Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy
Potential conflict of interest: Nothing to report.
Supported by Paujar Charity Foundation and Taiwan Children Liver Foundation.
Abstract
We investigated the utility of transient elastography (TE) for diagnosing biliary atresia (BA) in cholestatic infants and predicting the outcome of BA. Forty-eight cholestatic infants (9-87 days of age) with direct bilirubin level >1 mg/dL were enrolled. Liver stiffness measurement (LSM) by TE was performed during the cholestasis workup, and 15 subjects were diagnosed as BA. We assessed liver histology using liver biopsies from 36 subjects and graded fibrosis status using the METAVIR score. BA infants had significantly higher LSM values and METAVIR scores than non-BA cholestatic infants. A receiver operating characteristic (ROC) curve analysis showed that an LSM >7.7 kPa was predictive of BA among cholestatic infants (sensitivity = 80%; specificity = 97%; area under the curve [AUC] = 85.3%; P = 0.0001). Cholestatic infants with an LSM >7.7 kPa were more likely to be diagnosed with BA (odds ratio [OR] = 128; P < 0.001). Very early measurement of LSM after hepatoportoenterostomy (HPE) is associated with occurrence of thrombocytopenia, splenomegaly, and esophageal varices 6 months post-HPE. Five of the BA subjects were awaiting or had received liver transplantation (LT), and they had a significantly higher LSM measured 1 week post-HPE than that in the other BA subjects (26.0 vs. 10.8 kPa; P = 0.006). A Cox proportional analysis demonstrated that the need for LT was significantly higher in BA subjects with LSM >16 kPa measured 1 week post-HPE than other BA subjects (hazard ratio [HR] = 10.16; P = 0.04). Conclusion: LSM assessment during the workup of cholestatic infants may facilitate the diagnosis of BA. LSM post-HPE may predict complications and the need for early LT in infants with BA. (Hepatology 2018).
Abbreviations
-
- AUC
-
- area under the curve
-
- BA
-
- biliary atresia
-
- CART
-
- classification and regression decision tree
-
- CI
-
- confidence interval
-
- EV
-
- esophageal varices
-
- GGT
-
- gamma-glutamyl transferase
-
- HPE
-
- hepatoportoenterostomy
-
- HR
-
- hazard ratio
-
- IOC
-
- intraoperative cholangiography
-
- IQR
-
- interquartile range
-
- LF
-
- liver fibrosis
-
- LSM
-
- liver stiffness measurement
-
- LT
-
- liver transplantation
-
- NPV
-
- negative predictive value
-
- OR
-
- odds ratio
-
- PPV
-
- positive predictive value
-
- ROC
-
- receiver operating characteristic
-
- TE
-
- transient elastography
-
- TPN
-
- total parenteral nutrition
Biliary atresia (BA) is one of the most severe cholestatic liver diseases in infants. BA is caused by progressive fibroinflammatory cholangiopathy and is the most common indication for liver transplantation (LT) in children.1-3 The most common initial presentations of BA include persistent jaundice, hepatomegaly, and acholic stools, but these are very similar to the symptoms of other cholestatic liver diseases in early infancy.2, 3
Early and prompt diagnosis of BA with timely hepatoportoenterostomy (HPE) surgery is the gold standard for establishing good bile flow and improving long-term survival.4-6 Early HPE is associated with a jaundice-free status 3 months postsurgery, a higher rate of native liver survival, and better clinical outcomes.7 However, early diagnosis of BA remains challenging in infants with cholestasis.8 Imaging of the gallbladder and biliary tree under sonographic and magnetic resonance cholangiopancreatography examination are widely used to diagnose BA in cholestatic infants, but the diagnostic accuracy of these tests are unsatisfactory.9-14 Patent biliary scintigraphy can exclude a diagnosis of BA, but the absence of excretion may also be observed in infants with other cholestatic liver diseases.2, 15 Intraoperative cholangiography (IOC) remains the gold standard for confirming a diagnosis of BA, but is an invasive procedure and not suitable for the differential diagnosis of cholestatic infants.1, 2
BA patients are prone to developing liver fibrosis (LF) in early infancy, and the results of transient elastography (TE) correlate with LF status in BA patients.16-18 Thus, assessing liver stiffness in cholestatic infants may facilitate the differential diagnosis of BA. A recent case-control study suggested a diagnostic benefit of shear-wave elastography (SWE) to differentiate BA, cholestatic infants, and healthy newborn.19
In this study, we evaluated the role of TE in diagnosing BA in cholestatic infants and its relationship with the results of pathological pictures. We also investigated the utility of early TE measurement for predicting clinical outcomes post-HPE in BA patients.
Patients and Methods
SUBJECTS AND CLINICAL DATA
We recruited 48 cholestatic infants (31 males and 17 females) from the Department of Pediatrics of National Taiwan University Hospital (NTUH) from May 2015 to December 2017 to this study prospectively. All patients presented with cholestasis (serum direct bilirubin level >1 mg/dL and direct to total bilirubin ratio >20%). Subjects with ascites, septic shock, and previous abdominal surgery were excluded. All subjects underwent workup for cholestasis, including blood tests, urine test, metabolic workup, and abdominal sonogram. Liver biopsy was performed in 36 subjects (75%) for diagnostic purposes, and IOC was performed in 22 subjects (45.83%). The diagnosis of BA was confirmed by IOC in 15 subjects (31.25%), all of whom underwent HPE. All patients underwent regular follow-up after the workup and management in our institution. Tests for blood biochemical parameters (total and direct bilirubin levels; alanine aminotransferase [ALT], alkaline phosphatase [ALP], and gamma-glutamyl transferase [GGT] levels) and abdominal sonograms were performed regularly during the follow-up period. The study protocol was approved by the institutional review board of NTUH.
MEASUREMENT OF SPLEEN SIZE AND DIAGNOSIS OF ESOPHAGEAL VARICES IN BA PATIENTS
In subjects with BA post-HPE, we performed an abdominal sonogram every 6 months since 6 months of age or at the presence of palpated splenomegaly at physical examination. Longitudinal length of spleen was measured by the abdominal sonogram. Esophagogastroduodenoscopy was performed for the diagnosis of esophageal varices (EV) at the presence of positive stool occult blood with/without splenomegaly or hematemesis in these BA patients.
LF MEASUREMENT BY TE
Liver fibrosis measurement (liver stiffness measurement; LSM) by TE (Fibroscan 502 Touch; Echosens, Paris, France) was performed during the cholestatic workup before liver biopsy and IOC. We used the S1 probe (5 MHz) for LSM. Ten shots within 3-5 minutes were performed in each subject. Given the accuracy of TE, an interquartile range (IQR)/median LSM value <0.3 was defined as valid. In subjects with BA, LSM was performed weekly for 1 month post-HPE and then monthly until 6 months after the operation. Six months after the operation, LSM was performed in subjects with BA at 3-month intervals. A bedside abdominal sonogram was performed to define the ascites-free status before each LSM. All TEs were performed by W.J.F. using the same instrument.
LF ASSESSMENT
LF status was assessed histologically in specimens from 36 subjects obtained by needle biopsy during the cholestatic workup or wedge biopsy during the operation. The METAVIR score was evaluated by a pathologist (J.Y.M.) who was blind to the LSM data.20
STATISTICAL ANALYSIS
STATA (version 14; StataCorp LP, College Station, TX) and MedCalc (version 18; MedCalc Software, Ostend, Belgium) software packages were used for statistical analyses. The primary outcome was a diagnosis of BA, and the secondary outcome was the cholestatic complications (thrombocytopenia, splenomegaly, and EV 6 months post-HPE and the need for LT younger than 1.5 years of age in BA subjects). For continuous variables, the nonparametric Mann-Whitney U test was used to assess differences in IQRs and medians between the two groups. Fisher's exact test or the chi-square test was performed to assess differences in incidence between the groups. Receiver operating characteristic (ROC) curve analyses were performed to determine cutoff values. Logistic regression was used to assess the odds ratio (OR) and 95% confidence interval (CI) for predicting BA. Cox proportional analysis, Kaplan-Meier plot, and log-rank test were applied for survival analysis of the need of LT. Regression analysis was also used for data analysis. A P value <0.05 was regarded as statistically significant.
Results
GENERAL CHARACTERISTICS OF THE SUBJECTS
BA was diagnosed in 15 subjects, neonatal hepatitis in 18 subjects, total parenteral nutrition (TPN)-related cholestasis in 3 subjects, neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) in 1 subject, urinary tract infection (UTI) in 2 subjects, choledochal cyst in 2 subjects, liver-congestion–related cholestasis in 2 subjects, cystic fibrosis in 1 subject, glycogen storage disease in 1 subject, type I progressive familial intrahepatic cholestasis in 1 subject, Alagille syndrome in 1 subject, and inborn error of bile acid synthesis in 1 subject in these cholestatic infants (n = 48).
Subjects with BA (n = 15) had higher GGT, direct bilirubin levels, and LSM values than those subjects without BA of similar age (n = 33; P = 0.001, 0.004, and 0.0004, respectively; Table 1). There was no relationship between LSM value and total serum level of bilirubin (P = 0.76), LSM value and serum direct bilirubin level (P = 0.11), or LSM value and serum bile acid level (P = 0.68) during cholestasis workup in these 48 subjects. In the 15 BA patients, there was also no significant relationship between LSM value and serum total and direct bilirubin levels (P = 0.62 and 0.95, respectively) or serum bile acid level (P = 0.94) pre-HPE.
| None-BA (n = 33) | BA (n = 15) | P Value | |
|---|---|---|---|
| Age, median (IQR), days | 40 (27-56) | 45 (34.5-60.5) | 0.62 |
| T-bil, median (IQR), mg/dL | 6.68 (5.21-8.76) | 8.45 (6.87-9.71) | 0.15 |
| D-bil, median (IQR), mg/dL | 2.88 (2.41-4.34) | 4.25 (3.81-5.97) | 0.004 |
| GGT, median (IQR),U/L | 100 (72-180) | 396 (214.5-880.5) | 0.001 |
| ALP, median (IQR), U/L | 409 (343-547) | 461 (333.0-608.5) | 0.87 |
| AST, median (IQR), U/L | 65 (41-116) | 121 (74.0-178.5) | 0.11 |
| ALT, median (IQR), U/L | 37 (18-74) | 74 (34.0-129.5) | 0.20 |
| LSM, median (IQR), kPa | 4.60 (3.90-6.00) | 10.50 (8.50-20.90) | 0.0004 |
| Bile acid, median (IQR), uM | 105.5 (81-136) | 139 (102-181) | 0.25 |
| Male sex, n (%) | 24 (72.72) | 7 (46.67) | 0.08 |
| Liver biopsy, n (%) | 21 (63.64) | 15 (100) | 0.01 |
| METAVIR score, n (%) | (n = 21) | (n = 15) | |
| F0 | 8 (38.10) | 0 (0) | |
| F1 | 5 (23.81) | 0 (0) | |
| F2 | 8 (38.09) | 4 (26.67) | |
| F3 | 0 (0) | 6 (40) | |
| F4 | 0 (0) | 5 (33.33) | 0.0001 |
- Abbreviations: ALP, Alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; D-bil, direct bilirubin; GGT, gamma glutamyl transpeptidase; T-bil, total bilirubin.
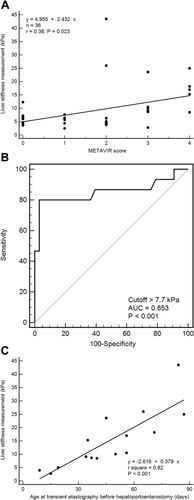
In total, 36 subjects received liver biopsy in this cohort, and the METAVIR score was graded as F0 in 8 subjects, F1 in 5 subjects, F2 in 12 subjects, F3 in 6 subjects, and F4 in the remaining 5 subjects. Among these 36 cholestatic infants with liver specimens obtained within 90 days of age, the median METAVIR score was F3 (range = F2-F4) in BA (n = 15), F1 (range = F0-F2) in neonatal hepatitis (n = 14), F1 (range = F0-F2) in UTI (n = 2), F0 in one choledochal cyst, F0 in one cystic fibrosis, F2 in one glycogen storage disease, F0 in one type I progressive familial intrahepatic cholestasis, and F2 in one TPN-related cholestasis. LSM value was significantly higher in METAVIR F3-F4 subjects (median = 10.5 kPa; IQR = 8.9-17.0 kPa; n = 11) than METAVIR F0-F2 subjects (median = 4.9 kPa; IQR = 4.0-6.4 kPa; n = 25; P = 0.002). LSM value was also correlated with the METAVIR score in these 36 subjects (P = 0.023; Fig. 1A).
There are 12 cholestatic subjects without liver biopsy received regular follow-up in our institution. Four of them were diagnosed as cytomegalovirus-related neonatal hepatitis and had a total serum level of bilirubin <2 mg/dL before 1 year of age. Two of the 12 cholestatic subjects were diagnosed as TPN-related cholestasis, 1 with NICCD according to genetic analysis, 1 with Alagille syndrome according to genetic analysis, 1 with inborn error of bile acid synthesis according to urine bile acid analysis, 1 with choledochal cyst, and 2 with liver congestion attributed to right heart failure.
DIAGNOSTIC UTILITY OF LSM IN BA
ROC curve analysis showed that an LSM cutoff >7.7 kPa was optimal for predicting BA measured by TE (sensitivity = 80%; specificity = 97%; area under the curve [AUC] = 85.30%; P = 0.0001; Fig. 1B). A GGT level >200 IU/L was used as a diagnostic predictor of BA in a recent study.8 A GGT level >200 IU/L and LSM >7.7 kPa were predictive of BA in univariate logistic regression analyses (OR = 10.21 and 128; P = 0.001 and <0.001, respectively; Table 2). In the multivariate logistic regression model that included both factors, only the LSM value >7.7 kPa was predictive of BA (OR = 83.62; P < 0.001; Table 2). The positive and negative predictive values (PPV and NPV) of an LSM value cutoff >7.7 kPa for BA were 92.31% and 91.43%, respectively.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Male (n = 31) versus female (n = 17) | 0.33 (0.09-1.67) | 0.090 | — | — |
| GGT >200 U/L (n = 18) versus ≤200 U/L (n = 30) | 10.21 (2.48-42.10) | 0.001 | 3.89 (0.48-31.55) | 0.230 |
| LSM >7.7 kPa (n = 13) versus ≤7.7 kPa (n = 35) | 128 (12.10-1,353.49) | <0.001 | 83.62 (7.54-927.70) | <0.001 |
- P value <0.017 was regarded as statistically significant, and between 0.017-0.035 as a trend in the univariate logistic regression analysis after Bonferroni correction. Only factors that achieved a trend (<0.034) were included into the multivariate model analysis.
RELATIONSHIP BETWEEN AGE AND LF
Pre-HPE, LSM value of BA subjects increased significantly with age (P < 0.001; Fig. 1C). Three subjects with BA (20%) had an LSM value <7.7 kPa, and all of them underwent TE before 21 days of age. Another 12 subjects with BA (80%) who underwent TE after 21 days of age had LSM values >7.7 kPa during the workup for cholestatic liver disease. In the non-BA cholestatic subjects (n = 33), the correlation between LSM value and age was not significant (correlation coefficient = 0.48; P = 0.80).
In all cholestatic infants more than 21 days of age (n = 43), the PPV and NPV for BA of an LSM value cutoff >7.7 kPa were 92.31% and 100%, respectively.
FOLLOW-UP PATTERN OF LSM PATTERN IN BA PATIENTS POST-HPE
The clinical data of the 15 subjects with BA are summarized in Table 3. Jaundice-free status (serum level of total bilirubin <2 mg/dL) ever achieved in 14 subjects at a median time of 35 days (IQR = 17.0-67.5 days) post-HPE. The follow-up period post-HPE in case 15 is 2 months to the end of this study, and no cholestatic complications at the final visit (4.5 months of age).
| Case | Sex | LSM Workup Age (days) | LSM at Workup (kPa) | HPE Age (days) | METAVIR Score | LSM at 3 Months Post-HPE (kPa) | Jaundice-Free Time Post-HPEa (days) |
|---|---|---|---|---|---|---|---|
| 1 | Male | 9 | 4 | 26 | F2 | 10.1 | 112 |
| 2 | Male | 15 | 2.8 | 20 | F3 | 5.7 | 13 |
| 3 | Female | 19 | 5.1 | 24 | F2 | 18.8 | 19 |
| 4 | Female | 34 | 9.1 | 48 | F3 | 64 | 37 |
| 5 | Female | 35 | 15.3 | 42 | F4 | 25 | 35 |
| 6 | Female | 22 | 8.7 | 22 | F3 | 54 | 15 |
| 7 | Female | 40 | 8.5 | 48 | F4 | 29 | 75 |
| 8 | Male | 45 | 23.6 | 45 | F3 | 4.6 | 20 |
| 9 | Male | 50 | 10.1 | 50 | F3 | 4.9 | 9 |
| 10 | Female | 56 | 10.5 | 60 | F3 | 39.7 | Persistent jaundice |
| 11 | Male | 56 | 17 | 57 | F4 | 18 | 20 |
| 12 | Male | 65 | 26 | 66 | F2 | 75 | 35 |
| 13 | Male | 71 | 18.2 | 76 | F4 | 33.4 | 60 |
| 14 | Female | 84 | 43.5 | 84 | F2 | 53.8 | 143 |
| 15b | Female | 87 | 25 | 90 | F4 | — | 20 |
- a Time interval between HPE and documented serum total bilirubin levels less than 2 mg/dL.
- b Case 15 was followed for 2 months post-HPE to the end of this study, hence the LSM at 3 months postsurgery remains unavailable.
Three patterns of the changes in LSM values post-HPE were evident (Supporting Table S1). LSM pattern 1 (n = 4, 26.67%; LSM resolving or stably low) involved a significant decline in LSM value and serum bilirubin level post-HPE that remained stable. The LSM value of case 9 pre-HPE was 10.1 kPa, and this patient underwent surgery at 50 days of age (METAVIR F3). His serum bilirubin levels and LSM declined and remained stationary after the 9 days after operation (Fig. 2A).
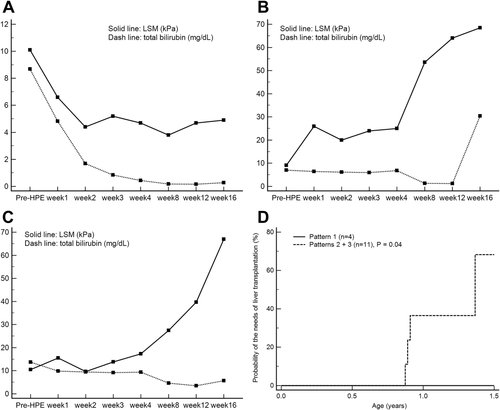
LSM pattern 2 (n = 10, 66.67%; LSM slowly elevated) involved elevated LSM values, even the jaundice-free status ever achieved post-HPE. The LSM value of case 4 was 9.1 kPa pre-HPE, and this patient underwent HPE at 48 days of age (METAVIR F3). Her LSM value remained elevated post-HPE, and her serum level of total bilirubin decreased to <2 mg/dL 37 days after the operation (Fig. 2B). She received LT at 11 months of age.
The interval between HPE and a jaundice-free status (total serum bilirubin level <2 mg/dL) in pattern 1 subjects (n = 4) was significantly shorter than that in pattern 2 subjects (n = 10; median = 16.5 vs. 36 days; IQR = 11-20 vs. 19.5-95.0 days; P = 0.03).
LSM pattern 3 (n = 1; 6.67%; LSM rapidly elevated) subjects showed persistent jaundice post-HPE and a rapid increase in the LSM values. The LSM value of case 10 was 10.5 kPa pre-HPE, and this patient underwent surgery at 60 days of age (METAVIR F3). Her jaundice persisted post-HPE, and her LSM value increased rapidly from 10.5 to 67.0 kPa (Fig. 2C). Now, she is waiting for LT.
The serial pattern of serum total bilirubin levels and LSM assessed before and after HPE in all BA infants are summarized in Supporting Table S1. Kaplan-Meier analysis demonstrated that need of LT is significantly higher in LSM patterns 2 and 3 BA subjects (n = 11) than pattern 1 BA subjects (n = 4; log-rank test, P = 0.04; Fig. 2D).
PREDICTIVE ROLES OF VERY EARLY SERIAL LSM MEASUREMENT POST-HPE ON CLINICAL OUTCOMES
In subjects with BA followed up for more than 6 months post-HPE (n = 14), LSM values declined or remained <7.7 kPa in 3 subjects with BA post-HPE and were elevated in another 11 subjects with BA compared to LSM values obtained during the cholestatic workup.
The LSM value measured 1 week post-HPE was positively correlated with longitudinal spleen length 6 months post-HPE (correlation coefficient = 0.74; P = 0.003; Supporting Fig. S1A), presence of EV 6 months post-HPE (correlation coefficient = 0.74; P = 0.003), and need for LT before 1.5 years of age (correlation coefficient = 0.74; P = 0.003; Table 4). Subjects with EV 6 months post-HPE (n = 5) had higher LSM values measured 1 week post-HPE than subjects without EV (n = 9; median = 26.0 vs. 10.8 kPa; IQR = 19.5-29.0 vs. 6.4-14.8; P = 0.006).
| LSM Measurement | Platelet Count 6 Months Post-HPE | Spleen Longitudinal Length 6 Months Post-HPE | Presence of EV 6 Months Post-HPE | Risk of LT Before 1.5 Years of Age |
|---|---|---|---|---|
| Before surgery |
r = –0.11 P = 0.72 |
r = 0.47 P = 0.09 |
r = 0.36 P = 0.20 |
r = 0.36 P = 0.20 |
| 1 week postsurgery |
r = –0.36 P = 0.20 |
r = 0.74 P = 0.003 |
r = 0.74 P = 0.003 |
r = 0.74 P = 0.003 |
| 2 weeks postsurgery |
r = –0.33 P = 0.26 |
r = 0.78 P = 0.001 |
r = 0.67 P = 0.009 |
r = 0.67 P = 0.009 |
| 3 weeks postsurgery |
r = –0.38 P = 0.18 |
r = 0.76 P = 0.002 |
r = 0.70 P = 0.005 |
r = 0.70 P = 0.005 |
| 4 weeks postsurgery |
r = –0.37 P = 0.19 |
r = 0.82 P < 0.001 |
r = 0.76 P = 0.002 |
r = 0.76 P = 0.002 |
| 8 weeks postsurgery |
r = –0.52 P = 0.06 |
r = 0.87 P < 0.001 |
r = 0.88 P < 0.001 |
r = 0.88 P < 0.001 |
| 12 weeks postsurgery |
r = –0.54 P = 0.04 |
r = 0.75 P = 0.002 |
r = 0.87 P < 0.001 |
r = 0.87 P < 0.001 |
Five subjects with BA who required or had undergone LT before 1.5 years of age had significantly higher LSM values measured 1 week post-HPE than that in the other subjects with BA (median = 26.0 vs. 10.8 kPa; IQR = 19.5-29.0 vs. 6.4-14.8; P = 0.006). The LSM value of BA subjects measured 3 months post-HPE was also negatively correlated with platelet count 6 months post-HPE (correlation coefficient = –0.54; P = 0.04; Supporting Fig. S1B; Table 4).
ROC analysis yielded the cutoff of LSM >16 kPa measured 1 week post-HPE for best prediction of EV 6 months post-HPE and need of LT before 1.5 years of age (P < 0.001 and P < 0.001, respectively; Supporting Fig. S2A,B). BA infants with LSM >16 kPa measured 1 week post-HPE were noted to have a higher risk of developing EV 6 months post-HPE (risk ratio [RR] = 10; P = 0.005) and need of LT before 1.5 years of age (RR = 10; P = 0.005). A Cox proportional analysis demonstrated that need for LT was significantly higher in BA subjects with LSM >16 kPa than other BA subjects with LSM ≤16 kPa 1 week post-HPE (hazard ratio [HR] = 10.16; 95% CI = 1.13-91.72; P = 0.04).
Discussion
Cholestatic liver diseases, including BA, are the main cause of LF in early infancy and the leading cause of LT in children. In this study, LF progressed more rapidly in subjects with BA than in those with other cholestatic liver diseases at similar age. A cut-off LSM value of 7.7 kPa would facilitate a differential diagnosis of BA in cholestatic infants. LSM value post-HPE is associated with the jaundice-free period. Thrombocytopenia, splenomegaly, EV, and risk of LT are also associated with very early LSM value post-HPE in BA patients.
Early diagnosis and HPE is the gold-standard treatment and improves the survival of BA patients.4-6 Early HPE is associated with an increased probability of a jaundice-free status 3 months postsurgery, as well as better clinical outcomes, but accurately diagnosing BA in cholestatic infants remains challenging.7, 8, 21 A recent multicenter study using the classification and regression decision tree (CART) predictive model reported an 81% true-positive rate and an 11% false-negative rate for BA.8 Despite the high accuracy of the optimized prediction models in the CART study, the high precision required for differentiating BA was not achieved.8 Liver biopsy may assist in the diagnosis and assessment of LF status in cholestatic infants, but is invasive.
In this study, infants with BA had higher METAVIR scores than other cholestatic infants of similar age and serum bilirubin levels. In infants with BA, LSM is positively correlated with the age at which TE is performed. A cross-sectional study of children with BA reported a significant correlation between LSM values assessed by TE and liver histological parameters.16 Our study demonstrated the diagnostic role of LSM by TE in the differential diagnosis of BA among cholestatic infants before 90 days of age in a prospective design study. A previous cross-sectional study demonstrated that liver stiffness assessed by SWE assessed by an Aixplorer ultrasound system (SuperSonic Imagine SA, Aix-en-Provence, France) is significantly different between BA and noncholestatic normal infants with a wide age range (16 days to 5 months).19 Our work and the previous cross-sectional study involved different study populations, dissimilar study ages, and different instruments to assess liver stiffness. Ours was a prospective longitudinal follow-up study. We further demonstrated the diagnostic accuracy of LSM above 7.7 kPa for BA among cholestatic infants before 90 days of age in this prospective study. The LSM value was correlated with age and METAVIR liver histology score in subjects with BA. An LSM value cutoff >7.7 kPa had an OR of 128 and a PPV and NPV of 92.31% and 91.43%, respectively, for diagnosing BA in cholestatic subjects before 90 days of age. NPV increased to 100% in subjects between 21 and 90 days of age, which suggests the possibility of false-negative results in infants with BA who are <21 days of age. A GGT cutoff of 204 IU/L was used as a diagnostic parameter in the CART predictive model.8 Applying the GGT levels into the multivariate analysis in this study, the LSM value cutoff >7.7 kPa is more suitable for predicting BA. Therefore, noninvasive assessment of liver stiffness by TE in cholestatic infants may facilitate the differential diagnosis of BA.
Sonography and blood tests are performed during the follow-up of patients with BA post-HPE, but they are not sufficiently sensitive to detect changes in the liver stiffness value.18 We found no obvious relationship between serum bilirubin or bile acid levels and LSM value in this study, and the degree of cholestasis is unlikely to interact with LF in LSM in this population. LSM value improved or normalized in 26.67% of patients with BA (pattern 1), whose cholestasis improved rapidly post-HPE. However, LSM value increased in 73.33% of patients with BA (pattern 2 or 3), who also showed a slower reduction in the severity of cholestasis or persistent cholestasis. The jaundice-free period post-HPE was significantly shorter in pattern 1 (LSM resolving or stably low) subjects than pattern 2 (LSM slowly elevated) subjects. The difference may be attributed to different mechanisms of pathogenesis and warrants a further mechanistic study.22-28 The risk of earlier LT was associated with an LSM value >16 kPa measured 1 week post-HPE in subjects with BA. Moreover, the need for LT was significantly higher in LSM pattern 2 or 3 than pattern 1 BA subjects.
Recent cross-section studies reported that TE values were associated with esophageal/gastric varices in BA patients, further support our findings.(18,29,30) Very importantly, this study demonstrated that a change in LSM value measured 1 week post-HPE may be predictive of cholestatic complications of BA in later life.
BA patients with portal hypertension tend to develop ascites during the follow-up, whereas the presence of ascites is a confounding factor of LSM. We performed bedside abdominal sonogram to confirm the “ascites free status” before LSM in this study to avoid the possible confounding effect of ascites in this study.
In conclusion, we report the utility of TE for the differential diagnosis and follow-up of cholestatic infants. Infants with BA had significantly higher METAVIR liver histology scores and LSM values than other cholestatic infants. The difference in LSM values between cholestatic infants with and without BA can be determined noninvasively using TE. Early high LSM value post-HPE in subjects with BA was associated with thrombocytopenia, splenomegaly, EV, and risk for LT in later life.




