The liver–gut microbiota axis modulates hepatotoxicity of tacrine in the rat
Potential conflict of interest: Nothing to report.
Supported by the Agency for Science, Technology, and Research (A*STAR) Biomedical Research Council (grant R-148-000-144-305), the National University of Singapore (grant C-148-000-003-001), and GSK R&D Centre in Singapore. Niranjan Nagarajan acknowledges financial support from the A*STAR Science and Engineering Research Council under the Industrial Microbiology Genome Informatics platform (project number 1124004038). Lian Yee Yip is supported by the President's Graduate Fellowship.
Current affiliation for Lian Yee Yip: Metabolomics, Bioprocessing Technology Institute, Agency for Science Technology and Research (A*STAR), Singapore, Singapore.
Abstract
The gut microbiota possesses diverse metabolic activities, but its contribution toward heterogeneous toxicological responses is poorly understood. In this study, we investigated the role of the liver–gut microbiota axis in underpinning the hepatotoxicity of tacrine. We employed an integrated strategy combining pharmacokinetics, toxicology, metabonomics, genomics, and metagenomics to elucidate and validate the mechanism of tacrine-induced hepatotoxicity in Lister hooded rats. Pharmacokinetic studies in rats demonstrated 3.3-fold higher systemic exposure to tacrine in strong responders that experienced transaminitis, revealing enhanced enterohepatic recycling of deglucuronidated tacrine in this subgroup, not attributable to variation in hepatic disposition gene expression. Metabonomic studies implicated variations in gut microbial activities that mapped onto tacrine-induced transaminitis. Metagenomics delineated greater deglucuronidation capabilities in strong responders, based on differential gut microbial composition (e.g., Lactobacillus, Bacteroides, and Enterobacteriaceae) and approximately 9% higher β-glucuronidase gene abundance compared with nonresponders. In the validation study, coadministration with oral β-glucuronidase derived from Escherichia coli and pretreatment with vancomycin and imipenem significantly modulated the susceptibility to tacrine-induced transaminitis in vivo. Conclusion: This study establishes pertinent gut microbial influences in modifying the hepatotoxicity of tacrine, providing insights for personalized medicine initiatives. (Hepatology 2018;67:282-295).
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- AUC
-
- area under the plasma concentration-time curve
-
- Cmax
-
- peak plasma concentration
-
- GC/TOFMS
-
- gas chromatography time-of-flight mass spectrometry
-
- LH
-
- Lister hooded
-
- MilR
-
- mild responder
-
- ModR
-
- moderate responder
-
- mRNA
-
- messenger RNA
-
- NonR
-
- nonresponder
-
- OH-THA
-
- 1-hydroxytacrine
-
- rRNA
-
- ribosomal RNA
-
- StrR
-
- strong responder
Elucidating the mechanisms underlying interindividual responses to pharmacotherapy is paramount in personalized medicine research. The superorganismic nature of the human host means that not all variations in therapeutic outcome can be attributed to host genetics.1-3 Indeed, the gut microbiota possesses vast metabolic activities that can either complement or oppose the host's enzymatic actions, thereby modulating drug disposition and therapeutic outcomes.3-5 To date, only a small fraction of marketed drugs are known to be metabolically influenced by the microbiota,3 and the contribution of gut microbiota toward interindividual variation in pharmacology remains poorly understood.
Heterogeneous responses to drug therapy are clinically important and poses a major challenge to both drug development and patient management. Tacrine is the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease. It exhibits significant interindividual variability in its pharmacokinetics and hepatotoxicity of yet unknown etiology. Whereas preclinical toxicology failed to provide clear evidence of tacrine-induced hepatotoxicity,6 tacrine elicits hepatocellular injury clinically, characterized by elevations in alanine aminotransferase (ALT) in about 50% of patients.7, 8 Furthermore, some patients developed jaundice and/or hepatocellular necrosis as confirmed by liver biopsy.7, 9, 10 Tacrine is metabolized extensively by hepatic cytochrome P450 enzymes (primarily CYP1A2) to form hydroxylated metabolites, which are further metabolized to glucuronides.11 A toxic reactive metabolite of tacrine has been postulated11, 12 but not identified. Although tacrine was suggested as a direct hepatotoxicant,13-15 the mechanism of its toxicity has not been explained. Consequently, the unpredictable toxicokinetics of tacrine led to its demise in light of safer and efficacious alternatives.
Currently, the contribution of gut microbiota to interindividual variability in pharmacology is poorly defined. The intricate relationship between the host and gut microbiota further complicate such investigation. Hence, a holistic systems biology approach is necessary to elucidate the complex host–gut microbiota interactions in pharmaceutical research. In the current study, we employed an integrated strategy combining pharmacokinetics, toxicology, metabonomics, genomics, and metagenomics to elucidate the molecular mechanism underpinning the hepatotoxicity of tacrine. Our analyses revealed 3.3-fold higher systemic exposure of tacrine and double peak plasma concentration (Cmax) phenomenon in strong responders that experienced tacrine-induced elevation in transaminase or transaminitis. The unique pharmacokinetics of the strong responders revealed an enterohepatic recycling pattern of deglucuronidated tacrine not attributable to variation in hepatic gene expression. Toxicity-related marker metabolites of microbial and comicrobial origins underscored the role of gut microbiota or its activity in characterizing tacrine-induced hepatotoxicity. Metagenomics delineated greater deglucuronidation capabilities in strong responders based on differential gut microbial composition (e.g., Lactobacillus, Bacteroides and Enterobacteriaceae) and β-glucuronidase gene abundance. Finally, the influences of orally administered β-glucuronidase and antibiotics on the occurrence of tacrine-induced transaminitis were validated in an independent group of tacrine-dosed rats. Our results confirm that the liver–gut microbiota axis regulates interindividual responses to tacrine-induced hepatotoxicity.
Materials and Methods
Expanded descriptions for reagents, animal husbandry, gas chromatography time-of-flight mass spectrometry (GC/TOFMS)-based metabonomics, analytical methods for pharmacokinetic studies, messenger RNA (mRNA) profiling, 16S ribosomal RNA (rRNA) gene sequencing, and statistical analysis are provided in the Supporting Information.
ANIMAL STUDY DESIGN
All experiments were conducted in compliance with the GSK Policy on the Care, Welfare, and Treatment of Laboratory Animals, the guidelines of the Institutional Animal Care and Use Committee (protocol number 080342).
MAIN STUDY
Thirty-eight male Lister hooded (LH) rats (Harlan, UK) (weight, 237-373 g) were used. Of these, 26 rats received by oral gavage a single dose of 20 mg · kg−1 tacrine, dissolved in 1% methylcellulose, at a volume of 10 mL · kg−1. This dose was chosen to induce liver injury without causing death.6, 13, 16 The remaining 12 rats in the control group were administered an oral dose of 1% methylcellulose vehicle. The sample size was estimated by assuming that 50% of tacrine-dosed rats exhibit transaminitis and using the estimated standard deviation of ALT of 79 U · L−1 from a pilot study. Plasma, urine, and feces samples were collected from each rat over a period of 176 hours. Livers (perfused with cold phosphate-buffered saline) were collected immediately at the end of the study, snap frozen, and stored at −80°C before analysis.
VALIDATION STUDY
Sixty-nine male LH rats (weight, 236-293 g) were divided into three treatment groups (sample size calculation is described in the Supporting Information). In group 1 (conventional treated), 23 rats were first given an oral gavage of vehicle used to prepare the β-glucuronidase enzymes as described previously.17 Five minutes later, 17 rats received a single oral dose of 20 mL · kg−1 tacrine and six rats were orally administered vehicle (1% methylcellulose). Group 1 mirrored the main study and served as a control for the other treatment group. In group 2 (β-glucuronidase treated), all 23 rats were given a single oral dose of 168,400 U · mL−1 of β-glucuronidase, at a dose of 0.6 mL · kg−1, before oral gavage of either 20 mg · kg−1 tacrine or 1% methylcellulose vehicle. To mitigate degradation, we calculated for a much higher dose of the β-glucuronidase enzyme (>1000-fold excess of β-glucuronidase required for conversion of the drug). In group 3 (antibiotics treated), all 23 rats were administered 50 mg/kg/d each of vancomycin and imipenem (given in drinking water) 3 days before similar treatment with tacrine. Plasma samples were prepared and collected for analysis.
CLINICAL CHEMISTRY AND TOXOCITY PHENOTYPING
Plasma aspartate aminotransferase (AST) and ALT were determined using a Cobas C111 analyzer (Roche Diagnostics, Basel, Switzerland). Based on LiverTox grading of drug-induced liver injury (https://livertox.nih.gov/Severity.html), tacrine-dosed rats with AST more than 3 times the mean AST of controls over the entire study duration were classified as responders to tacrine-induced transaminitis, while rats not meeting this criterion were classified as nonresponders (NonR). The responders were further arbitrarily classified as strong responders (StrR, AST elevation ≥3 measurements), moderate responders (ModR, AST elevation >2 measurements) or mild responders (MilR, AST elevation >1 measurement) based on the duration of transaminitis.
GC/TOFMS-BASED METABONOMICS
Urine and fecal metabonomic experiments were performed using the established in-house protocol as described by Chan et al.18 Detailed descriptions of the sample preparation, GC/TOFMS metabonomics and data analysis are provided in the Supporting Information.
PHARMACOKINETICS
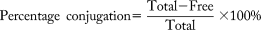
PROFILING OF HEPATIC mRNA EXPRESSION
Total RNA was isolated from approximately 10 mg of tissue from each liver sample using RNeasy Mini Kit, according to the manufacturer's protocol. The hepatic expression of a panel of 17 candidate genes potentially involved in tacrine's disposition based on literature review were profiled using quantitative polymerase chain reaction, and their respective primer sequences are reported in Supporting Table 1. Details of the mRNA profiling experiments are described in the Supporting Information.
MICROBIAL DNA ISOLATION FROM RAT FECES
Microbial DNA was isolated from 84 LH rat fecal samples collected at –24-0, 0-8, 8-24, and 72-96 hours (Supporting Table 2) using the QIAamp DNA Stool Minikit by following a modified Stool Pathogen Detection protocol to maximize DNA extraction. Isolated DNA was checked for purity spectrophotometrically to assess 260/280 and 260/230 values. Concentration of double-stranded DNA (dsDNA) was quantified using Quant-iT PicoGreen kit.
16S rRNA GENE SEQUENCING
Polymerase chain reaction amplification of the V3-V4 region was performed, using the primer pair as described by Klindworth et al.19 The samples were prepared following the protocol in Nextera XT DNA Sample Preparation kit. Paired-end 2 × 250 bp sequencing was performed on the Illumina MiSeq using a 500-cycle MiSeq Reagent Standard Kit (version 2). Details of sample preparation and data processing are provided in the Supporting Information.
METAGENOMIC LIBRARY PREPARATION AND SEQUENCING
DNA libraries were prepared and paired-end sequencing (2 × 76 bp reads) was performed on the Illumina HiSeq2000 platform as detailed in the Supporting Information.
METAGENOMIC ASSEMBLY, OPEN READING FRAME (ORF) PREDICTION AND ANNOTATION
Low-quality bases (base quality <3) were trimmed and read pairs with short reads (<60 bp) were filtered out. De novo assembly was performed on the trimmed reads using SOAPdenovo20 (version 1.05) with parameters “-K 41 -M 3 -R -d -D” and open reading frame (ORF) prediction was performed using Prodigal21 (parameter: metagenomic mode). Genes were annotated based on matches to the KEGG database using RapSearch22 (default parameters).
ORFs annotated as β-glucuronidase were extracted from all metagenome assemblies and merged into a single reference. Rat genomic reads were identified and filtered out by mapping to the rat genome (rn6) using BWA-MEM with default parameters (bwa-0.7.9a). Filtered reads (microbiome reads) were then mapped using BWA-MEM to β-glucuronidase reference sequences to compute relative abundance. For computing the abundance of the uidA gene, mapping was performed with the uidA gene sequence as reference. Differential abundance testing (StrR versus NonR group) was performed using the webtool EDDA (version 1.4.0) (http://edda.gis.a-star.edu.sg/; upper-quartile normalization, cuffdiff mode).23
Metaphlan24 (default parameters) was used to profile community abundance at the genus level. Differential abundance testing (StrR versus NonR) was performed with the webtool EDDA (version 1.4.0) (upper-quartile normalization, cuffdiff mode)23 using the mapped clade-specific (genus) reads produced by metaphlan. An false discovery rate (FDR) cutoff (< 0.05) was used to filter for significant differentially abundant genera (StrR versus NonR). Bacteria genera that are present in less than 1% (defined by metaphlan) in all analyzed samples were filtered out (StrR versus NonR) as we regard their contributions to the microbiome as minimal. The raw sequencing reads in this study have been deposited in National Center for Biotechnology Information's Sequence Read Archive. The project number is PRJNA268906 and the Sequence Read Archive project number is SRP051029.
DATA AND STATISTICAL ANALYSIS
Details of data and statistical analysis are presented in the Supporting Information.
Results
TACRINE HEPATOXICITY CORRELATED WITH PHARMACOKINETICS BUT NOT RAT GENETICS
We administered an acute oral gavage of tacrine at 20 mg · kg−1 body weight to 26 LH rats, while 12 control rats received 1% methylcellulose vehicle (Fig. 1a). Clinical chemistry measurement of plasma ALT and AST revealed variable susceptibility to tacrine-induced transaminitis (Fig. 1b; Supporting Fig. S1a,b). Based on the severity of transaminitis, 10 tacrine-dosed rats with elevation of AST >3× mean of controls were classified as StrR (elevations over ≥3 time points; n = 4), ModR (elevations over 2 time points; n = 3), or MilR (elevations over 1 time point; n = 3) while 16 rats with AST <3× mean of controls were classified as NonR to reflect the graded responses. For the responders, the development of transaminitis began at 0-8 hours, peaked at 8-24 hours, and recovered from 24 hours post-administration of tacrine (Fig. 1b). Good correlation observed between the plasma ALT and AST markers at the peak of transaminitis (r2 > 0.8), and necrosis and fatty changes in the midzonal and pericentral regions of the liver lobules in LH rat liver sections confirmed the occurrences of hepatocellular injury after tacrine administration (Supporting Fig. S1c-g). The differential susceptibility to tacrine-induced transaminitis in LH rats corroborated its clinical prevalence7, 10, 25 and validated the suitability of LH rats for further investigation.
Because several studies have indicated a link between tacrine-induced hepatotoxicity and its pharmacokinetics,13, 25 we next sought to validate such an association. Large interindividual variation in the plasma concentration versus time profiles of tacrine was apparent despite controlled preclinical experimental conditions (Fig. 1c). The first maximum plasma concentration (Cmax) and the initial absorption kinetic were found to be similar among the tacrine-dosed rats, thus ruling out variation in intestinal drug absorption (Fig. 1c). Interestingly, several rats exhibited a “double-Cmax” profile that was most prominent in the StrR (Fig. 1c). In addition, the StrR were found to have the highest Cmax of tacrine (Cmax = 638 ± 184 ng · mL−1), largest area under the curve (AUC0-∞) (AUC0-∞ = 5494 ± 1714 h · ng · mL−1), and lowest clearance (1272 ± 337 mL · h−1) compared with all other toxicity phenotypes (Fig. 1d). Taken together, the higher systemic exposure to tacrine in the StrR was linked to their double-Cmax profile, which is in turn suggestive of secondary intestinal drug absorption. Our results suggest that tacrine is a direct hepatotoxicant and corroborated previous findings.13-16
Enterohepatic recycling is a common mechanism yielding double-Cmax pharmacokinetic profiles.26, 27 We hypothesize that the glucuronidated metabolites of tacrine are excreted into the intestine via active biliary secretion, where it is subsequently deconjugated by gut bacteria and reabsorbed intestinally as the parent drug. The augmented enterohepatic recycling of tacrine among the StrR increases its systemic exposure and enhances their susceptibility to transaminitis. Consequently, one would expect differential fecal excretion patterns of glucuronidated metabolites. To test our hypothesis, we compared the levels of free and conjugated tacrine and hydroxytacrine metabolites (OH-THA) in StrR and NonR. No significant differences were observed in the excretion of OH-THA between the StrR and NonR (Fig. 1e). However, the mean amount of total tacrine (free tacrine and tacrine-N-glucuronides) excreted by the StrR over 24 hours was significantly higher than that for the NonR (127.4 ± 69.9 nmol · mg−1 dose vs 40.4 ± 17.8 nmol · mg−1 dose; P < 0.05 [Mann-Whitney U test]) (Fig. 1e). Tacrine-N-glucuronide is a phase 2 conjugated metabolite of tacrine generated in the liver. 58.8% ± 10.0% of the total tacrine were excreted as tacrine-N-glucuronide in the feces of StrR, whereas this proportion was significantly lower (15.6% ± 3.4%) for the NonR (P < 0.05 [Mann-Whitney U test]) (Fig. 1f). In the intestines, tacrine-N-glucuronide might undergo deglucuronidation catalyzed by microbial β-glucuronidase to yield free tacrine for completion of enterohepatic recycling. In summary, vastly different fecal excretion patterns were observed between the StrR and NonR that was consistent with their variable pharmacokinetics.
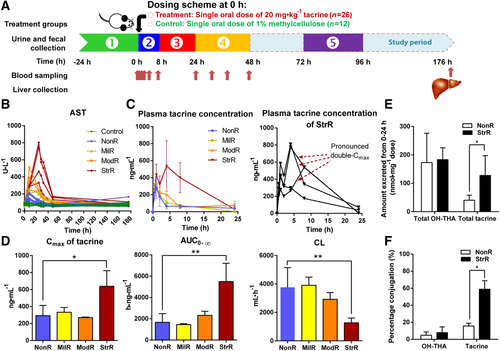
The liver is a major organ involved in drug disposition and detoxification processes. We next profiled the mRNA expression levels of disposition-related genes in rat livers to determine the potential role of host factors in defining the toxicological outcome. No significant difference was found in the mRNA expression levels of the panel of 17 genes (uptake and efflux transporters, phase 1 and 2 metabolizing enzymes, biliary excretion transporter, and nuclear receptors) relevant to the pharmacokinetics of tacrine between the tacrine-dosed rats (n = 26) and controls (n = 12). The expression were also generally similar between each toxicity phenotypes (n = 10) and the NonR (n = 16) (Fig. 2). This suggests that beyond the host's hepatic gene expression, there may be other factors underlying tacrine-induced transaminitis.
MICROBIAL METABOLITES OF TACRINE-INDUCED HEPATOTOXICITY
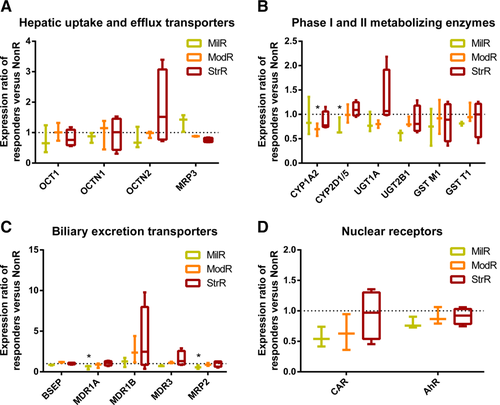
Metabonomics has been demonstrated to provide mechanistic insights in toxicology and reveal information about the crosstalk between the host and gut microbiota in systems biology.4, 28, 29 We performed GC/TOFMS-based metabonomics to screen for transaminitis-related fecal and urinary marker metabolites. Unsupervised principal component analysis revealed similar predose urinary and fecal metabolomes among all control and tacrine-dosed rats (Supporting Fig. S2a,b). A careful analyses of the StrR revealed a metabolic trajectory that followed the progression and resolution of transaminitis over −24-96 hours (Fig. 3a,b). At 0-8 hours, both urinary and fecal metabolomes of tacrine-dosed rats were found to cluster distinctly from the vehicle-dosed controls, indicative of metabolic changes after tacrine administration (Supporting Fig. S2c,d). At the peak of transaminitis (8-24 hours), the StrR were found to form a distinct cluster from the rest of the rats (Supporting Fig. S2e,f).
To screen for transaminitis-related metabolites, we compared the metabolomes of the StrR with the NonR during the peak of transaminitis (8-24 hours). Our analysis uncovered 43 unique urinary marker metabolites and 62 unique fecal metabolites associated with tacrine-induced transaminitis (variable importance in the projection > 1 and/or P < 0.05, |r| > 0.6) (Fig. 3c,d; Supporting Tables S3 and S4). One toxicity hallmark of tacrine is mitochondrial dysfunction.14, 15 We observed an overall depletion in citrate acid cycle and oxidative phosphorylation intermediates such as citrate, cis-aconitate, 2-ketoglutarate, succinate, fumarate, and malate in the urine of StrR associated with tacrine-induced transaminitis (Fig. 3e). This finding is consistent with other earlier mechanistic studies that demonstrated the impact of tacrine on the hepatic mitochondria. The perturbation of these key pathways may hence contribute toward the decline in ATP production by tacrine as observed by Berson et al.14
Notably, 56% and 45% of the respective urinary and fecal marker metabolites are of cometabolic origin,2, 3, 28-31 suggesting the implication of gut microbiota in tacrine-induced transaminitis. We observed an elevated abundance of fecal bile acids such chenodeoxycholic acid and lithocholic acid in StrR. Chenodeoxycholic acid is formed from cholesterol in the liver and biliary excreted into the intestine. Lithocholic acid is a secondary bile acid formed in the intestines by bacterial 7-α dehydroxylation of chenodeoxycholic acid32 and it is a well-established hepatotoxicant when present at high levels.33, 34 Members of the Bacteroides, Clostridium, and Eubacterium genera are known to possess 7-α dehydroxylation activity.35, 36 The distinction in the levels of these bile acids suggested differential regulation of bile acid metabolism or an augmented enterohepatic recycling in the StrR (Supporting Fig. S3). Elevated levels of metabolites such as phenylethylamine, p-hydroxyphenylacetic acid and indoleacetic acid were detected in the feces of StrR compared to NonR. They are major colonic fermentation products of phenylalanine, tyrosine and tryptophan and several Clostridium and Bacteroides species were reported to be responsible for the metabolism of these aromatic amino acids.37 Several long chain fatty acids such as oleic acid, stearic acid and linoleic acid were found in higher abundance in feces of StrR. These fatty acids possess either bactericidal or bacteriostatic activities.38-40 Gram-negative bacteria (e.g., Escherichia coli) generally have higher resistance to the toxic effects of these long chain fatty acids than gram-positive bacteria.41, 42 The elevations of these fatty acids in rats that are highly susceptible to tacrine-induced transaminitis suggest structural changes in the microbial community within the gut of these rats. Collectively, our metabonomics findings suggest a distinctive gut microbiota associated with tacrine-induced transaminitis and provide the impetus for a deeper understanding of the specific bacteria associated with this phenomenon.
GREATER DEGLUCURONIDATION POTENTIAL IN HEPATOTOXICTY
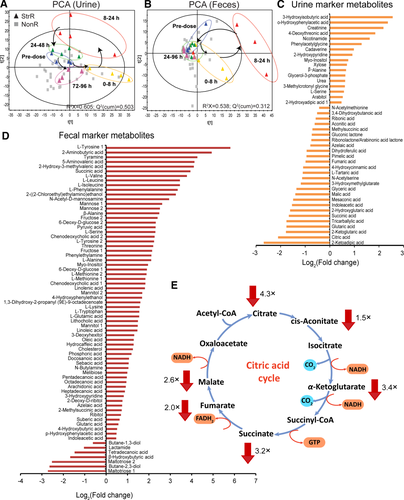
To further characterize changes in the gut microbiome that are associated with tacrine-induced transaminitis, we used 16S rRNA sequencing to profile rat gut microbiota composition before and after tacrine dosing (Supporting Table S5). A high proportion of gut microbiome was determined to be common between LH rats and humans (Supporting Fig. S4). Our data showed increased levels of Bacteroides and Enterobacteriaceae (both known to exhibit β-glucuronidase activity43, 44) in the StrR group compared with the NonR group during peak transaminitis (8-24 hours) (Fig. 4a,b; Supporting Fig. S5). Nevertheless, at baseline levels (before dosing), their abundance was similar for both StrR and NonR (Fig. 4a,b), suggesting that Bacteroides and Enterobacteriaceae may have a preferential growth pattern associated with tacrine administration. In contrast, Lactobacillus, a genus reported to inhibit bacterial β-glucuronidase activity45 and associated with hepatoprotection46 was found at a lower level in the StrR versus NonR groups (Fig. 4c; Supporting Fig. S5).
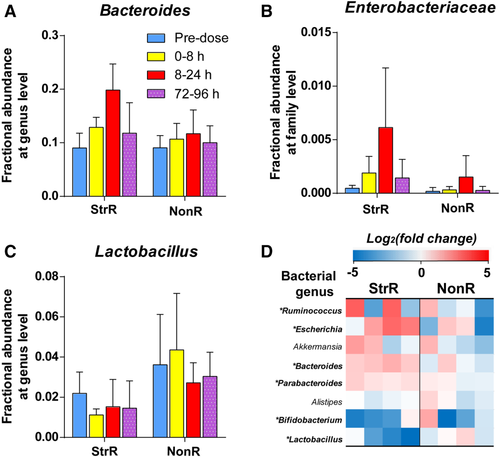
To study the differences in the functional composition of the microbiomes at the gene level, we employed shotgun sequencing of fecal samples collected during peak transaminitis (Supporting Table S6). We first used these data to confirm differences in microbial composition observed using 16S profiling, validating the higher abundance of β-glucuronidase producing bacterial genera in the StrR versus the NonR group (Fig. 4d). At the gene level, we first used the abundance of the uidA gene (encoding an extensively characterized β-glucuronidase) as a proxy and found the gene to be 6.1-fold higher in the StrR than in the NonR group (P < 8.41 × 10−12). Furthermore, we performed an unbiased analysis of all β-glucuronidases genes through a metagenome assembly, gene prediction, and mapping approach, and this also revealed their increased abundance within the microbiome in the StrR versus NonR groups (approximately 9% higher; P < 2.2 ×10−16). Taken together, our results demonstrate an association between gut microbial composition, β-glucuronidase abundance, and tacrine-induced transaminitis.
ORAL β-GLUCURONIDASE AND ANTIBIOTICS MODULATE SUSCEPTIBILITY
To validate the influence of intestinal β-glucuronidase activities on the susceptibility of LH rats to tacrine-induced transaminitis, we administered an oral gavage tacrine (20 mg · kg−1) to LH rats pretreated with a single oral dose of 168,400 units · mL−1 of β-glucuronidase 5 minutes before the administration of tacrine (n = 17) and compared this group with a conventional tacrine-dosed group without β-glucuronidase pretreatment (n = 17) (Fig. 5a). 41% of the conventional LH rats were classified as responders based on AST elevation > 3× average AST of the vehicle-dosed controls. Coadministration of oral β-glucuronidase significantly manipulated and augmented the susceptibility to tacrine-induced transaminitis from 41% to 76% (Supporting Fig. 6a–c) (P < 0.05; one-tailed Fisher's exact test). To confirm the strong correlation of tacrine systemic exposure with susceptibility to tacrine-induced transaminitis, in vivo pharmacokinetics studies of tacrine was further performed. β-glucuronidase treated tacrine-dosed rats were found to have a significantly higher systemic exposure to tacrine than the conventional tacrine-dosed rats (AUC0-last of β-glucuronidase treated rats: 4135 ± 2055 h · ng · mL−1 versus AUC0-last of conventional rats: 2446 ± 1921 h · ng · mL−1; P < 0.05 [Mann-Whitney U test]). Cmax of tacrine was also found to be higher in the former group compared with the latter group (Cmax of β-glucuronidase–treated rats: 608 ± 363 ng · mL−1 versus Cmax of conventional rats: 343 ± 316 ng · mL−1; P < 0.05 [Mann-Whitney U test]). The difference in pharmacokinetic profiles between the two treatment groups corroborated with the higher susceptibility to tacrine-induced transaminitis in the LH rats supplemented with β-glucuronidase.
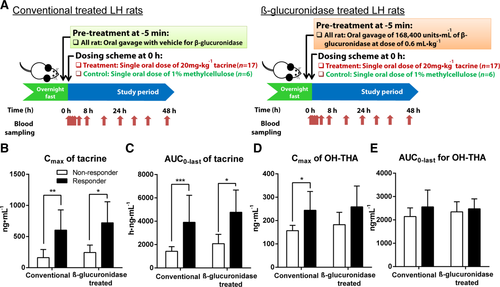
We next investigated the differences in the pharmacokinetic profiles between the toxicity phenotypes derived from the two treatment groups. For both groups of rats, the responders were found to have a significantly higher Cmax and exposure (AUC0-last) of tacrine compared with the NonR (Fig. 5b,c). In addition, these two pharmacokinetic parameters were comparable among the responders from both groups of rats. Cmax and AUC0-last of OH-THA metabolites were generally similar between the responders and NonR in both groups of rats except for a slightly higher Cmax in the responders of conventional rats compared with their NonR (Fig. 5d,e). Because pretreatment of tacrine-dosed LH rats with β-glucuronidase did not alter the pharmacokinetics parameters among the responders from both groups, our findings suggest the kinetics of enterohepatic recycling of tacrine is saturable. Whereas the pretreatment of rats with vancomycin and imipenem did not influence the susceptibility of LH rats to tacrine-induced transaminitis based on AST measurement (47% versus 41%), the susceptibility was significantly decreased from 53% to 35% based on ALT measurement (Supporting Fig. 6a) (P < 0.05; one-tailed Fisher's exact test). In terms of pharmacokinetics profiles for the antibiotics-treated rats, similar observations were made where classification of responder phenotype was consistent with pharmacokinetics data.
Discussion
Understanding the factors that dictate drug disposition and therapeutic outcome is pertinent for optimal pharmacotherapy. Tacrine was selected as our probe for investigation, as it elicits hepatotoxicity that is unpredictably variable. Its clinical withdrawal rendered it an interesting case study. Using LH rats as our animal model, we reproduced the clinically observed variation in tacrine-induced transaminitis.
A systems biology approach that integrates pharmacokinetics, genomics, metabonomics, and metagenomics was adopted. Through a longitudinal temporal pharmacokinetics study, we observed for the first time the occurrence of double-Cmax profile of tacrine in plasma of StrR, which is characteristic of enterohepatic recycling.26, 27 Taken together, higher systemic exposure of tacrine in StrR correlated with the hepatotoxic outcome. With enhanced enterohepatic recycling of the tacrine-N-glucuronide, there was an overall increase in its elimination in the feces due to its incomplete deconjugation within the gut. The seemingly counterintuitive increased rates of fecal glucuronide excretion in the StrR is accounted for by the tandem effects of enhanced enterohepatic recycling of tacrine and its mechanism-based inactivation of hepatic CYP1A2. The detailed modeling of these tandem effects is provided in the Supporting Information (Supporting Data, Supporting Figs. S1-S3). Notably, our hepatic mRNA expression study and Alfirevic et al.'s analysis of single nucleotide polymorphisms in 19 candidate genes associated with tacrine-induced hepatotoxicity47 revealed a lack of host genetic effects on tacrine toxicity. Our metabonomic analyses unveiled marker metabolites associated with the postulated mechanisms of tacrine toxicity,14, 15 reaffirming the presence of liver injury due to tacrine. In addition, the discovery of marker metabolites of microbial and cometabolic origin illuminated the role of gut microbiota in defining the hepatotoxicity of tacrine.
Microbiome analysis revealed an enrichment of β-glucuronidase–producing bacteria such as Bacteroides and Enterobacteriaceae and correspondingly higher β-glucuronidase gene abundance in StrR versus NonR after tacrine administration. Our results suggested the gut microbiome of StrR conferred a greater deglucuronidation capability than NonR that in turn promoted intestinal microbial-mediated deglucuronidation of tacrine-N-glucuronide. The greater augmented enterohepatic recycling of tacrine in the StrR (Fig. 1c,d) resulting from its augmented enterohepatic recycling explains their higher susceptibility to transaminitis.
To validate our findings, we coadministered microbial β-glucuronidase with tacrine in LH rats. The higher susceptibility of these rats to tacrine-induced transaminitis experiencing approximately 1.7-fold higher exposure to tacrine henceforth affirmed the influence of enhanced β-glucuronidase activities on augmenting both tacrine-induced transaminitis and systemic exposure to tacrine. The dosage regimen of the vancomycin and imipenem cocktail is known to possess broad spectrum activity, acting against both gram-positive and gram-negative bacteria in a complementary manner (Supporting Table 7). A 3-day intake of this antibiotic cocktail by rats is evident to 1) reduce bacterial load by 10-fold, 2) reduce bacterial phylotype richness from 217 to 21 operational taxonomic units on average, and 3) lead to near extermination of Bacteroidetes and significant decrease in Firmicutes, which constitute the two major phyla in rats.48 Based on the pretreatment of LH rats using the antibiotics cocktail and ALT measurement, we validated that the removal of gut bacteria modulated and decreased the susceptibility of rats to tacrine-induced transaminitis, which is consistent with our postulation. Taken together, these key findings confirmed the causative role of gut microbiota in defining tacrine-induced hepatotoxicity.
While it is clear from our study that the gut microbiota and their microbial β-glucuronidase activity influence the susceptibility to tacrine-induced transaminitis, the precise factors responsible for microbial perturbation on the administration of tacrine remain to be defined. Given that the abundances of key microbial genera (i.e., Bacteroides, Escherichia, and Lactobacillus) were not significantly different between StrR and NonR groups before dosing, the introduction of tacrine could have resulted in the structural divergence of the gut microbiota. One possibility is that tacrine increases sympathetic activity in the liver leading to vascular constriction and hepatic ischemia.13 When the vascular constriction is relieved, the consequent reperfusion disturbs the ecological balance in the gut.49 Xing et al.49 demonstrated in rats that ischemia/reperfusion liver injury leads to disturbance in gut microbiota where there is a decrease in Bifidobacteria and Lactobacilli and an increase in intestinal Enterobacterium and Enterococcus. Higher abundance of Lactobacilli in the NonR might have protected the rats against the proliferation of Enterobacteriaceae and other microbes that could potentially exacerbate tacrine-induced transaminitis. Delineation of hepatoprotective effect of probiotics in treating drug-induced transaminitis would require further investigation.
In conclusion, our study confirms that the liver–gut microbiota axis modulates the hepatotoxicity of tacrine in the rat. We envisage that the systems biology approach employed here will facilitate our investigation of the complex host–gut microbiota interactions in pharmacology and toxicology. While this study is focused on tacrine, our findings hold potential implications for other therapeutic yet hepatotoxic drugs, particularly those that undergo phase 2 glucuronidation and extensive enterohepatic recycling, demonstrate large interindividual variation in pharmacokinetics and exhibit narrow therapeutic indices.
Acknowledgment
We thank L. S. New and W. P. Yau for technical advice and support for in vivo pharmacokinetic analysis; K. K. Pasikanti, P. W. Goh, and Y. M. Tan for technical support with sample preparation for GC/MS analysis; L. C. Phua and H. T. Chng for technical advice on profiling mRNA expression; A. Sampathkumar for technical help with bioinformatics analysis of mRNA expression results; E. Y. Koh, S. M. Tham, and K. T. Chen for technical advice and support for microbiome analysis; J. C. Y. Chan for technical advice and assistance with LC/MS analysis and helpful discussions; W. S. Chen and C. W. Goh for technical support of animal work; J. K. Nicholson for expert advice on the study and manuscript; S. E. Ng for procurement, technical assistance, and instrument access; K. Lawless for training and guidance on the next generation sequencing experiment; and L. He for the help with primer design for profiling hepatic mRNA expression.




