Synchrony between suprachiasmatic nucleus-driven signals and the light/dark cycle is essential for liver homeostasis
*[Correction added July 14, 2017, after original online publication: Author Natali Guerrero Vargas's family name was changed to “Guerrero-Vargas.”]
Potential conflict of interest: Nothing to report.
The suprachiasmatic nucleus (SCN) our biological clock drives all rhythms in our body. Through synchronized rhythms in hormonal and autonomic output, the SCN prepares the physiology of our body for the changes in activity associated with the light/dark cycle. That this needs to be prepared for activity is so fundamental that all cells in our body are equipped with clock genes that are driven by the SCN in order to prepare the cellular machinery for its daily task. In our modern society, we do not follow sunrise and sunset anymore for activity and food intake, but live in a cycle dictated by work demands, intercontinental travel schedule, and our watches. Slowly, the realization dawns that such a lifestyle is associated with a higher risk of disease, such as the development of obesity, hypertension, and cancer.1 Consequently, there is a strong interest and need to study the penalty of an activity pattern that does not follow the signals of the SCN. For example, the development of nonalcoholic fatty liver disease (NAFLD) has been associated with desynchronization given that it not only develops as a consequence of an excess of food intake, but also following food intake during the rest period or attributed to chronic short sleep.
A recent article by Kettner et al.2 describes the development of a mouse model to study NAFLD and the ensuing hepatocellular carcinoma (HCC), by evaluating the consequences of long-term desynchronization by repeated jet lag. Kettner et al. simply transferred animals weekly, for 90 weeks, between two quarters with a difference in light onset of 8 hours. Such repeated jet lag induces long-term desynchronization whereby the light/dark cycle does not coincide with the activity-food intake pattern of the animals, resulting in accelerated tumor growth.3 The Kettner et al. study used mouse mutants, allowing a view on cellular processes involved in the development of HCC. Interestingly, a nonfunctional clock in the liver as a result of liver knockout of BMAL1 induced equally high liver pathology both in control and in jet-lagged animals, in line with current evidence that disruption of clock gene expression is associated with tumorgenesis. This observation was confirmed with complete clock mutants. Surprisingly, clock mutant animals, in spite of their capacity to immediately follow the changed light/dark cycle in their activity, developed severe liver pathology when regularly shifted, indicating that in contrast with activity, liver metabolism cannot follow such fast changes. Importantly, all animals subjected to jet lag showed not only NAFLD, but also cholestasis before HCC development. The strong element of the article is that the researchers linked these observations with the nuclear receptors, farnesoid X receptor (FXR) and constitutive androstane receptor (CAR), and demonstrated that these nuclear-receptor–controlled cholesterol and bile acid production pathways were deregulated in jet-lagged wild-type (WT) mice. A protective role of FXR was demonstrated; animals without this nuclear receptor rapidly developed NAFLD and showed HCC even without jet lag. Missing CAR, however, lowered bile acids and protected from HCC even after jet lag, indicating that CAR could be an important driver for HCC by stimulating NAFLD to nonalcoholic steatohepatitis (NASH) transition.
Of clinical interest are the elevated serum and hepatic bile acid levels reported in all conditions where more HCC is observed. Such increased levels of bile acids in the liver may induce a cascade of events driving the progression of NAFLD to NASH, fibrosis, and HCC. The loss of bile acid homeostasis may be a direct cause of increased HCC risk, which is supported by the observation of at least 5-fold increased bile acids in FXR–/– and decreased levels in CAR–/– animals as compared to WT. Given that bile acid production and release are mainly driven by food intake and parasympathetic output, the observed changes in the sympathetic output rather indicate that the whole autonomic balance in the jet-lagged animals is changed.
These observations tally well with recent studies,4 indicating that development of tumors elsewhere in the body may produce disturbed metabolic conditions in the liver, resulting in high circulating glucose and low liver free fatty acid levels. Interestingly, such disruption is completely independent of the clock gene rhythm in the liver and relates more to increased inflammatory molecules in the circulation. What can be envisioned from these two studies is that metabolic disturbances may promote tumor growth, indicating that the susceptibility for cancer development is beyond the liver when subjects are desynchronized.
The question arises with the current attention to the role of the biological clock and desynchronized lifestyle for our health; what is the significance of these observations for the role of the SCN, the clock genes, and the so-called clock controlled genes (CCGs) in liver physiology? First, these and numerous other observations show that CCGs can (and should be) influenced by many other processes that take place in the cells. For example, Vollmers et al.5 describe that in constant darkness, around 15% of genes in the liver have a rhythm; however, they conclude: “In the absence of feeding, only a small subset of transcripts continued to express circadian patterns,” suggesting that SCN-driven behavioral and hormonal signals are very important for expression of rhythmicity in the liver and not necessarily the clock genes. This is supported by a recent study using caloric restriction demonstrating increased amplitude of clock gene expression, but a loss of rhythm in several CCGs,6 demonstrating that other factors may have stronger effects on CCGs. Moreover, clock gene expression itself is dependent on many different factors, of which food intake and corticosterone secretion are the most important ones.7 Consequently, as can be concluded from the Kettner et al. study, clock gene disturbance does not necessarily indicate a clock-gene–driven mechanism for induction of HCC; a clock mutant may have an altered cellular homeostasis without pointing to direct linked molecular mechanisms for tumor development.
Under normal conditions, the circadian clock has at least two different, but synchronized, avenues through which bile acid production and release are influenced: (1) Through SCN-driven autonomic output, the parasympathetic system induces bile acid production and release. (2) Through SCN-driven rhythms in food intake also, bile acid production and release is stimulated (Fig. 1). Disruption of this pattern by jet lag or desynchronized feeding, that is, food intake during the sleep phase, produces signals that conflict with those of the SCN and change bile acid production and secretion, followed by accumulation of bile acids in the liver and in NAFLD. The present study adds to this, the development of HCC, and indicates the involvement of genes associated with bile acid production and detoxification in the progression of nonalcoholic fatty liver (NAFL) to HCC. This may reveal novel therapeutic targets to explore; however, lifestyle strategies that curb circadian, and hence metabolic, disruption in order to control NAFL progression and associated diseases are urgently needed.
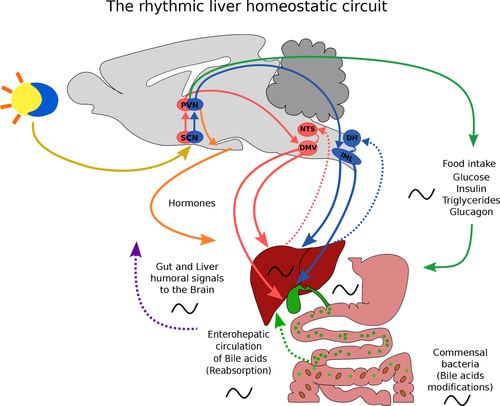
Rhythmic liver homeostatic circuit. Abbreviations: DMV, dorsal motor nucleus of the vagus; DH, dorsal horn; IML, intermediolateral cell column; NTS, nucleus tractus solitarius; PVN, paraventricular nucleus of the hypothalamus.
-
Ruud M. Buijs, Ph.D.1
-
Natali Guerrero-Vargas, Ph.D.2*
-
1Department of Cell Biology and Physiology
-
Institute for Biomedical Research
-
Universidad Nacional Autónoma de México, UNAM México City, México
-
2Department of Anatomy
-
Faculty of Medicine
-
Universidad Nacional Autónoma de México, UNAM México City, México




