secondary lymphoid tissue or in the circulation but rather resides in solid tissues.20 These cells are thought to be important in acting as first responders to infections attempting to breech and invade solid organs in many settings. Consistent with a memory function for these cells in iPALF livers, they are also CCR7/CD45RA low (Fig. 4A), and the fact that CD8+ T-cells almost uniformly express perforin (Fig. 3B and Table 2) argues for effector functions more consistent with a Trm phenotype rather than a regulatory phenotype.
Additionally, iPALF intrahepatic T-cells showed increased clonality, suggesting that an antigen-specific T-cell response may be leading to their increased numbers. We propose a model by which an antigen-driven process leads to the accumulation of Trm CD8+ T-cells in iPALF livers that are armed for effector function. The nature of such an antigen trigger remains unknown and could be either an autoimmune response to self or a maladaptive response to pathogen, perhaps hepatotropic given the tissue resident phenotype of liver CD8+ T-cells. Future investigation should be directed at understanding this antigen response and characterizing events that lead to immune overactivation or ineffective down-regulation. Because our data are only correlative, attempts to causally connect this excessive CD8 infiltration to disease are needed including identification of which effector functions are necessary.
Eight iPALF cases developed AA, while no dPALF cases experienced this complication. The etiology of acquired AA in the setting of ALF remains unknown but is thought to be immune-mediated, involving CD8+ T-cell injury to hematopoietic stem cells and high systemic levels of cytokines including interferon-gamma.21-23 In our study, the majority of iPALF cases with AA had dense CD8 staining (7 dense, 1 moderate), and we suspect that immune dysregulation due to activated CD8 cells could also be contributing to their bone marrow suppression. Similarly, Patel et al. reported that 7 pediatric cases of hepatitis-associated AA had a CD8 predominant hepatic lobular infiltrate by IHC staining with elevated peripheral blood CD8:CD4 T-cell ratios. Three of these patients were treated with immunosuppressive therapy including antithymoglobulin, cyclosporin A, and prednisone, with subsequent improvement in symptoms and liver tests. Follow-up liver biopsies in 3 patients noted a significant decrease in CD8+ lymphocytes.19 These findings support the hypothesis that CD8+ T-cells are involved in mediating the hepatocyte injury and that recovery is associated with a decrease in their numbers within the liver. Furthermore, this study suggests that a number of different immunosuppressive therapies may be effective at reducing CD8+ inflammation, and further research is indicated to determine optimal therapy for these patients. We found that all 3 iPALF patients in our study who developed AA and had peripheral blood flow cytometry available had a decreased CD4:CD8 ratio (due to an increase in CD8 cells), and this finding has been reported in other studies of hepatitis-associated AA.24, 25 In McKenzie et al., all iPALF and acute hepatitis patients had a low CD4:CD8 ratio by peripheral blood flow cytometry, 3 of whom developed AA.8 We propose that a low CD4:CD8 ratio is an additional biomarker of the iPALF immune dysregulation phenotype as well as of those iPALF patients who are more likely to develop AA. Future studies are necessary to determine whether identifying and treating these patients early in their disease course, before they progress to severe bone marrow failure, may improve outcomes.
Currently the established treatment of iPALF patients is limited to general supportive medical care and possible LT. However, several large pediatric centers have started treating selected iPALF patients with steroids. In our series, 8 iPALF patients received intravenous steroids for treatment of presumed immune dysregulation. The only published experience of treating pediatric iPALF patients with steroids specifically for their liver dysfunction is by McKenzie et al., in which patients were given intravenous immunoglobulin and methylprednisolone and all reportedly improved (decrease in alanine aminotransferase and total bilirubin), with 3 undergoing LT and 5 recovering with their native liver.8 When considering steroid therapy for children with ALF, it is important to note that the majority of studies in adult patients with ALF have failed to show any survival benefit of steroid treatment.26-29 However, recent single-center retrospective reports from China and Japan suggest that intravenous steroids may improve spontaneous survival in adult ALF.30, 31 In the pediatric population, indeterminate ALF is more prevalent in young children, who may mount different innate and adaptive immune responses compared to adults or who may be susceptible to a type of immune-mediated ALF not seen in adults and therefore may respond differently to immunosuppressive therapy. Multiple case reports and case series of iPALF and coincident AA have noted improvement in liver function when AA was treated with immunosuppression.21, 32-34 It remains unknown whether steroids or other immunosuppressive therapy will improve outcomes for iPALF, and the risks and benefits should be evaluated with a controlled clinical trial. A dense CD8 hepatic infiltrate appears to be a signature of immune dysregulation in iPALF patients and may help identify those patients who are more likely to respond favorably to immunosuppressive therapy. Future studies will examine whether flow cytometry of peripheral blood could similarly identify this group, by markers such as a predominance of CD103+CD8+ T-cells or a decreased CD4:CD8 ratio, which has the benefit of being an easily obtainable noninvasive test. Ultimately, as we learn more about immune cells, cytokines, and other signaling pathways involved in iPALF, the hope is that a more targeted treatment could be developed.
It is important to mention that not all iPALF cases had dense CD8+ inflammation: 4 had moderate and 3 had minimal staining. A possible explanation is that the immune dysregulation in iPALF occurs along a continuum and the inflammatory response may be variable, with milder cases or cases identified early in the disease course having fewer CD8 cells. Minimal or moderate CD8 cases may also represent cases of iPALF due to different nonimmune disease processes or to a known diagnosis that was missed despite best efforts at a complete workup. Similarly, not all dPALF cases had minimal staining. There was 1 patient with dense CD8 staining who had liver injury secondary to a drug hypersensitivity reaction, which is thought to be an inflammatory and immune-active process. We found that the CD8 staining pattern was variable in the AIH group, which is not surprising given that this is a condition characterized by a range of inflammatory activity depending on timing and severity of disease presentation. Despite this variation, CD8 staining density was still significantly increased in the iPALF compared to the AIH group. Several AIH patients received immunosuppressive therapy, primarily a short course of steroids, prior to IHC staining of their liver tissue. These cases exhibited a range of CD8 density scores, and it is unknown how this therapy may have altered the pattern of CD8 T-cell infiltration. CD8 staining density is not a pathognomonic test and should be considered and interpreted in the context of a patient's other clinical, serologic, and histopathologic features. Other disease conditions that may exhibit dense CD8 T-cell hepatic infiltration but were not represented in our study include acute viral hepatitis, hemophagocytic lymphohistiocytosis, and macrophage activation syndrome.35-37
All dPALF cases had minimal staining for both perforin and CD103. Because these cases also had minimal CD8 staining, this indicates that the few CD8 or CD4 T-cells present are not armed for effector functions and do not appear to be an expanded population of Trm cells. Interestingly, while the AIH cases exhibited a range of CD8 density staining, they were less likely to have dense or moderate perforin and CD103 staining compared to iPALF cases, suggesting that CD8 cells in iPALF and AIH may have different phenotypes. Thus, the most specific marker for the iPALF diagnosis, which would differentiate it from both dPALF and AIH, may be a panel including CD8, CD103, and perforin IHC staining to identify the Trm CD8+ T-cell phenotype of iPALF.
A study limitation was that our case selection was biased toward PALF patients who underwent LT as they were more likely to have liver tissue available for IHC staining. Therefore, we cannot make any statements about the association between CD8 density and PALF outcomes. In addition, not all investigators were blinded to PALF diagnosis when performing subjective stain scoring. To address this limitation, we measured computer-generated CD8 percent density and confirmed that those scores agreed with the subjective scores.
In summary, iPALF cases are characterized by a perforin+CD103+CD8+ T-cell predominant hepatic infiltrate consistent with expansion of a Trm CD8+ T-cell phenotype, which is clearly different from noninflammatory causes of PALF. Given our demonstrated clonality of the iPALF T-cell response, we suspect that the T-cell expansion is antigen-driven, and identification of the nature of this response may allow us to refine a more targeted therapy. We propose that dense CD8 staining is a biomarker of the iPALF immune dysregulation phenotype and can be used to help identify and define this group. Future studies will build on these findings with the goal of better understanding the pathophysiology of iPALF and hepatic CD8 T-cell infiltration and identifying potential immunosuppressive therapies that may improve outcomes for these patients.
Acknowledgment
The authors are grateful for support from Dr. Elizabeth Rand for sharing specimens from the Children's Hospital of Philadelphia liver biorepository and from Dr. Pierre Russo at the Children's Hospital of Philadelphia for his assistance with review of immunohistochemical stains.




